Abstract
Background
Social defect and chronic pain are 2 major health problems and recent data has demonstrated that they generally exist concurrently. However, a powerful evaluation model on the behavioral change is lacking. This study was designed to evaluate the behavioral curves using a statistically modeled trajectory analysis in neuropathic animals with or without social defect exposure.
Material/Methods
After approval by the institutional animal care committee, Sprague-Dawley rats were randomized into different interventional groups with 15 animals each. Sprague-Dawley rats underwent spared nerve injury (SNI) to establish the neuropathic pain model, of which the mechanical withdrawal threshold was measured using von Frey filaments for a period of 105 days. Otherwise, a modified version of the resident (Long-Evans rats)-intruder paradigm was applied to produce a social defect animal model through the elevated plus maze (EPM). After raw data collection, we modeled them into a powerful statistical effects analysis to build up the behavioral change tendency in single SNI or in combined SNI and social defect animals.
Results
The random and fixed effects analyses of the pain behavior after SNI were successfully modeled and demonstrated a gradient recovery tendency during the 15-week post-injury observational period. Correspondingly, SNI rats exhibited increased social defected symptoms, as indicated by the increased anxiety-like behavior in the EPM test. In addition, continuous social defect stress for 5 days or 10 days, respectively, partially attenuated and exacerbated SNI-induced allodynia in both random and fixed effects models. Five days but not 10 days social defect ameliorated SNI-associated anxiety-like behavior.
Conclusions
These data suggest that statistically powerful analysis of nerve injury-induced neuropathic pain is a highly sensitive model to determine the behavioral change tendency and distinguish them among behavior curves with or without social defect, and the combination of SNI with resident-intruder paradigm may be a suitable model for behavior evaluation of neuropathic pain with social defect.
MeSH Keywords: Chronic Pain, Models, Animal, Neuralgia, Social Determinants of Health
Background
Social defect underlies a combination of a series of severe psychiatric conditions such as anxiety, depression, autism, schizophrenia, or suicidal tendency [1]. It is estimated that 20% of people worldwide have varying degrees of social defect disorders, with another 30% at some point in life experiencing different social defect symptoms [2]. In recent years, social defect disorders abruptly increased and may even affect younger generations, significantly impairing human wellbeing, economic development, and social stabilization, and contributing a high public health cost [3].
Studies have shown that patients with social defected disorders may suffer from chronic pain [4–6]. Chronic pain is one of the risks involved in a series of physical, psychological, familiar, or social adverse events, with 20% classified to be neuropathic pain. However, chronic neuropathic pain still lacks a definite relief paradigm, with an effective treatment rate of only 6% or even lower [7]. Interestingly, a considerable proportion of patients with chronic neuropathic pain have been observed to have an increased risk of social defect symptoms, including anxiety, depression, and suicidal ideation [8]. This high comorbidity rate of social defect and chronic pain suggest that there may be certain coexisting underlying physiologic or pathophysiological mechanisms between social defect and chronic pain.
In this study we introduced the modeled analyses of the pain behavior using a more powerful means of evaluating rats with peripheral nerve injury. Otherwise, social defect was induced in some of these rats with the resident-intruder paradigm [9]. We observed anxiety-like behavioral changes and severity of neuropathic pain in SNI rats, as well as in SNI rats following various durations of social defect stress exposure. It is supposed that this type of statistically modeled behavioral study not only facilitates the pain behavioral assessment in a more effective way, but also assists further investigation of the etiological and pathophysiological mechanisms of the combination of chronic pain and social defect, and also verifies the therapeutic efficacy of possible target interventions on the comorbidity.
Material and Methods
Animals
Five-week-old male Sprague-Dawley (S-D) rats weighing 275–300 g and 20 Long-Evans rats (L-E rats) weighing 400–500 g were used (from Nanjing Medical University, Nanjing, China). All rats were singly housed with a 12-h light-dark cycle in a climate-controlled room with ad libitum food and water. All experiments were conducted in accordance with the animal behavioral guidelines, using approved protocols from the institutional animal care committee.
Chronic neuropathic pain animal model establishment
The animal model of chronic neuropathic pain was established by spared nerve injury (SNI) practiced in our previous study [10]. Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and placed in the prone position. A suture thread was tied around tibial and common peroneal branches of the sciatic nerve, both of which were ligated 2 cm distal to the suture. The sural branch of the sciatic nerve was not manipulated. In the sham control rats, the same surgical procedure was performed except for the ligation of any branches of the sciatic nerve.
Mechanical withdrawal threshold analysis
The mechanical allodynia was evaluated by withdrawal response using von Frey filaments (Stoelting, Chicago, IL, USA) from the first day following the SNI model was established for a period of 105 days. Briefly, animals were placed on an elevated wire grid and the plantar surface of the paw stimulated with a series of ascending force using von Frey filaments [10]. The threshold was defined as the lowest filament that evoked a brisk withdrawal response to 1 of 3 repetitive stimuli.
Social defeat animal model establishment
The social defeat (SD) paradigm used in our study involves intimidations and aggressive encounters by a large, aggressive male rat (resident L-E) toward a smaller male rat (intruder S-D) [9]. During each episode of social stress, 1 intruder was placed into the home cage territory of 1 unfamiliar resident previously screened for high aggression for 15 min. A typical agonistic encounter resulted in intruder subordination or defeat, signaled by the intruder assuming a supine position for approximately 3 sec. After defeat, a wire mesh enclosure was placed in the cage to prevent physical contact between the resident and intruder but allowing visual, auditory, and olfactory contact for the remainder of the 30-min defeat session. Finally, all S-D intruders were used for behavioral assessment.
Elevated Plus Maze (EPM) test
Social-defect associated anxiety-like behavior in rodents was tested with EPM test immediately after the SNI model was established in rats, followed with or without various duration of social defect exposure. The EPM test is a widely used and extensively validated animal model of anxiety based on the natural aversion of rodents for open spaces of the maze [11]. The apparatus was made of wood; it was painted gray, consisted of 4 arms (30×10 cm) shaped in the form of a cross, and was elevated 50 cm above the floor. The connecting (open) center area measured 55 cm. Each S-D rat was placed into the center area of the plus maze facing an open arm. The rats were then permitted to explore the maze freely for a 5-min period while being videotaped. The total time spent in open arms and closed arms were evaluated for each rat. Either a decrease of time spent on the open arms or an increase of time spent in the closed arm is indicative of high anxiety-like behavior.
Statistical analysis
In our study, we use “S-D” to denote Sprague-Dawley rats, but “SD” to represent social defect. In order to build up a more powerfully characteristic recovery tendency, we, in this study, modeled the pain behavioral data using both random and fixed effects analysis methods via SAS® v9.4 software (SAS Institute Inc., Cary, NC, USA). In the random effects analysis, behavior was determined whether different recovering patterns existed within each group of animals. The fixed effects model was followed to see if distinct behavior recovery presented among different groups of animals. Based on these modeled analyses, we also showed the behavioral curves with corresponding 95% confidence intervals to set-up a possible changing tendency.
Statistical analyses and graphs were done using GraphPad Prism 4.0 software. Random effects and fixed effects distribution of mechanical threshold in rats were analyzed with a non-linear regression model. Times recorded in the EPM test are expressed as mean ±SEM. Difference between groups was compared using the t test or one-way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis when appropriate. A value of P<0.05 was considered statistically significant.
Results
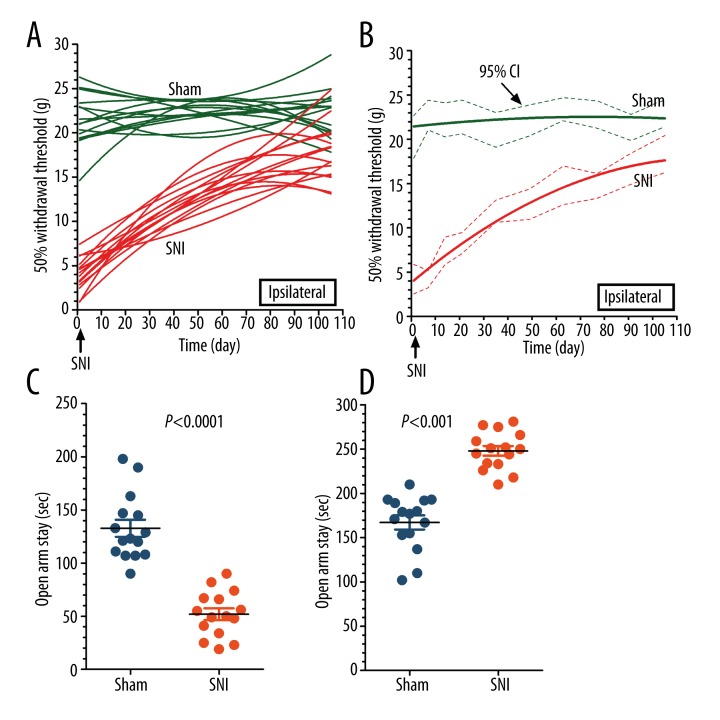
Figure 1A shows a random effects distribution of mechanical withdrawal threshold, with obvious difference among rats in each group, as well behavioral changes at each observational point. The mechanical threshold of sham-operated rats (Sham) was essentially unchanged throughout the observation period of 105 days. However, rats with spared nerve injury (SNI) exhibited a significant reduction in the mechanical threshold and showed a progressive recovery during the 105-day observational period. Figure 1B illustrates the fixed-effects distribution of mechanical threshold in both groups, showing that if individual difference among rats was excluded, the regressive effect of non-linear fixed-effects was apparent. The increased pain level of rats in the SNI group recovered progressively, however, it id not completely reach the baseline level of rats in the sham group at the end of the 105-day observational period. The above statistical results indicated it was a chronic process of behavioral changes after SNI, although existing a wide range of recovery times. Otherwise, the consistent recovery trend was apparent and almost reached the baseline level.
Figure 1.
Modeled analyses of 50% mechanical withdrawal threshold in rats with SNI. An SNI neuropathic pain animal model was established with ligation of tibial and common peroneal branches of the sciatic nerve, while the left sural branch of the sciatic nerve was left intact. Mechanical withdrawal threshold was measured with von Frey filament (A). Random-effects distribution of mechanical threshold in both groups was calculated with a non-linear regression model. The results show an obvious difference among rats in each group, as well as behavioral changes at each observational point. However, the intragroup non-linear regression curve clearly shows a progressive recovery trend in the 105-day observational period after SNI (B). Fixed-effects distribution of mechanical threshold in both groups showed that when individual differences among rats were excluded, the regressive effect of the non-linear fixed-effects model was apparent. The increased pain level of rats in the SNI group exhibited a progressive recovery trend, but not reaching the baseline level of rats in the sham group at the end of the 105-day observational period. An SNI neuropathic pain animal model was established with ligation of tibial and common peroneal branches of the sciatic nerve, while the left sural branch of the sciatic nerve was left intact. The total time spent in open arms and closed arms by rats after SNI were recorded during the 5-min observational time window. The results showed that SNI rats spent significantly less time in the open arms of the maze than sham control rats (C). SNI rats spent significantly more time in the closed arms of the maze than sham control rats (D) P<0.001 SNI versus Sham, n=15.
In the EPM test, SNI rats spent significantly less time in the open arms of the maze than sham control rats (Figure 1C). SNI rats spent significantly more time in the closed arms of the maze than did the sham control rats (Figure 1D). Rats treated with SNI exhibited heightened social defect symptoms, as indicated by the increased anxiety-like behavior in the EPM test.
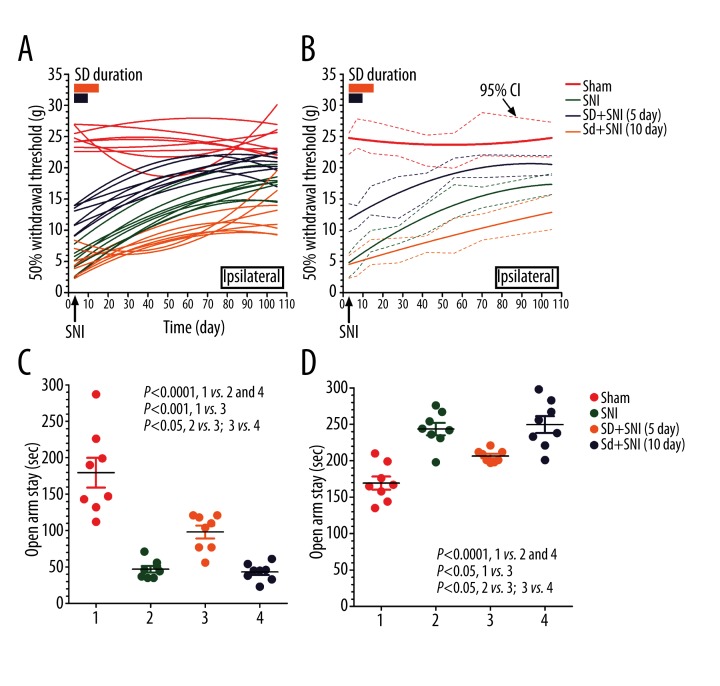
Social defect produced a different effect on chronic neuropathic pain recovery following various durations of stimulation. If chronic pain was induced following continuous social defect stress for 5 days (SD+SNI 5d), we would observe an ameliorated neuropathic pain, although still inferior to normal control rats, during the 105-day observational time window, as demonstrated by the elevated mechanical withdrawal threshold compared to SNI rats without social defect stress (Figure 2A). However, if social defect anxiety stress persisted for 10 days (SD+SNI 10d), neuropathic pain deteriorated in SNI rats, demonstrated by the decreased mechanical withdrawal threshold compared to SNI rats without social defect stress, indicating exacerbated allodynia (Figure 2B). It is likely that SNI-induced neuropathic pain is highly susceptible to social defect stress duration, meaning that a shorter duration (5-day stress exposure) may ameliorate SNI-induced chronic neuropathic pain, whereas a longer duration (10-day exposure) may worsen it.
Figure 2.
Behavioral analyses of neuropathic rats with social defect. We creatively established the social defected neuropathic pain animal model with resident-intruder paradigm and SNI. Mechanical withdrawal threshold was measured with von Frey filament during the 105-day observational window. The results showed that a shorter duration (5 days of social defect stress) may increase the pain threshold of SNI neuropathic pain rats, but a longer duration (10 days of social defect stress) may lower the pain threshold of SNI neuropathic pain rats both in a random-effects regression analysis (A) and in fixed-effects regression analysis (B). We creatively established the social defected neuropathic pain animal model with resident-intruder paradigm and SNI. The total time spent in open arms and closed arms of rats after SNI were recorded during the 5-min observational time window. The results show that if SNI was established followed by continuous social defect stress for 5 days, anxiety-like behavior ameliorated, as demonstrated by increased time in the open arms and decreased time in the closed arms compared to SNI rats without social defect stress (C, D). Although the ameliorated anxiety-like behavior was still significantly worse than that of the sham control rats (Sham), if rats were exposed to social defect anxiety stress for 10 days, the social defect behavior was not different from that of SNI rats without SD anxiety stress (SNI) (C, D). P<0.001 Sham versus SNI and SD+SNI 10 day; P<0.05 Sham versus SD+SNI 5 day; P<0.05 SNI versus SD+SNI 5 day, SD+SNI 5 day versus SD+SNI 10 day; n=15.
Social defect stress exposure duration had a different effect on SNI-associated anxiety-like behavior. When nerve ligation was followed with continuous social defect stress for 5 days (SD+SNI 5d), we observed an significantly ameliorated anxiety-like behavior, as demonstrated by the increased time in the open arms and the decreased time in the closed arms of the EPM test compared to SNI rats without social defect stress (SNI) (Figure 2C, 2D). Although the ameliorated anxiety-like behavior was worse than that of the sham control rats, social defect behavior was not different from that of SNI rats without SD anxiety stress (SNI) if rats were exposed to social defect anxiety stress for 10 days (SD+SNI 10d) (Figure 2C, 2D). It is interesting that the duration of social anxiety stress exposure is involved in the regulation of SNI-associated anxiety-like behavior. Five days, but not 10 days, of social defect stress may ameliorate SNI-associated anxiety-like behavior.
Discussion
We successfully established the neuropathic pain animal model with SNI and observed the increased anxiety-like social defect symptoms in rats with neuropathic pain. It is interesting that SNI-induced neuropathic pain was highly sensitive to varying degrees of social defect stress; at the same time, social defect exposure duration was involved in the regulation of SNI-associated anxiety-like behavior. We propose that a combination of SNI and the resident-intruder paradigm may be a suitable animal model for the study of comorbid neuropathic pain and social defect, and may help to explore the underlying mechanism and possible novel treatment approaches.
Neuropathic pain can be established by a series of animal models, such as chronic constriction injury of the sciatic nerve, spinal nerve ligation, partial spinal nerve ligation, partial sciatic nerve ligation, and chronic compression of dorsal root ganglion, most of which were created by loose ligation of a whole spinal segmental nerve or a tight ligation of a partial spinal segmental nerve[12]. However, in recent years, the spared nerve injury model, which is produced by partial sciatic nerve injury, has been developed, as presented in our previous study [10]. The SNI model involves a lesion of 2 of the 3 terminal branches of the sciatic nerve and permits behavioral testing of the non-injured skin territories adjacent to the denervated areas. The SNI model has been suggested to result in early (<24 h), prolonged (>6 months), and robust behavioral modifications. Indeed, in our study, SNI successfully induced chronic neuropathic pain in rats, as demonstrated by immediately, significantly, and prolonged allodynia to mechanical stimuli.
Rats with induced neuropathic pain exhibited increased social defect symptoms, as indicated by increased anxiety-like behavior in the EPM test. Social defect stress in our study was established with the resident-intruder paradigm, which is regarded as one of the most robust models of post-traumatic stress disorder, depression, and other stress-related illnesses [13]. Social defect stress in rats is known to induce long-lasting, adverse physiological, behavioral, and neuronal deficits, which seem to resemble certain human psychopathologies of depression and anxiety [14]. SNI-induced neuropathic pain is highly sensitive to varying degrees of social defect stress and social defect stress exposure duration is involved in the regulation of SNI-associated anxiety-like behavior.
In clinical practice, chronic pain is prevalent among patients with social defect symptoms such as anxiety, depression, or attention problems [15,16]. Social support or psychiatric therapy may attenuate associated chronic pain [17]. Usually, it is suggested that patients with chronic pain should be examined with respect to their social defect comorbidity [18]. In our study, a shorter duration (5 days of social stress exposure) may ameliorate SNI-induced chronic neuropathic pain, whereas a longer duration (10 days of exposure) may worsen it. Five days, but not 10 days, of social defect stress may ameliorate SNI-associated anxiety-like behavior. We proposed that 5 days of social stress has a preconditioning effect on ensuing chronic pain and anxiety-like behaviors, and that a longer exposure (10 days of social stress) ultimately induce certain functional changes and worse the chronic pain. However, it is still not known why 10 days of social stress has no effect on SNI-associated anxiety-like behavior. Although the exact underlying mechanisms still need to be explored in future work, a combination of SNI and resident-intruder paradigm successfully simulated the phenomenon of comorbidity of social defect and chronic pain in our study.
Considering the interaction of neuropathic pain and social defect stress in our study or in clinical practice, we propose that social defect and chronic pain may have certain similar or coexisting etiologies and pathophysiology mechanisms. We suggest that the myriad neurotransmitters and other substances involved in the development and maintenance of neuropathic pain also play a part in certain neurobiological disorders [19]. Otherwise, social defect affects normal neuronal, immune, cardiovascular, and metabolic functions, and ultimately promote the development of chronic pain [20]. However, for the purpose of perfecting the comorbid animal model of chronic pain and social defect, other than behavioral changes, molecular or imaging data are also needed.
Conclusions
SNI and the resident-intruder paradigm provide new insights into the animal model of comorbid chronic pain with social defect. It may help to explore the underlying mechanism and possible novel treatment approaches for this highly prevalent comorbidity.
Footnotes
Conflict of interests
None.
Source of support: National Natural Scientific Foundation of China (NSFC, 81271242 and 81371248); Nanjing Municipal Outstanding Young Scientist Grant (JQX12009)
References
- 1.Matsuo T, Jusup M, Iwasa Y. The conflict of social norms may cause the collapse of cooperation: indirect reciprocity with opposing attitudes towards in-group favoritism. J Theor Biol. 2014;346:34–46. doi: 10.1016/j.jtbi.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Chatel-Goldman J, Schwartz JL, Jutten C, et al. Non-local mind from the perspective of social cognition. Front Hum Neurosci. 2013;7:107. doi: 10.3389/fnhum.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saavedra-Rodriguez L, Feig LA. Chronic social instability induces anxiety and defective social interactions across generations. Biol Psych. 2013;73(1):44–53. doi: 10.1016/j.biopsych.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rush AJ, Polatin P, Gatchel RJ. Depression and chronic low back pain: establishing priorities in treatment. Spine. 2000;25(20):2566–71. doi: 10.1097/00007632-200010150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Allely CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. ScientificWorldJournal. 2013;2013:916178. doi: 10.1155/2013/916178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnot O, Tordjman S. [Schizophrenia and pain reactivity]. Press Med. 2008;37(11):1561–68. doi: 10.1016/j.lpm.2008.05.013. [in French] [DOI] [PubMed] [Google Scholar]
- 7.Frank JW, Bair MJ, Becker WC, et al. Update in pain medicine for primary care providers: a narrative review, 2010–2012. Pain Med. 2014;15(3):425–31. doi: 10.1111/pme.12337. [DOI] [PubMed] [Google Scholar]
- 8.Vachon P, Millecamps M, Low L, et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behav Brain Funct. 2013;9:22. doi: 10.1186/1744-9081-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood SK, Walker HE, Valentino RJ, et al. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151(4):1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen X, Liu Y, Xu S, et al. Menin regulates spinal glutamate-GABA balance through GAD65 contributing to neuropathic pain. Pharmacol Rep. 2014;66(1):49–55. doi: 10.1016/j.pharep.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Pellow S, Chopin P, File SE, et al. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 12.Jaggi AS, Jain V, Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol. 2011;25(1):1–28. doi: 10.1111/j.1472-8206.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 13.Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354(1):107–18. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- 14.Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PloS one. 2009;4(1):e4265. doi: 10.1371/journal.pone.0004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skrove M, Romundstad P, Indredavik MS. Chronic multisite pain in adolescent girls and boys with emotional and behavioral problems: the Young-HUNT study. Eur Child Adolesc Psychiatry. 2014 doi: 10.1007/s00787-014-0601-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Cheatle MD, Wasser T, Foster C, et al. Prevalence of suicidal ideation in patients with chronic non-cancer pain referred to a behaviorally based pain program. Pain Physician. 2014;17(3):E359–67. [PubMed] [Google Scholar]
- 17.Peilot B, Andrell P, Samuelsson A, et al. Time to gain trust and change-Experiences of attachment and mindfulness-based cognitive therapy among patients with chronic pain and psychiatric co-morbidity. Int J Qual Stud Health Well-being. 2014;9:24420. doi: 10.3402/qhw.v9.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annagur BB, Uguz F, Apiliogullari S, et al. Psychiatric disorders and association with quality of sleep and quality of life in patients with chronic pain: a SCID-based study. Pain Med. 2014;15(5):772–81. doi: 10.1111/pme.12390. [DOI] [PubMed] [Google Scholar]
- 19.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. doi: 10.1136/bmj.f7656. Erratum in: BMJ, 2014; 348: g2323. [DOI] [PubMed] [Google Scholar]
- 20.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]