Abstract
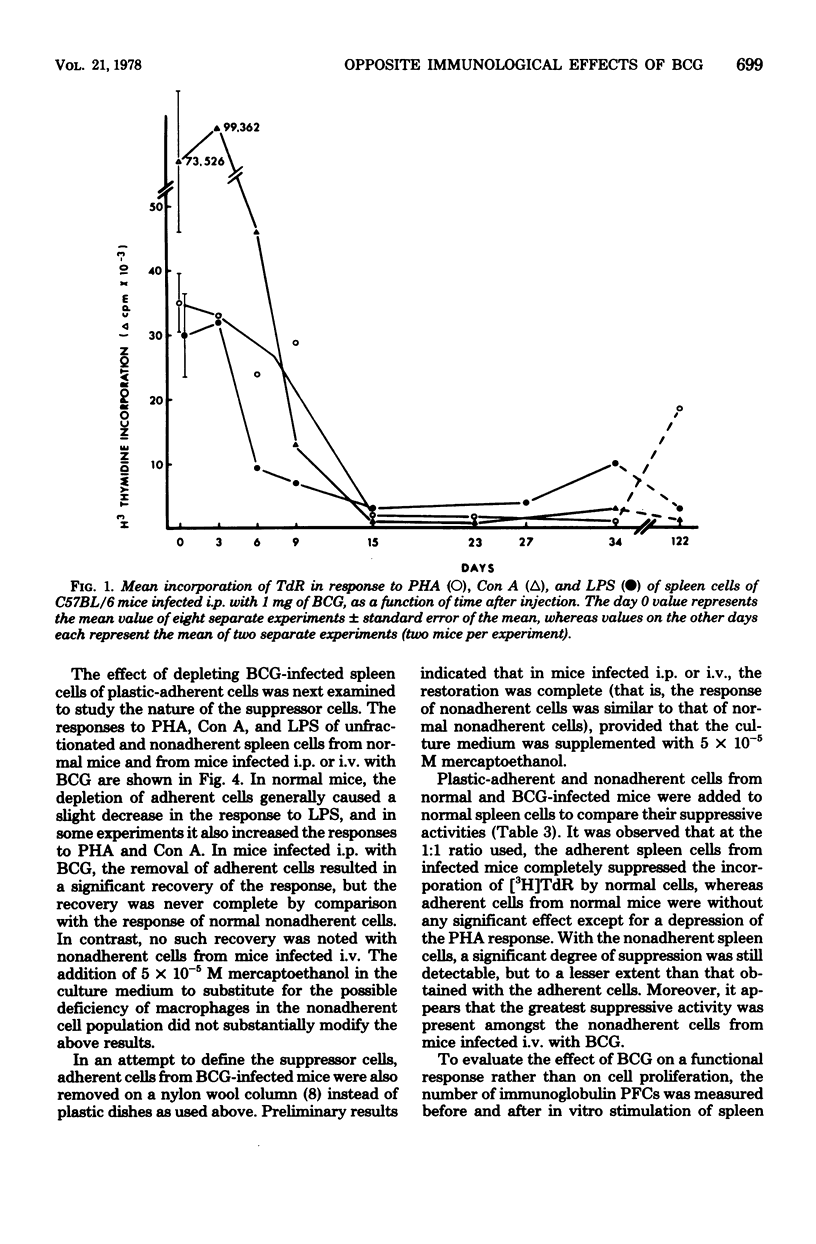
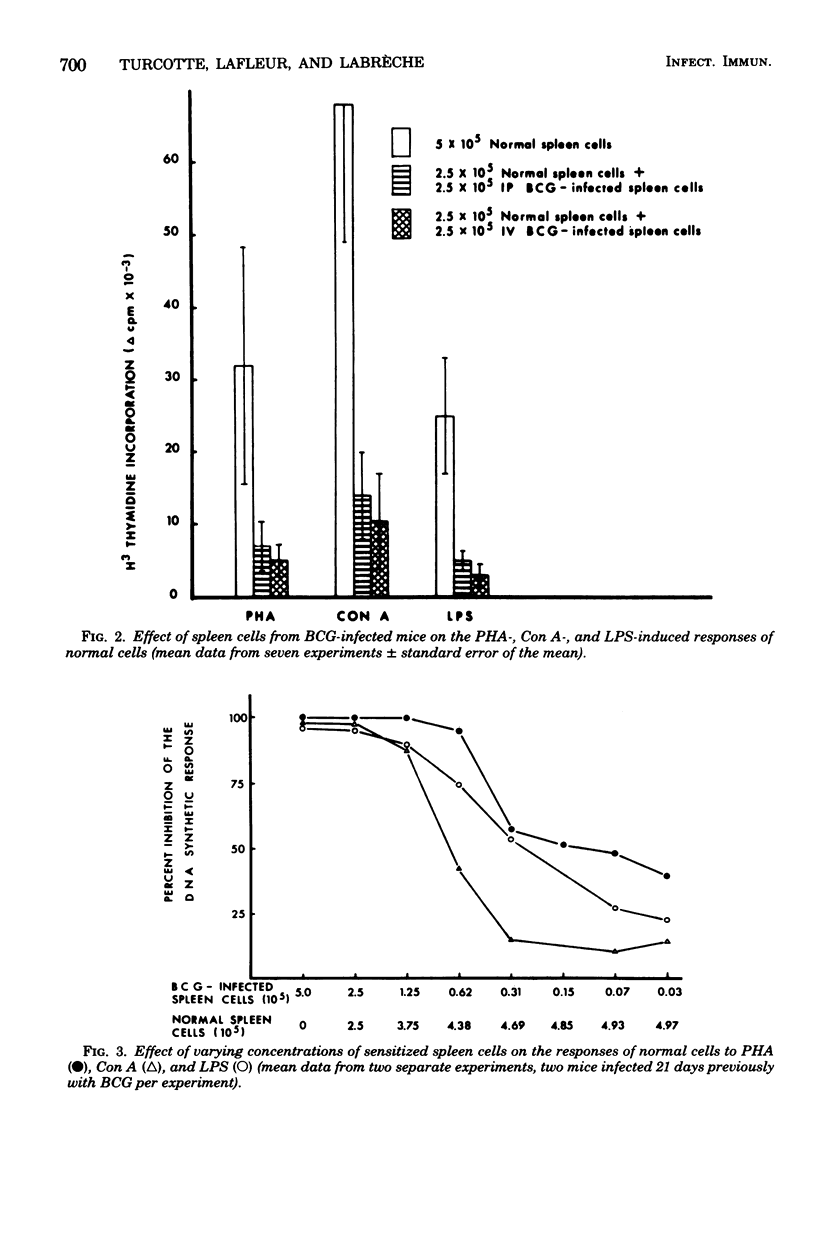
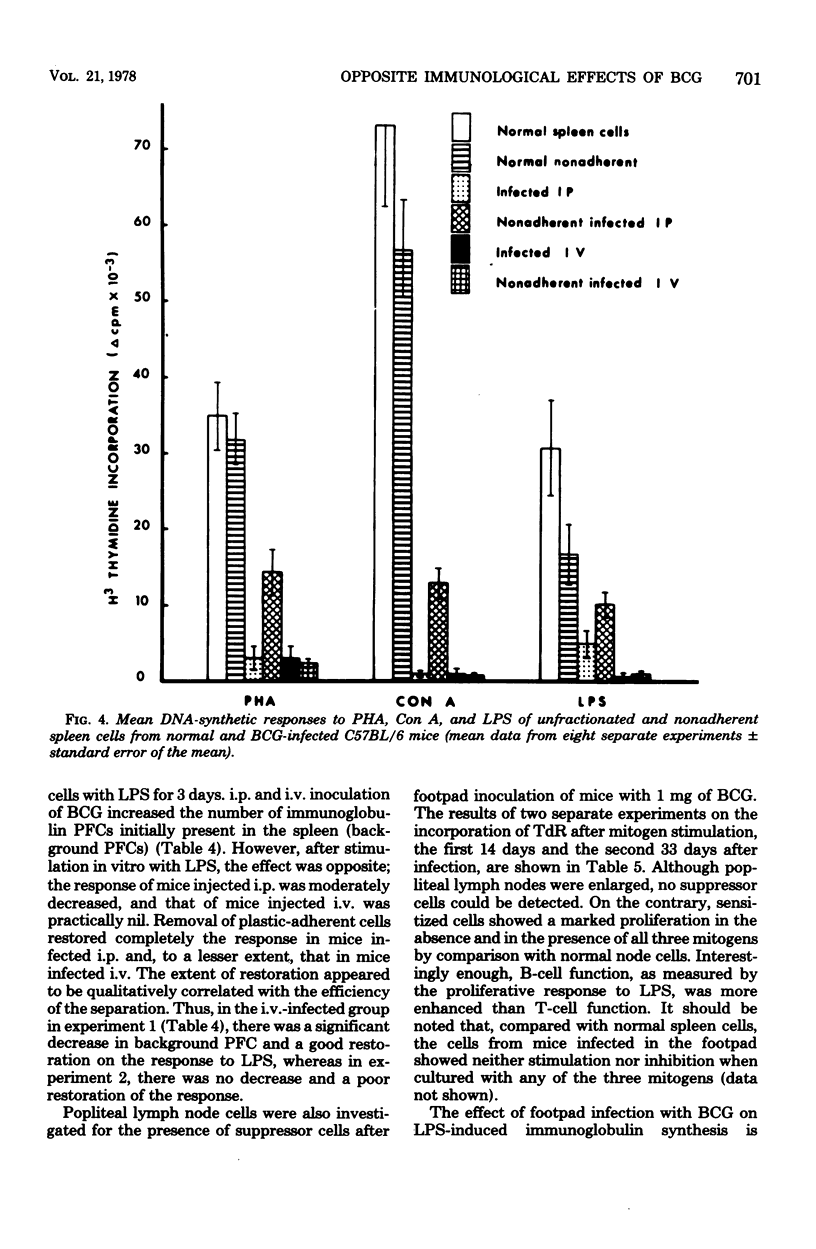
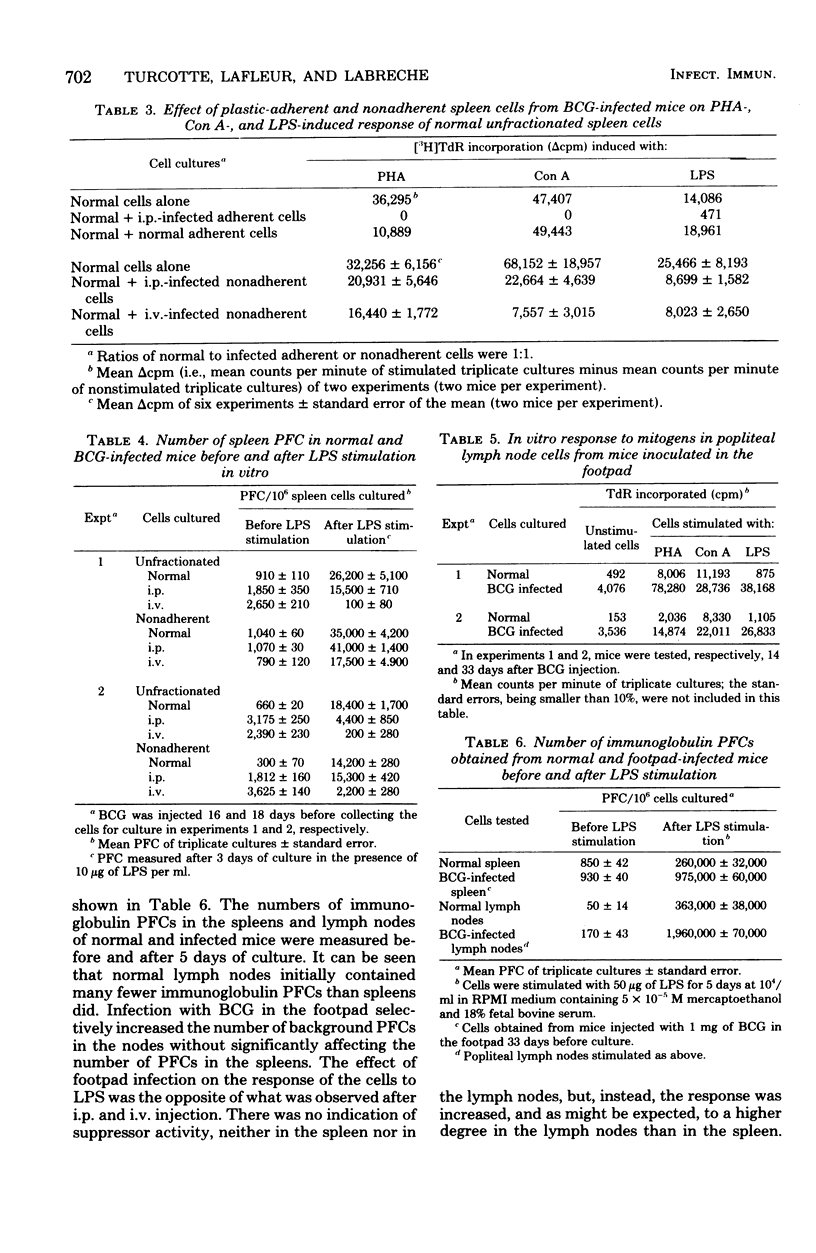
C57BL/6 mice were immunized intravenously (i.v.), intraperitoneally (i.p.), or subcutaneously with one dose of Bacillus Calmette-Guérin (BCG). At various time intervals after injection, the lymphocyte response, as measured by thymidine incorporation into DNA, and the number of immunoglobulin-secreting cells were determined in vitro before and after mitogenic stimulation with phytohemagglutinin, concanavalin A, or lipopolysaccharide. In unstimulated cultures, the spontaneous thymidine incorporation and immunoglobulin synthesis of spleen cells were increased to some extent in mice infected i.p. or i.v. with BCG, as compared with noninfected mice. In contrast, after mitogenic stimulation, a marked depression of the proliferative response of spleen cells to both T- and B-cell mitogens and a marked inhibition of LPS-induced immunoglobulin secretion were observed in mice infected i.v. and to a lesser extent in those infected i.p. The depression of lymphoblastogenesis in spleens was fully established 15 days after infection and persisted for a long period of time. When unfractionated or plastic-adherent spleen cells from BCG-infected mice were cultured with normal spleen cells, a strong depression of their reactivity to phytohemagglutinin, concanavalin A, and lipopolysaccharide was observed. After the removal of cells adherent to plastic, the response was partially restored in the nonadherent population from mice infected i.p., but not in that from mice infected i.v. After mitogenic stimulation, lymph node cells of mice inoculated subcutaneously showed a response to mitogen higher than that of normal cells. These results thus demonstrate that, depending on the route of administration, BCG exerts very different effects.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Zbar B., Borsos T., Rapp H. J. BCG and cancer (first of two parts). N Engl J Med. 1974 Jun 20;290(25):1413–1420. doi: 10.1056/NEJM197406202902506. [DOI] [PubMed] [Google Scholar]
- Doft B. H., Merchant B., Johannessen L., Chaparas S. D., Sher N. A. Contrasting effects of BCG on spleen and lymph node antibody responses in nude and normal mice. J Immunol. 1976 Nov;117(5 Pt 1):1638–1643. [PubMed] [Google Scholar]
- Hanna M. G., Jr, Zbar B., Rapp H. J. Histopathology of tumor regression after intralesional injection of Mycobacterium bovis. I. Tumor growth and metastasis. J Natl Cancer Inst. 1972 May;48(5):1441–1455. [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Glaser M., Holden H. T., Fernbach B. R., Herberman R. B. Suppressor cells in tumor bearing mice and rats. Biomedicine. 1976 Dec 15;24(6):371–374. [PubMed] [Google Scholar]
- Kirchner H., Holden H. T., Herberman Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975 Nov;115(5):1212–1216. [PubMed] [Google Scholar]
- Lamoureux G., Poisson R. Letter: B.C.G. and immunological anergy. Lancet. 1974 May 18;1(7864):989–990. doi: 10.1016/s0140-6736(74)91296-3. [DOI] [PubMed] [Google Scholar]
- Laucius J. F., Bodurtha A. J., Mastrangelo M. J., Creech R. H. Bacillus Calmette-Guerin in the treatment of neoplastic disease. J Reticuloendothel Soc. 1974 Dec;16(6):347–373. [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Lagrange P. H., Ishibashi T. The modifying effect of BCG on the immunological induction of T cells. J Exp Med. 1974 Jun 1;139(6):1540–1552. doi: 10.1084/jem.139.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. E., Mackaness G. B., Lagrange P. H. Immunopotentiation with BCG. II. Modulation of the response to sheep red blood cells. J Natl Cancer Inst. 1973 Nov;51(5):1669–1676. doi: 10.1093/jnci/51.5.1669. [DOI] [PubMed] [Google Scholar]
- Mitchell M. S., Kirkpatrick D., Mokyr M. B., Gery I. On the mode of action of BCG. Nat New Biol. 1973 Jun 13;243(128):216–218. doi: 10.1038/newbio243216a0. [DOI] [PubMed] [Google Scholar]
- Molinaro G. A., Maron E., Eby W. C., Dray S. A general method for enumerating single cells secreting antigen: albumin-secreting hepatocytes detected as plaque-forming cells. Eur J Immunol. 1975 Nov;5(11):771–774. doi: 10.1002/eji.1830051108. [DOI] [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. I. Inhibition of PHA, mixed lymphocyte and GVH reactivity. Cell Immunol. 1972 Nov;5(3):459–468. doi: 10.1016/0008-8749(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Waksman B. H. Tolerance, the thymus, and suppressor T cells. Clin Exp Immunol. 1977 Jun;28(3):363–374. [PMC free article] [PubMed] [Google Scholar]


