Abstract
Aims and Hypothesis
To describe the associations between age, sex and body mass index (BMI) at diagnosis of type 2 diabetes (T2DM) and test the hypothesis that men are diagnosed with diabetes at lower average BMI than women of similar age.
Research Design and Methods
Linear regression was used to estimate and compare the relationship between age and BMI at diagnosis among 51,920 men and 43,137 women included in a population based diabetes register in Scotland for whom an index BMI measurement was taken within one year of diabetes diagnosis. We also examined HBA1c values by gender within same timescale.
Results
Mean BMI closest to date of diagnosis of T2DM was 31.83 kg/m2 (SD 5.13) in men and 33.69 kg/m2 (SD 6.43) in women. The inverse relationship between age and BMI at T2DM diagnosis was significantly steeper in women than in men (slope estimate in men −0.12 kg/m2 per year [95% CI: −0.13 to −0.12] women −0.18 kg/m2 per year [95% CI: −0.18 to −0.17], p<0.0001 for formal test of interaction). Mean BMI difference was most marked at younger ages and narrowed with advancing age. However, HBA1c levels within year of diagnoses were broadly similar in men and women.
Conclusions
Men are diagnosed with type 2 diabetes at lower BMI than women across the age range. This observation may help explain why type 2 diabetes is more common among middle-aged men in populations of European extraction. Whether the same pattern is also observed in other ethnic groups requires confirmation.
Keywords: Body Mass Index, Diagnosis, Gender, Insulin resistance
Introduction
Greater adiposity is the major risk factor for development of type 2 diabetes. However, as multiple factors including age and genetics (captured in part via family history) influence risk of type 2 diabetes there is a range of body mass at diagnosis. Hellier and Pedula [1] have previously reported an inverse linear relationship between body mass and mean age at diagnosis, indicating that, in general, young people that develop type 2 diabetes have higher body mass indices at diagnoses than do older people.
More recently, it has become apparent that middle-aged men are at significantly higher risk of diabetes than women in several different populations [2-4]. One possible explanation for this observation is that men, on average, may have to gain less weight to develop T2DM than women, in part because men without diabetes are generally more insulin resistant than women [5]. Thus we hypothesised that men may develop diabetes at lower average BMIs across the age spectrum.
Research Design and Methods
Data were drawn from a 2008 extract of the Scottish Care Information Diabetes Collaboration (SCI-DC) dataset, a population-based register holding clinical and demographic data on people diagnosed with diabetes in Scotland. Generation of a linked dataset of SCI-DC data to other health data was approved by the SCI-DC steering committee, the Scottish multi-centre research ethics committee, the Privacy Advisory Committee of NHS National Services Scotland (NHS NSS) and Caldicott guardians of all Health Boards in Scotland. The research database held no identifiable information. Individuals included in this analysis were those with recorded type 2 diabetes for whom BMI was recorded within one year of diagnosis of diabetes (index BMI). BMI was determined in all centres by clinical staff using height and weight determinations.
Patients for whom the index BMI measurement was recorded after 31st December 2007 were excluded, as were those whose died within the first two years of diagnosis of diabetes, those with an index BMI <25 kg/m2 age <30 years (both factors to limit inclusion of those potentially misclassified with type 1 diabetes), or where the smoking status was not recorded. Other relevant variables (HbA1c, and smoking status) recorded within one year after diagnosis of diabetes were also extracted. HBA1c was measured by routine NHS clinical laboratories all with DCCT aligned methodologies. Where multiple measurements were recorded within one year after diabetes diagnosis, the chronologically earliest was used.
Statistical analyses
The association of interest was estimated for each sex using linear regression (BMI predicted by age at diagnosis) and we modelled the slopes separately for each sex. No transformations were applied to the data prior to analysis. The influence of smoking status on BMI was assessed by extending the original regression model (in which BMI was predicted by age) to include an additional predictor of smoking status (binary: current / former smoker vs. never smoked). A p < 0.05 was considered significant, and 95% confidence limits for parameter estimation are given. Analysis was performed using SAS software (Version 9.2).
Results
Characteristics of the population are presented in Table 1. The relationship between BMI and age at diagnosis of type 2 diabetes was based on data from 95,057 individuals (51,920 men; 43,137 women). This sample represents 35.1% of eligible people because BMI or other relevant data were missing for 64.9%. Of importance, the percentage of women in those included versus excluded in this analysis was similar at 45.4% and 46.6%, respectively. Similarly, whilst those excluded were somewhat younger than those included (in part due to our exclusion of those below age of 30 years to limit inclusion of potentially misclassified type 1 diabetes), men in both included and excluded groups were significantly younger than comparable females. For included women mean age was 61.6 yr (SD 12.1 yr) but 59.2 yr (SD 11.5 yr) for men, whereas for excluded women mean age was 57.3 yr (SD 20.2 yr) but 53.7 yr (SD 18.9 yr) for excluded men.
TABLE 1. Characteristics of study population by BMI category.
| MEN | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 25 to < 30 kg/m2 n = 21,998 | 30 to < 35 kg/m2 n = 18,874 | 35 to < 40 kg/m2 n = 7,353 | 40 to < 45 kg/m2 n = 2,460 | 45 to < 50 kg/m2 n = 820 | 50+ kg/m2 n = 415 | p [note 1] | |
|
|
|||||||
| Age (yrs) [note 2] mean (SD) |
61.9 (11.3) | 59.0 (11.0) | 55.3 (10.9) | 52.3 (10.6) | 50.7 (9.8) | 48.8 (9.6) | −0.26 (< 0.0001) |
|
|
|||||||
| HbA1c (%) | |||||||
| mean (SD) | 8.02 (2.06) | 7.97 (1.95) | 8.03 (1.92) | 8.11 (1.88) | 8.12 (1.84) | 8.37 (2.08) | 0.02 (< 0.0001) |
| mmol/mol | 64 | 64 | 64 | 65 | 65 | 68 | |
| p [note 3] | |||||||
| Smoking status: | 25.9 | 24.3 | 24.6 | 23.5 | 22.0 | 21.1 | <0.0001 |
| % current | 39.8 | 42.0 | 39.7 | 35.5 | 31.0 | 32.2 | |
| % former | 34.3 | 33.8 | 35.8 | 41.0 | 47.0 | 46.7 | |
| % never | |||||||
| WOMEN | |||||||
|---|---|---|---|---|---|---|---|
| 25 to < 30 kg/m2 n = 14,249 | 30 to < 35 kg/m2 n = 13,871 | 35 to < 40 kg/m2 n = 8,379 | 40 to < 45 kg/m2 n = 4,021 | 45 to < 50 kg/m2 n = 1,614 | 50+ kg/m2 n = 1,003 | p [note 1] | |
|
|
|||||||
| Age (yrs)[note 2]mean (SD) | 65.8 (11.3) | 62.3 (11.5) | 58.7 (11.7) | 55.9 (11.2) | 53.3 (10.8) | 51.0 (10.4) | −0.33 (< 0.0001) |
| HbA1c (%) | |||||||
| mean (SD) | 8.00 (2.05) | 7.92 (1.96) | 7.89 (1.88) | 7.96 (1.84) | 7.91 (1.75) | 7.91 (1.70) | 0.00 (0.69) |
| mmol/mol | 64 | 63 | 63 | 64 | 63 | 63 | |
| p [note 3] | |||||||
| Smoking status: | 24.6 | 23.4 | 23.3 | 23.0 | 23.4 | 21.5 | 0.01 |
| % current | 29.5 | 31.0 | 30.1 | 31.0 | 29.6 | 28.6 | |
| % former % never |
45.9 | 45.6 | 46.6 | 46.0 | 47.0 | 49.9 | |
NOTES: 1) Rank correlation of row variable with BMI (correlation coefficient followed by p value).
2)Within one year of diagnosis with diabetes.
3)Value returned by Mantel-Haenszel chi-square test (alternative hypothesis is linear association between row variable and column variable). Smoking status assumed to reflect approximate degree of exposure i.e. ‘never’ < ‘former’ < ‘current’
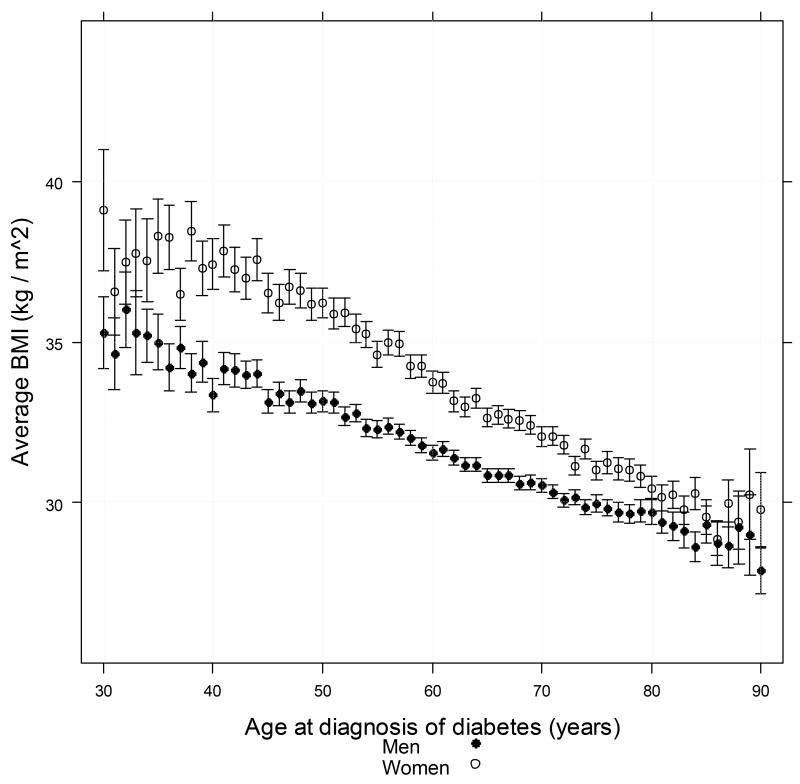
The mean BMI recorded within a year of diagnosis of type 2 diabetes in men, was 31.83 kg/m2 (SD 5.13) and 33.69 kg/m2 (SD 6.43) in women. The relationship between age and BMI at diagnosis is shown in Figure 1. The inverse relationship between age and BMI within a year of diagnosis of T2DM was significantly steeper in women than in men (slope estimate in men −0.12 kg/m2/year [95% CI: −0.13 to −0.12]; women −0.18 kg/m2/year [95% CI: −0.18 to −0.17], p<0.0001 by formal test of interaction). This gender differential in BMI was also most marked at younger ages [Figure 1].
FIGURE 1. Distribution of average BMI value by age at diagnosis with Type 2 diabetes (horizontal axis) for patients aged between 30 years and 90 years at diagnosis and with BMI<25kg/m2.
Patients who died within two years of BMI determination were excluded. Vertical bars represent 95% confidence interval around mean. Vertical axis is truncated at lower limit of 25 kg / m2 to maximize effective resolution of the chart.
We found no marked effect or confounding by smoking since essentially the slope estimates were unchanged for men and negligibly altered for women to −0.178 from −0.177 kg/m2/year when smoking was added.
Discussion
Results from this population based diabetes cohort of over 95000 people confirms an inverse association between BMI and age at diagnosis of type 2 diabetes, substantially extending a previous report based on data for 2437 adults [1]. We show that this relationship varies by sex, so that at most ages men have lower BMI around the time of diagnosis of diabetes than women, though the sex differential decreases with age. Importantly, there is no evidence that men are diagnosed earlier in the course of their diabetes: earliest HbA1c levels after diagnosis were broadly similar in men and women. Our findings provide an explanation of why, despite higher prevalence of obesity in women, prevalence of diabetes in middle-aged men appears to exceed that of women in some parts of the world [2-4], although further studies in other ethnic groups are needed.
Potential mechanisms for this observation include the fact that, for a given BMI, men are less insulin sensitive than women [5]. Middle-aged men generally have higher fasting glucose levels, higher triglyceride and lower HDL-cholesterol levels than women of a similar age even after adjusting for BMI [4, 6]. Fat distribution differs by sex and, in general, men have greater visceral and hepatic fat compared to women [5]. Women have greater amounts of ‘safe’ subcutaneous fat than men and thus the present observations are consistent with the hypothesis that women need to accumulate greater total adiposity to develop harmful ectopic fat deposition and produce the extent of insulin resistance required to develop diabetes.
The mechanism(s) behind a narrowing of the BMI difference at diagnosis between sexes with advancing age require further study but speculatively could include factors such as survival bias, hormonal changes (possibly post-menopausal changes, although there is no obvious point of inflection) or differential changes in lifestyle behaviours.
Potential clinical ramifications of the findings merit brief discussion. Lovejoy and Sainsbury [7] point out that research is urgently needed to determine whether current weight loss programmes, largely developed and tested on women, are appropriate for men. Intensive lifestyle intervention appears equally effective in men and women participating in diabetes prevention trials [8]. However significantly fewer men volunteer for such trials and less than one third of the participants in the Diabetes Prevention Programme were men [8], the same being true in other major trials. Of further interest, future bariatric surgery guidelines developed for patients with diabetes may need to take account of higher diabetes risk at any given BMI in middle-aged men versus women.
Our study is limited by the fact that the population studied was mainly of white European ancestry and further corroborative work is needed in other ethnic groups. We also lacked waist circumference measurements (which enhance prediction of diabetes in women but not men [9]) and consequently whether waist circumference has to increase more in women versus men to convert to diabetes requires further study. Finally, our study employed strict inclusion criteria and required BMI to be available within a year of diagnosis. Thus many patients did not have sufficient data. Nevertheless the comparisons of those included versus excluded in terms of similar percentage of females, and similar age differential by gender suggests our exclusions are unlikely to have biased the relationship seen in Figure 1 based on results from over 95,000 individuals. Moreover, recent results generated in two populations of older men and women recruited from multiple towns in United Kingdom have provided broadly concurrent results, albeit from a smaller (n=7529) study using data from those with and without established diabetes [10]. In this latter study, waist circumference and body mass index levels along with related risk factors differed significantly more between diabetic and non-diabetic women than between diabetic and non-diabetic men, suggesting once again that women may need to undergo much larger metabolic perturbances (driven in part or largely by greater weight gain) to transit from non-diabetes to diabetes [10]. Clearly, further mechanistic studies are needed to confirm or refute this hypothesis.
In summary, we confirm an inverse relationship between age and BMI at diagnosis of diabetes, but importantly extend existing knowledge to show that men across the age spectrum are diagnosed with diabetes at a lower BMI than women. This novel observation could explain the higher male prevalence of type 2 diabetes in populations of European extraction.
Acknowledgments
These data are available for analysis by members of the Scottish Diabetes Research Network epidemiology group thanks to the hard work of numerous NHS staff who enter the data and people and organisations (the Scottish Care Information -Diabetes Collaboration [SCI-DC] Steering Group, the Scottish Diabetes Group, the Scottish Diabetes Survey Monitoring Group, the managed clinical network managers and staff in each Health Board) involved in setting up, maintaining and overseeing SCI-DC. This work was supported by the Wellcome Trust through the Scottish Health Informatics Programme (SHIP) Grant (Ref WT086113). SHIP is a collaboration between the Universities of Aberdeen, Dundee, Edinburgh, Glasgow and St Andrews and the Information Services Division of NHS Scotland. Funding for diabetes register linkage was provided by the Scottish Government and the authors acknowledge the financial support of NHS Research Scotland (NRS), through the Scottish Diabetes Research Network
Footnotes
Duality of interest: None of the authors have any relevant conflict of interest to disclose.
References
- 1.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24:1522–7. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- 2.Lipscombe LL, Hux JE. Trends in diabetes prevalence, incidence, and mortality in Ontario, Canada 1995-2005: a population-based study. Lancet. 2007;369:750–6. doi: 10.1016/S0140-6736(07)60361-4. [DOI] [PubMed] [Google Scholar]
- 3.Choi YJ, Kim HC, Kim HM, Park SW, Kim J, Kim DJ. Prevalence and management of diabetes in Korean adults: Korea National Health and Nutrition Examination Surveys 1998-2005. Diabetes Care. 2009;32:2016–20. doi: 10.2337/dc08-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. Erratum in: Lancet. 2010;376:958. Hillage, H L [corrected to Hillege, H L] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerging Risk Factors Collaboration. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovejoy JC, Sainsbury A. Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009 Mar;10(2):154–67. doi: 10.1111/j.1467-789X.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Papacosta O, Whincup PH, Carson C, Thomas MC, Lawlor DA, Ebrahim S, Sattar N. Assessing prediction of diabetes in older adults using different adiposity measures: a 7 year prospective study in 6,923 older men and women. Diabetologia. 2010;53:890–8. doi: 10.1007/s00125-010-1670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Papacosta O, Lawlor DA, Whincup PH, Lowe GD, Ebrahim S, Sattar N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than do men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia. 2012;55:80–87. doi: 10.1007/s00125-011-2284-4. [DOI] [PubMed] [Google Scholar]