Abstract
The common pathogenetic pathway of progressive injury in patients with chronic kidney disease (CKD) is epitomized as normal kidney parenchymal destruction due to scarring (fibrosis). Understanding the fundamental pathways that lead to renal fibrosis is essential in order to develop better therapeutic options for human CKD. Although complex, four cellular responses are pivotal. (1) An interstitial inflammatory response that has multiple consequences—some harmful and others healing. (2) The appearance of a unique interstitial cell population of myofibroblasts, primarily derived from kidney stromal cells (fibroblasts and pericytes), that are the primary source of the various extracellular matrix proteins that form interstitial scars. (3) Tubular epithelial cells that have variable and time-dependent roles as early responders to injury and later as victims of fibrosis due to the loss of their regenerative abilities. (4) Loss of interstitial capillary integrity that compromises oxygen delivery and leads to a vicious cascade of hypoxia–oxidant stress that accentuates injury and fibrosis. In the absence of adequate angiogenic responses, a healthy interstitial capillary network is not maintained. The fibrotic ‘scar' that typifies CKD is an interesting consortium of multifunctional macromolecules that not only change in composition and structure over time, but can be degraded via extracellular and intracellular proteases. Although transforming growth factor beta appears to be the primary driver of kidney fibrosis, a vast array of additional molecules may have modulating roles. The importance of genetic and epigenetic factors is increasingly appreciated. An intriguing but incompletely understood cardiorenal syndrome underlies the high morbidity and mortality rates that develop in association with progressive kidney fibrosis.
Keywords: extracellular matrix, interstitial capillaries, kidney fibrosis, macrophages, myofibroblasts
The high prevalence and burden of chronic kidney disease (CKD) is well established. It is generally accepted that all primary causes of CKD share a common pathogenetic pathway of progressive injury due to the destructive consequences of scarring (fibrosis). Despite extensive research efforts to identify the critical cellular and molecular mediators of fibrosis, this rapidly expanding body of new knowledge has yet to be translated into clinical practice. Failure to do so has resulted in an estimated 13–16% of the adult population living with CKD (defined as individuals with an estimated glomerular filtration rate (eGFR) of <60 ml/min per 1.73 m2) who face the possible need of renal replacement therapy with dialysis and/or a kidney transplant at some point in the future. Unfortunately, the majority will never reach that point as CKD confers at least a fivefold increased risk of premature death due to accelerated cardiovascular disease. Recent and ongoing advances in basic science research provide the necessary platform for designing and testing novel therapies to change the unfortunate fate of CKD patients. The potential therapeutic repertoire is vast. It is conceivable that the next generation of personalized medicine will make it possible to offer specific therapeutic protocols comprising multiple agents that are selected based on the specific molecular signature of the scar-generating response that uniquely defines the CKD response in an individual. The key participants are now known, and therapeutically targetable steps are rapidly emerging. Four distinct cellular participants are known and the ideal therapeutic protocol should significantly regulate each of them (Figure 1).
Multifunctional inflammatory cells, especially macrophages;
myfibroblasts that determine the accumulation rate and molecular composition of the extracellular matrix proteins that constitute the kidney scars;
microvascular endothelial cells;
tubular epithelial cells.
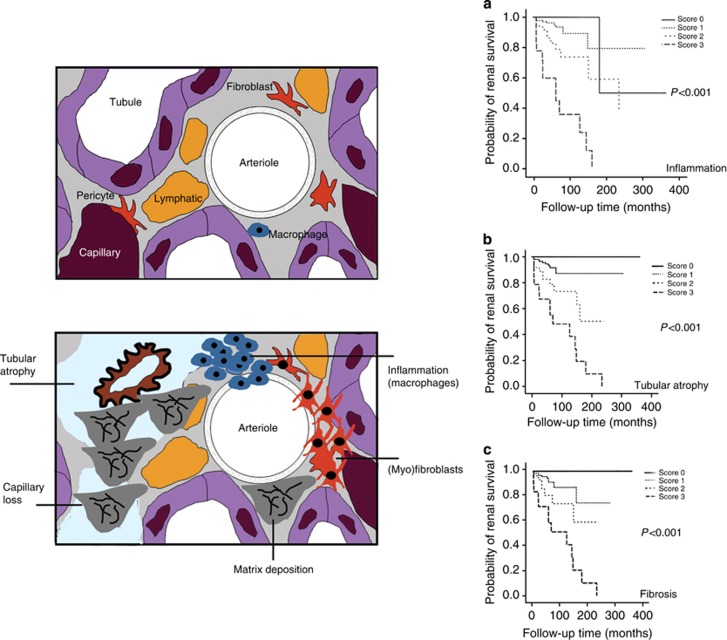
Figure 1.
Schematic overview of the primary fibrogenic events. The upper figure depicts the key elements of the tubulointerstitium in normal kidneys. The myeloid cell population may be designated as dendritic cells rather than macrophages. The lower panel represents the key changes that are discussed further in this review: de novo appearance of an interstitial population of unique fibroblastic cells that also express alpha smooth muscle actin and are the primary source of the expanded extracellular matrix that leads to destructive interstitial scarring; an interstitial inflammatory response composed of primarily of blood-borne lymphohematopoietic cells (macrophages in particular are key drivers of both fibrosis and tissue repair); and progressive renal parenchymal loss due to interstitial capillary rarefaction and tubular atrophy. Graphs illustrate the statistically significant histopathological indicators of renal survival (free of end-stage kidney disease or serum creatinine doubling) with follow-up (shown in months) in a study of 313 patients with biopsy-confirmed lupus nephritis. The severity scores for (a) interstitial inflammation, (b) tubular atrophy, and (c) interstitial fibrosis were based of the following: 0 (nil), normal; 1+ (mild), <25% of the interstitial area affected; 2+ (moderate), 25–50% of the tubulointerstitial are involved; 3+ (severe), >50% of the interstitial area involved. (The graphs are reproduced with permission from Macmillan Publishers Ltd: Yu F et al. Kidney Int 2010;77:820–829.)
Transforming growth factor beta (TGFβ) is thought to be the grand master that elicits numerous signals that culminate in fibrosis and renal parenchymal loss.
INFLAMMATORY CELLS
All CKD is characterized by an interstitial infiltrate of macrophages, the density of which correlates inversely with kidney survival.1 Depending upon local environmental cues, macrophages can synthesize and secrete a variety of products that influence fibrogenesis. These include growth factors and cytokines (TGFβ, platelet-derived growth factor, fibroblast growth factor, tumor necrosis factor alpha, interferon gamma, hepatocyte growth factor), enzymes and their inhibitors (angiotensin-converting enzyme, plasminogen activators, plasminogen activator inhibitor-1, collagenases, tissue inhibitor of metalloproteinases), matrix proteins (collagen, fibronectin, thrombospondin), and many others (complement proteins, coagulation factors, bioactive lipids, reactive oxygen species, nitric oxide, endothelin, etc.)2 A variety of experimental cell depletion strategies have shown that reducing the number of interstitial macrophages reduces kidney fibrosis. The most recent experiments have taken advantage of genetic engineering strategies to selectively deplete macrophages and have shown remarkable benefit, even in genetic models such as polycystic kidney disease, a common cause of human CKD for which the important pathological interstitial changes have lived in the shadows of cystogenesis until recently.3
Although the multifunctional potential of macrophages associated with tissue injury has long been recognized, important scientific advances over the past decade, derived in large part from studies in mice, have begun to clarify the molecular basis of their functional diversity.4 Classically activated ‘M1' macrophages and alternatively activated ‘M2' macrophages appear to originate from monocytes that are exposed to distinct and non-overlapping local stimuli. Best characterized are interferon gamma, lipopolysaccharide, tumor necrosis factor and granulocyte–macrophage colony–stimulating factor for the M1 subset that are primarily associated with tissue injury, whereas interleukin-4, interleukin-13, interleukin-10, corticosteroids, vitamin D, macrophage colony–stimulating factor and TGFβ preferentially polarize to the M2 subset that are more likely to promote tissue repair and injury resolution. Further work is needed to delineate the differential effector functions between M1 injury induction and M2 injury resolution. Some clues may come from gene-, protein- and metabolic profiling studies. For e.g., mannose receptor I, Yim-1 and arginase 1 appear restricted to M2 cells. The role of macrophages in injury repair was clearly demonstrated in a model of reversible liver injury: macrophage depletion during the phase of injury induction lessened the degree of fibrosis, but when their depletion was delayed until the phase of injury resolution, fibrosis severity was worse.5 As fibrosis is an integral component of wound healing, further investigation is necessary to understand the differences between M2 responses that lead to ‘adaptive' tissue repair with minimal scarring and restoration of normal parenchyma versus ‘maladaptive' tissue repair with irreversible parenchyma loss that leads to CKD. However, this emerging body of knowledge highlights the therapeutic potential of cell-based therapies using macrophages that are primed ex vivo to mediate adaptive repair of injured kidney tissue. Proof of concept experiments using animal models support this hypothetical approach in the future.6
MYOFIBROBLASTS
Myofibroblasts are a unique population of cells that appear de novo in the renal interstitium during fibrosis.7 The presence of these cells appears essential for scar formation and despite limited human data, their numbers appear to correlate with renal outcomes. Expression of alpha smooth muscle actin (αSMA) by interstitial cells with a characteristic fibroblastic morphology is their defining feature. A variety of in vitro studies and mRNA in situ hybridization studies in kidney tissues identify myofibroblasts as the primary source of the scar-forming matrix proteins, suggesting that their presence is essential for fibrosis. Given their central importance, the cellular origin of these cells is a critical question that has been extensively investigated in animal models, but conflicting data leave this as a subject of an ongoing debate. Recent lineage-tracing studies have employed unique genetic engineering strategies and a variety of cell-tracing methodologies and, perhaps not surprisingly, contradictory results have been reported.7, 8, 9, 10 What is clear is that matrix-producing αSMA+ interstitial cells have several potential cellular origins. It is likely that each has unique recruitment and activation pathways in addition to TGFβ. Furthermore, αSMA expression itself may not be a critical fibrosis-promoting protein, as renal fibrosis was reported to be more severe in mice with genetic αSMA deficiency.11 Subsets of these cells also express receptors that can internalize and degrade extracellular matrix, as has recently been shown for mannose receptor 2.12 Whether cellular origin has a role in myofibroblasts functional heterogeneity remains to be determined.
Although the jury is still deliberating, it appears that the majority of the interstitial myofibroblasts are derived from a pool of endogenous kidney cells that migrate, proliferate, and transform. Resident kidney fibroblasts and microvascular pericytes are the favored leading contenders as the primary myofibroblast source(s).13 Small numbers of matrix-producing interstitial cells also originate from myeloid lineage bone marrow cells (fibrocytes), whereas in severely damage kidneys, local cells (tubular epithelium, endothelium, and perhaps macrophages) may transdifferentiate into αSMA+ matrix-producing cells, but overall their numbers appear to be small and their presence delayed until the advanced stages of CKD.14
TUBULAR EPITHELIA
During the induction phase of chronic kidney injury, tubular epithelial cells actively participate in injurious pathways through their ability to synthesize products, such as reactive oxygen species, and inflammatory mediators, such as chemokines, that find their way into the interstitium via basolateral secretion or via paracellular pathways by escaping through tight junction barriers. A variety of abnormally filtered urinary proteins derived from the systemic plasma pool or upstream glomerular cells may engage tubular epithelia in these events.15 Urinary proteins such as members of the complement cascade or cytokines may activate specific cellular responses by binding to their cognate receptors on tubular apical membranes. An alternative activation pathway that is triggered by biochemically modified or conjugated urinary albumin involves proximal tubular megalin receptors that mediate protein endocytosis and activate specific signaling responses in partnership with its co-receptors cubilin and amnionless.16 This latter pathway has been associated with stimulated synthesis of inflammatory chemokines (monocyte chemoattractant protein-1, regulated on activation normal T-cell expressed and secreted, interleukin-8, fractalkine), profibrotic molecules (TGFβ, endothelin), and the transdifferentiation of tubular epithelial into αSMA+ cells. Extensively investigated in cell culture systems, the degree to which proteinuria triggers these tubular cell responses in vivo is still not clear, but this paradigm is thought to explain in part the undisputed fact that the degree of proteinuria closely correlates with chronic inflammatory and fibrosis pathways that typify CKD.
As fibrosis severity increases, tubular epithelia that normally have a potent regenerative capacity lose this ability and succumb due to apoptosis or accelerated senescence. The reason for this transition from regenerative to dying cells is not well understood, but appears to involve cell-cycle specific factors, autophagy failure, endoplasmic reticulum stress, oxidative stress, and the loss of unknown ‘regenerative signals'.17, 18, 19 Tubular cell death is a hallmark feature of renal parenchymal damage that leads to serious negative outcomes, as it leaves behind non-functional atubular glomeruli. Histological measures of tubular cell area closely correlate with renal function.20 An essential ingredient of effective human CKD treatment will be the ability to preserve and/or regenerate functional epithelia to preserve intact and functional nephrons. These regenerative responses are likely to recapitulate normal nephrogenic pathways of kidney development. Important clues to new candidate regenerative pathways are likely to emerge now that next-generation sequencing technologies are delineating genetic mutations that cause human renal hypodysplasia, such as wingless-type MMTV integration family, member 4.21
INTERSTITIAL CAPILLARIES
Given the high oxygen needs to support the metabolic activities of the kidney, an analogy has been made between CKD due to progressive fibrosis and suffocation.22 One of the early events in chronic kidney injury is an increase in the permeability of the interstitial microvasculature.23 As a result, many normally excluded plasma proteins such as fibrinogen and albumin conjugates leak into the interstitium (the kidney capillary leak syndrome) and trigger an inflammatory and potentially profibrotic response. The critical plasma proteins have not been identified in CKD but one of them appears to be fibrinogen (ogen), as genetic fibrinogen deficiency reduces the number of αSMA+ interstitial cells in experimental CKD.24 Although many chronic pathological disorders are characterized by excessive angiogenesis, CKD suffers from the opposite problem—failure of reparative angiogenesis and a progressive decline in the surface area of interstitial capillaries. This has led investigators to consider the possibility of pro-angiogenic factors as therapy for CKD or, alternatively, blockade of anti-angiogenic factors that are presumed to be unregulated and harmful during the course of progressive kidney scarring.25
The hypoxia–oxidant stress connection is thought to be closely coupled with the damaging consequences of kidney fibrosis. Evidence of significant tubular cell oxidant stress is a universal feature of chronically damaged kidneys, which is likely a consequence of both the excessive generation of reactive oxygen species and inadequate antioxidant defenses. Much remains to be learned about the identity and effects of the specific molecular targets of reactive oxygen species that promote kidney fibrosis, something we are likely to learn more about in the near future, as metabolomic studies are defining the specific profile of sugars, nucleotides, amino acids, and lipids in normal and fibrotic kidneys. A role for oxygen species as cell signaling molecules has been recognized and may also prove relevant to fibrogenic pathways.26 With the growing application of high-throughput screening technologies to screen large drug libraries, the possibility that drugs with the ability to alter the redox potential in damaged kidneys should be therapeutically beneficial. For e.g., our group has recently shown that the drug cysteamine (approved for human use to prevent nephropathic cystinosis) also significantly reduces kidney fibrosis in non-cystinotic experimental kidney disease models, an effect that is associated with a reduction in oxidant generation and a reduction in protein oxidation.27
MODELING AND REMODELING OF INTERSTITIAL KIDNEY SCARS
Far from being a boring conglomerate of collagen, the fibrotic ‘scar' that typifies CKD is an interesting consortium of multifunctional macromolecules that change in composition and structure over time. Although the fibrillar collagens I and III often predominate, additional members include other collagens, traditional basement membrane proteins (collagen IV, laminin, nidogen, heparin sulfate proteoglycan), large proteoglycans (aggrecan, versican), small proteoglycans (decorin, biglycan, fibromodulin), glycoprotein (fibronectin, tenascin), and many others (secreted protein acidic and rich in cysteine, which is also known as SPARC or osteonectin, thrombospondin, vitronectin, hensin, etc). The area of the fibrotic interstitium is the best negative histological correlate of renal function and long-term renal prognosis. Within this destructive maze of molecules are some that serve beneficial roles. Examples include decorin that can block the activity of TGFβ and biglycan, which inhibits the conversion of fibroblasts into myofibroblasts.28, 29 A variety of cellular receptors engage specific extracellular matrix proteins to activate intracellular signaling pathways and cellular responses. Although collagen deposition has become synonymous with fibrosis, it is not yet known whether it is the key determinant of parenchymal destruction associated with progressive fibrosis, or whether other molecules serve an essential role. This is an important consideration, as many antifibrotic therapies have been designed with the goal of reducing collagen synthesis or enhancing its turnover.
Assuming the collagen is the primary culprit of fibrosis-induced cellular loss, it is remarkable that the in vivo key enzymatic pathways that remodel and degrade collagen are still unclear. Despite high synthesis rates, collagen does not accumulate in normal kidneys. For e.g., in normal mouse kidneys, ∼20% of the total collagen content is newly synthesized every 2 weeks. Five enzyme groups have identified collagenase activities: certain matrix metalloproteinases, serine proteases, the ADAMTS family and lysosomal enzymes (cysteine and aspartic proteases). It was widely assumed that specific extracellular enzymes of the matrix metalloproteinase family were primarily responsible for degrading collagen molecules during wound healing to prevent excessive scarring. But remarkably, none of the specific matrix metalloproteinases that have been tested in knockout mice have been shown to reduce fibrosis severity, whereas some (matrix metalloproteinase-2, -7, -9) actually worsen fibrosis. These adverse outcomes are likely a consequence of their pleitropic effects that include proteolytic activation of several latent growth factors and cell receptor–dependent activities. It has only been in recent years that the importance of cell-dependent pathways of collagen remodeling have been recognized. In a sequence of steps that involve collagen endocytosis, lysosomal transport and cathepsin-medicated proteolysis, extracellular collagen molecules can be degraded. In experimental models of kidney fibrosis, the mannose receptor 2 (Mrc2) (also known as urokinase receptor–associated protein and Endo180), which is expressed by ^15% of interstitial myofibroblasts and macrophages, significantly reduces fibrosis severity compared with mice with genetic Mrc2 deficiency.12 The appearance of these Mrc2+ cells is unique to damaged kidneys, and the specific inducers of Mrc2 expression are still under investigation. Other receptors known to endocytose and degrade collagen include mannose receptor 1, α1β1 and α2β1 integrins and milk fat globule epidermal growth factor 8.30 The recent recognition that lysosomal enzymes of the cathepsin family appear to serve an important antifibrotic role suggests another way that specifically engineered cellular therapies might find a role in the clinical arena in the future.
MOLECULAR DRIVES OF FIBROSIS: DOES EVERYONE NEED TGFβ?
TGFβ is the prototypic fibrogenic growth factor. Engagement of its cognate receptors (II and I) has the potential to activate a variety of canonical and non-canonical intracellular signaling and regulating pathways that have been extensively investigated and numerous fibrosis-promoting target genes have been identified.31 Both tubular and interstitial cells can synthesize TGFβ and, although not the only cellular targets, fibroblasts and myofibroblasts are particularly responsive to TGFβ stimulation. Although TGFβ is theoretically the ideal molecular target for antifibrotic therapies, it has multiple cellular functions including essential immunoregulatory actions. At least in mice, genetic TGFβ1 deficiency is not compatible with life. An ever-growing panel of molecules are known to elicit fibrosis-promoting effects and, for the majority, effects on the TGFβ pathway are directly or indirectly involved. For most of them, it remains unknown whether fibrosis could develop in the complete absence of TGFβ. Some of the best known fibrogenic molecules are members of the rein-angiogenesis system, reactive oxygen species, other growth factors (connective tissue growth factor, platelet-derived growth factor, fibroblast growth factor, epidermal growth factor, tumor necrosis factor alpha), plasminogen activator ihibtor-1, endothelin-1, a variety of proteases, protease inhibitors, chemokines, adhesion molecules (especially certain integrins), specific matrix molecules, cluster of differentiation 36 (a class B scavenger receptor), the Wnt1/β catenin pathway, Notch, hedgehog legends, KCa3.1 channel, lysophosphatidic acid, homeodomain-interacting protein kinase 2, the myeloid differentiation primary response gene 88 pathway, and parathyroid hormone–related protein. Similarly, the extent to which TGFβ is an essential target of the various antifibrotic molecules is unclear. Some of better known antifibrotic molecules that have been considered candidates for the treatment of human CKD are hepatocyte growth factor, bone morphogenic protein-7, bradykinin, relaxin, heme oxygenase, interferon gamma, vitamin D, adiponectin, adenosine A2A, klotho, kielin/chordin-like, rapamycin pathway, transient receptor potential cation channel subfamily V member 1, Wnt7 and lipoxins.
GENETIC AND EPIGENETICS REGULATION OF THE FIBROTIC RESPONSE
The high degree of variability in the outcome and long-term prognosis among groups of patients with the same primary kidney disease is well recognized. The quest to understand this variability is a fundamental step if personalized medicine is ever to become a reality for CKD patients. There is no question that genetics plays a significant role, perhaps best illustrated by ethnicity-dependent variations in kidney outcomes. The apolipoprotein L1 genotype (G1 and G2 risk alleles) influences prognosis in African Americans is a recent example of genetic determinants of fibrosis severity.32 New insights have emerged recently through the use of genome-wide association studies to establish significant associations with the risk and severity of CKD, although these associations do not establish causality and gene polymorphisms do not necessarily result in changes in protein function. Nonetheless, genome-wide association studies in human CKD represent an unbiased approach to identify new candidates that deserve further investigation for their potential role in the fibrogenic responses that led to CKD. One such example is uromodulin (UMOD), identified in several recent human genome-wide association studies as a significant determinant of CKD risk and severity (reviewed in ref. 33). UMOD encodes a protein that is uniquely expressed in the thick ascending limb of the loop of Henle and the early distal tubule. Although the primary function of UMOD remains unknown, it is the most abundant protein found in normal human urine, and protein-encoding genetic mutations are a known cause of familial CKD characterized as chronic tubulointerstitial nephritis. With the rapidly declining cost and analysis time for human genetic studies, it is anticipated that significant new insights into the genetic basis of CKD risk and prognosis will soon emerge.
Other heritable factors may also influence fibrogenic responses without altering the protein-coding gene sequences. Specific mechanisms may include DNA methylation, histone modification, and microRNA activities. Each of these processes has the potential to modify renal fibrotic responses and specific examples have recently been published, with many more likely to emerge. For e.g., in experimental models of kidney fibrosis, the number of interstitial myofibroblasts was significant reduced by a demethylating agent, a histone deacetylase inhibitor, and anti-microRNA-21 therapy, noting that microRNA-21 unregulation has been reported in several animal and human CKD tissue samples.34, 35, 36, 37 Epigenetic regulation of fibrotic responses provides a new way to investigate environmental influences on genetically regulated pathways, such as kidney fibrosis.
CARDIORENAL SYNDROME
Ending where this overview began, it is important to remember that most CKD patients will never reach the point of needing renal replacement therapy to sustain life; they are more likely to die prematurely due to accelerated cardiovascular diseases. The CKD risk rises almost exponentially as renal function declines. In a study by DuBose et al.,38 the age-specific risk of cardiovascular events was 17-fold higher in individuals with an eGFR<15 ml/min per 1.73 m2 compared with these with an eGFR>60 ml/min per 173 m2. Several factors are thought to contribute to the pathogenesis of this cardiorenal syndrome, including many that are injurious for both organs and cause endothelial cell dysfunction, inflammation, smooth muscle cell proliferation, oxidative stress, and vascular calcification. Therapeutic interventions that attenuate kidney fibrosis, hypertension, and/or proteinuria should also diminish the incidence and severity of cardiovascular disease and improve patient survival.
Over the past two decades, basic science research has greatly advanced our understanding of the cellular and molecular pathways that transform a kidney with normal structure and function into one of compromised function that is likely to worsen over time due to a relentless process of fibrosis. Although many questions remain to be answered, we must now do a better job of translating this knowledge into new and effective strategies to prevent, treat and perhaps even cure human CKD.
Acknowledgments
I am grateful for research funding from the Child and Family Research Institute (Vancouver, BC), the National Institutes of Health, Seattle Children's Research Institute, Cystinosis Research Foundation, Juvenile Diabetes Foundation, the Canadian Institutes of Health Research, the Kidney Foundation of Canada, and the SickKids Research Foundation (Toronto, ON) for current and past grant funding that has enabled active engagement in research studies on the cellular and molecular basis of kidney fibrosis over the past 28 years. I also acknowledge the contribution of many trainees, research assistants, colleagues, and collaborators to our work that was cited in this review. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association. Dr Eddy, with Seattle Children's Research Institute, has filed a patent for the use of cysteamine for the treatment of kidney fibrosis.
The author declared no competing interests.
References
- Yu F, Wu LH, Tan Y, et al. Tubulointerstitial lesions of patients with lupus nephritis classified by the 2003 International Society of Nephrology and Renal Pathology Society system. Kidney Int. 2010;77:820–829. doi: 10.1038/ki.2010.13. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17:2964–2966. doi: 10.1681/ASN.2006070704. [DOI] [PubMed] [Google Scholar]
- Karihaloo A, Koraishy F, Huen SC, et al. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2011;22:1809–1814. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Harris DC. Macrophages in renal disease. J Am Soc Nephrol. 2011;22:21–27. doi: 10.1681/ASN.2010030269. [DOI] [PubMed] [Google Scholar]
- Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol. 2009;5:319–328. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu VS, Taduri G, O'Connell J, et al. Origin and function of myofibroblasts in kidney fibrosis. Nat Med. 2013;19:1047–1053. doi: 10.1038/nm.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeji M, Moriyama T, Oseto S, et al. Smooth muscle alpha-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J Biol Chem. 2006;281:40193–40200. doi: 10.1074/jbc.M602182200. [DOI] [PubMed] [Google Scholar]
- Lopez-Guisa JM, Cai X, Collins SJ, et al. Mannose receptor 2 attenuates renal fibrosis. J Am Soc Nephrol. 2012;23:236–251. doi: 10.1681/ASN.2011030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy AA. The origin of scar-forming kidney myofibroblasts. Nat Med. 2013;19:964–966. doi: 10.1038/nm.3299. [DOI] [PubMed] [Google Scholar]
- Reich BJ, Smith LB. Bayesian quantile regression for censored data. Biometrics. 2013;69:651–660. doi: 10.1111/biom.12053. [DOI] [PubMed] [Google Scholar]
- Zandi-Nejad K, Eddy AA, Glassock RJ, et al. Why is proteinuria an ominous biomarker of progressive kidney disease. Kidney Int Suppl. 2004;92:S76–S89. doi: 10.1111/j.1523-1755.2004.09220.x. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H, Storm T, et al. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda) 2012;27:223–236. doi: 10.1152/physiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zepeda-Orozco D, Black R, et al. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–1778. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Czerniak S, DiRocco DP, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackensen-Haen S, Bohle A, Christensen J, et al. The consequences for renal function of widening of the interstitium and changes in the tubular epithelium of the renal cortex and outer medulla in various renal diseases. Clin Nephrol. 1992;37:70–77. [PubMed] [Google Scholar]
- Vivante A, Mark-Danieli M, Davidovits M, et al. Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol. 2013;24:550–558. doi: 10.1681/ASN.2012010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- Yamaguchi I, Tchao BN, Burger ML, et al. Vascular endothelial cadherin modulates renal interstitial fibrosis. Nephron Exp Nephrol. 2012;120:e20–e31. doi: 10.1159/000332026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen I, Susnik N, Inhester T, et al. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011;80:1035–1044. doi: 10.1038/ki.2011.214. [DOI] [PubMed] [Google Scholar]
- Long DA, Norman JT, Fine LG. Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura DM, Bahrami NM, Ren S, et al. Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD. J Am Soc Nephrol. 2014;25:43–54. doi: 10.1681/ASN.2012090962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Melchior-Becker A, Dai G, Ding Z, et al. Deficiency of biglycan causes cardiac fibroblasts to differentiate into a myofibroblast phenotype. J Biol Chem. 2011;286:17365–17375. doi: 10.1074/jbc.M110.192682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen DH, Leonard D, Masedunskas A, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202:951–966. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Wasser WG, Tzur S, Wolday D, et al. Population genetics of chronic kidney disease: the evolving story of APOL1. J Nephrol. 2012;25:603–618. doi: 10.5301/jn.5000179. [DOI] [PubMed] [Google Scholar]
- Eddy AA. Scraping fibrosis: UMODulating renal fibrosis. Nat Med. 2011;17:553–555. doi: 10.1038/nm0511-553. [DOI] [PubMed] [Google Scholar]
- Luo Y, Wang C, Chen X, et al. Increased serum and urinary microRNAs in children with idiopathic nephrotic syndrome. Clin Chem. 2013;59:658–666. doi: 10.1373/clinchem.2012.195297. [DOI] [PubMed] [Google Scholar]
- Wang G, Kwan BC, Lai FM, et al. Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol. 2012;36:412–418. doi: 10.1159/000343452. [DOI] [PubMed] [Google Scholar]
- Ben-Dov IZ, Muthukumar T, Morozov P, et al. MicroRNA sequence profiles of human kidney allografts with or without tubulointerstitial fibrosis. Transplantation. 2012;94:1086–1094. doi: 10.1097/TP.0b013e3182751efd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacki F, Savary G, Gnemmi V, et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One. 2013;8:e58014. doi: 10.1371/journal.pone.0058014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose TD., Jr. American Society of Nephrology Presidential Address 2006: chronic kidney disease as a public health threat—new strategy for a growing problem. J Am Soc Nephrol. 2007;18:1038–1045. doi: 10.1681/ASN.2006121347. [DOI] [PubMed] [Google Scholar]