Abstract
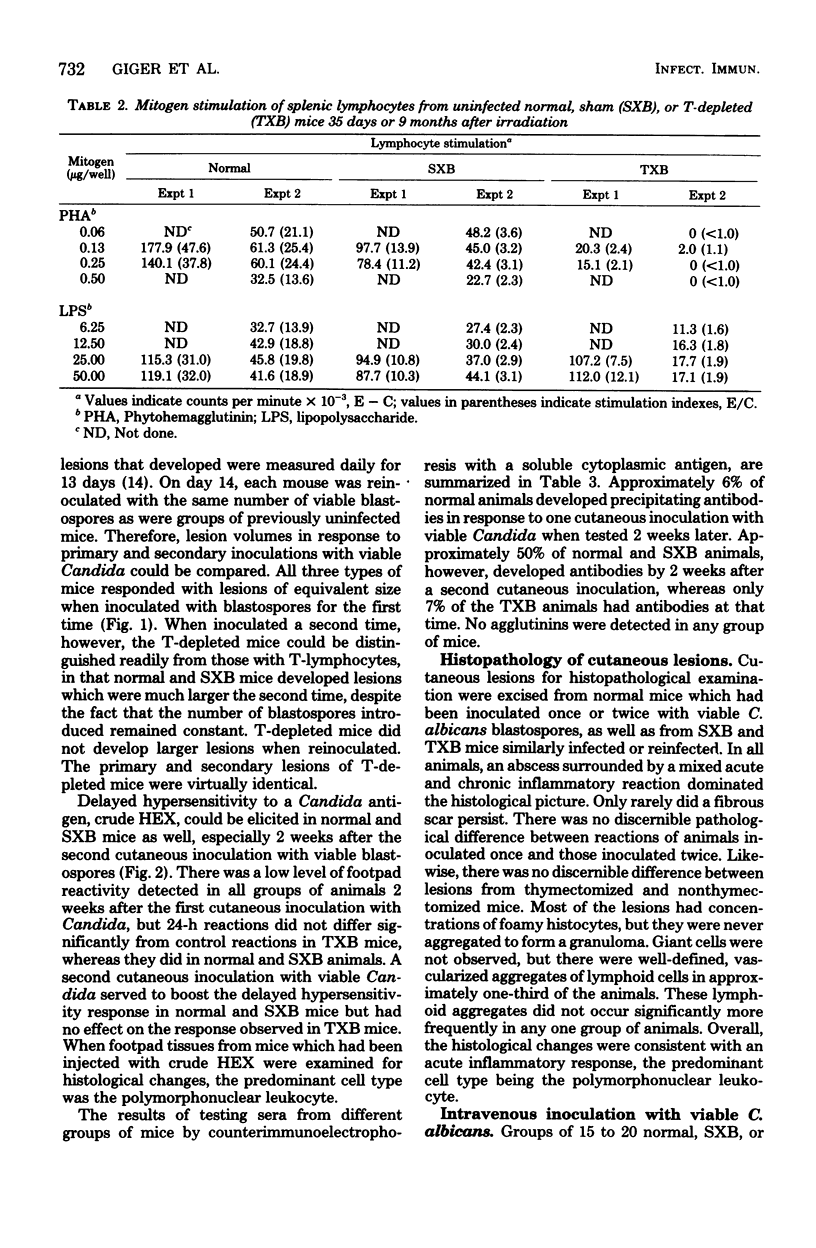
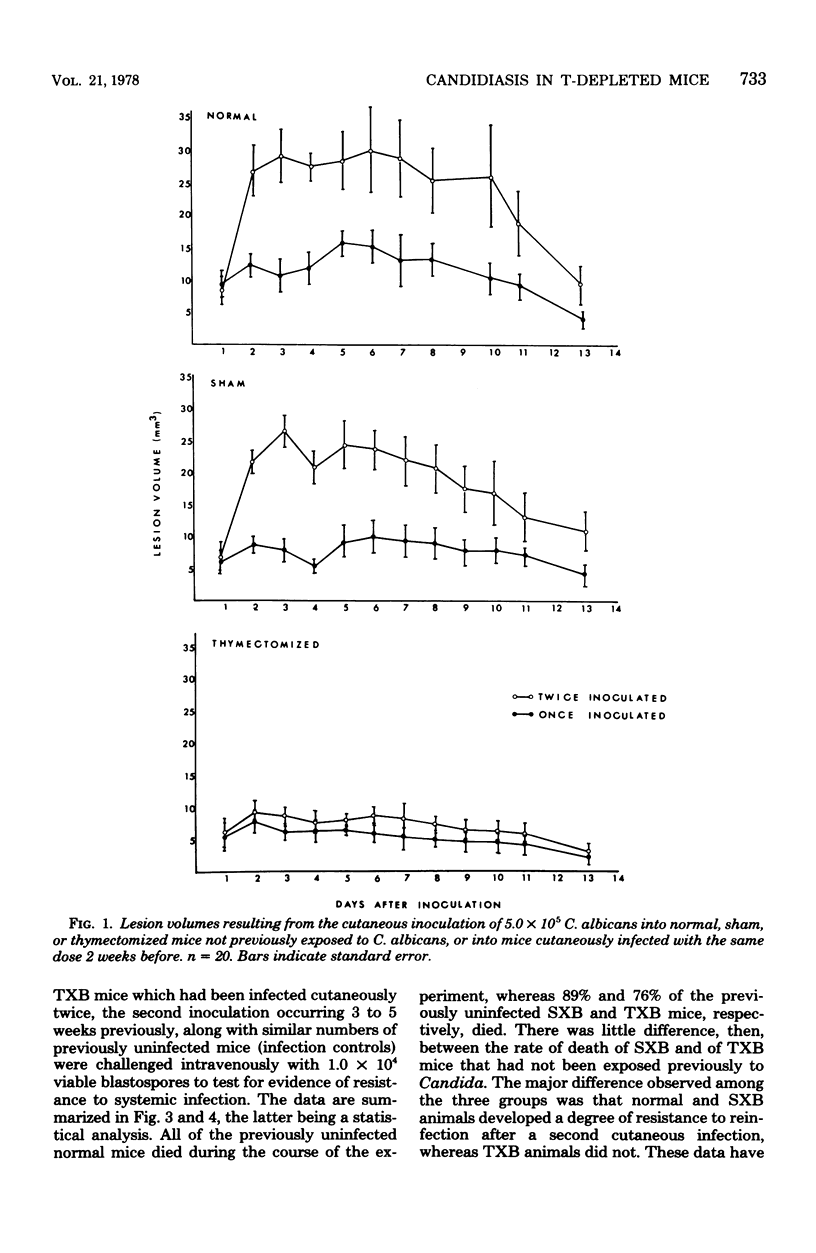
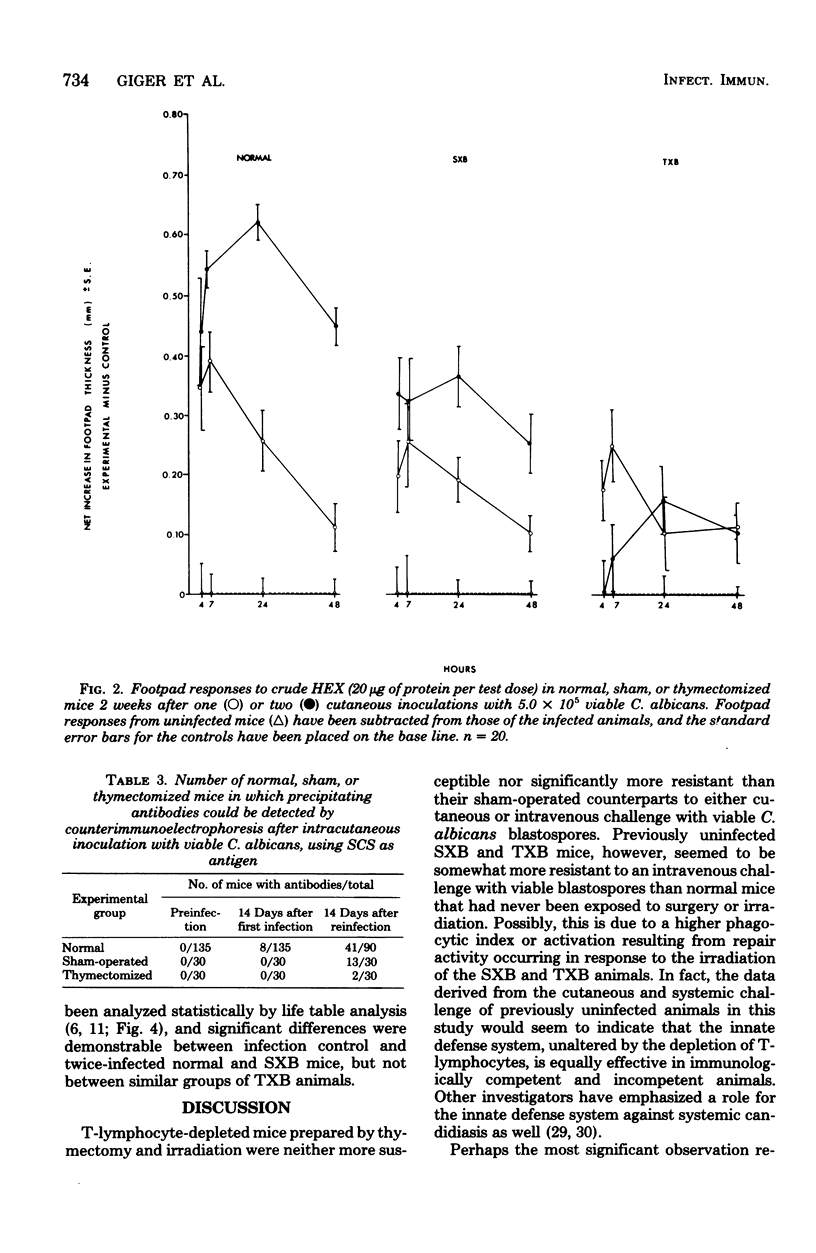
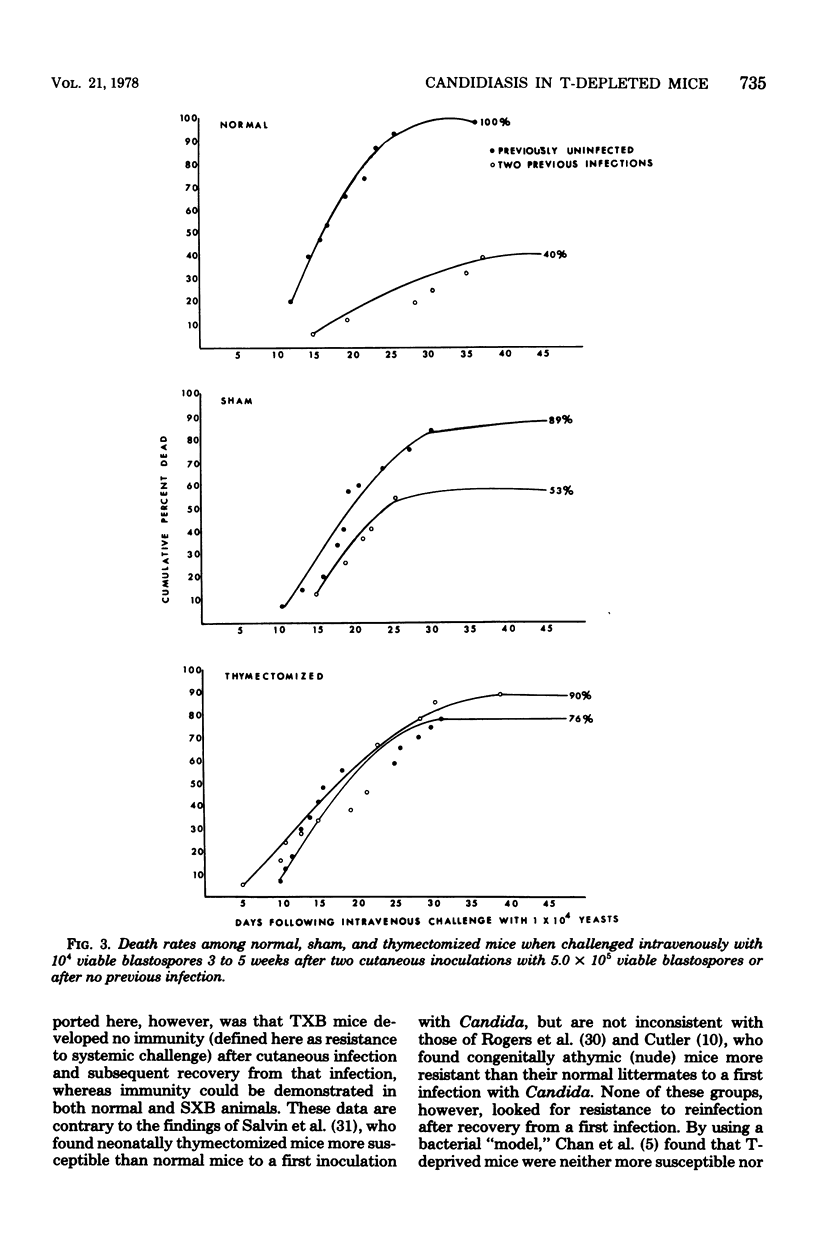
Mice depleted of T-lymphocytes by thymectomy and irradiation (TXB) and immunologically competent mice were compared for gross and histological pathology as well as immune responses after cutaneous and/or intravenous challenge with Candida albicans. In response to a first cutaneous inoculation with viable Candida, TXB, sham-operated (SXB), and unmanipulated (normal) mice, all developed lesions of comparable size, duration, and histopathology. When challenged a second time cutaneously, normal and SXB mice developed lesions which were greatly increased in size when compared with those produced by a first cutaneous infection, whereas TXB mice developed lesions comparable in size to those initiated by the first infection. Histologically, the first and second lesions in all animals were acute abscesses predominantly comprised of polymorphonuclear leukocytes. The larger second lesions in SXB and normal mice were accompanied by detectable circulating antibody and by delayed hypersensitivity. Neither circulating antibody nor delayed hypersensitivity were stimulated in the TXB mice. When challenged intravenously, all previously uninfected mice, regardless of T-cell status, were equally susceptible to C. albicans. Contrary to SXB or normal mice, however, TXB mice which had been infected cutaneously were not more resistant to a subsequent intravenous challenge as judged by 6-week survival. The results suggest that T-cells do not play a significant role in innate resistance of mice to systemic candidiasis, but that such cells are important in the development of acquired resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. C., Sahyoun A., Adler J. L., Schlesinger R. M., Breman J., Madras P., P'eng F., Monaco A. P. High incidence of fungus infections in renal transplantation patients treated with antilymphocyte and conventional immunosuppression. Transplant Proc. 1973 Mar;5(1):549–553. [PubMed] [Google Scholar]
- Benner R., van Oudenaren Antibody formation in mouse bone marrow. V. The response to the thymus-independent antigen Ecsherichia coli lipopolysaccharide. Immunology. 1976 Jan;30(1):49–57. [PMC free article] [PubMed] [Google Scholar]
- CUTLER S. J., EDERER F. Maximum utilization of the life table method in analyzing survival. J Chronic Dis. 1958 Dec;8(6):699–712. doi: 10.1016/0021-9681(58)90126-7. [DOI] [PubMed] [Google Scholar]
- Cahill L. T., Ainbender E., Glade P. R. Chronic mucocutaneous candidiasis: T cell deficiency associated with B cell dysfunction in man. Cell Immunol. 1974 Nov;14(2):215–225. doi: 10.1016/0008-8749(74)90207-x. [DOI] [PubMed] [Google Scholar]
- Chan C., Kongshavn P. A., Skamene E. Enhanced primary resistance to Listeria monocytogenes in T cell-deprived mice. Immunology. 1977 Apr;32(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Chronic mucocutaneous candidiasis. J Cutan Pathol. 1974;1(5):211–229. [PubMed] [Google Scholar]
- Cohen S. The role of cell-mediated immunity in the induction of inflammatory responses. Parke-Davis Award Lecture, 1977. Am J Pathol. 1977 Sep;88(3):502–528. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. G. Delayed-type hypersensitivity in the mouse. I. Induction and elicitation by Salmonella adelaide flagellin and its derivatives. Scand J Immunol. 1972;1(2):167–178. doi: 10.1111/j.1365-3083.1972.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Cutler J. E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976 Feb;19(2):121–124. [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTER R. V., COLLINS H. S. The occurrence of opportunistic fungus infections in a cancer hospital. Lab Invest. 1962 Nov;11:1035–1045. [PubMed] [Google Scholar]
- Hart P. D., Russell E., Jr, Remington J. S. The compromised host and infection. II. Deep fungal infection. J Infect Dis. 1969 Aug;120(2):169–191. doi: 10.1093/infdis/120.2.169. [DOI] [PubMed] [Google Scholar]
- Hermans P. E., Ulrich J. A., Markowitz H. Chronic mucocutaneous candidiasis as a surface expression of deep-seated abnormalities. Report of a syndrome of superficial candidiasis, absence of delayed hypersensitivity and aminoaciduria. Am J Med. 1969 Oct;47(4):503–519. doi: 10.1016/0002-9343(69)90181-8. [DOI] [PubMed] [Google Scholar]
- Hodes R. J., Handwerger B. S., Terry W. D. Synergy between subpopulations of mouse spleen cells in the in vitro generation of cell-mediated cytotoxicity: evidence for the involvement of a non-T cell. J Exp Med. 1974 Dec 1;140(6):1646–1659. doi: 10.1084/jem.140.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Smith T. K. Chronic mucocutaneous candidiasis: immunologic and antibiotic therapy. Ann Intern Med. 1974 Mar;80(3):310–320. doi: 10.7326/0003-4819-80-3-310. [DOI] [PubMed] [Google Scholar]
- Myerowitz R. L., Pazin G. J., Allen C. M. Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology. Am J Clin Pathol. 1977 Jul;68(1):29–38. doi: 10.1093/ajcp/68.1.29. [DOI] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Gaines J. D., Gilmer M. A. Demonstration of Candida precipitins in human sera by counterimmunoelectrophoresis. Lancet. 1972 Feb 19;1(7747):413–413. doi: 10.1016/s0140-6736(72)90860-4. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D., Marchioro T. L., Schneck S. A., Hill R. B., Jr Systemic fungal infections complicating renal transplantation and immunosuppressive therapy. Clinical, microbiologic, neurologic and pathologic features. Am J Med. 1967 Jul;43(1):28–38. doi: 10.1016/0002-9343(67)90146-5. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to experimental renal candidiasis in rats. Infect Immun. 1978 Feb;19(2):737–740. doi: 10.1128/iai.19.2.737-740.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- SALVIN S. B., PETERSON R. D., GOOD R. A. THE ROLE OF THE THYMUS IN RESISTANCE TO INFECTION AND ENDOTOXIN TOXICITY. J Lab Clin Med. 1965 Jun;65:1004–1022. [PubMed] [Google Scholar]
- Schoch E. P., Jr Thymic conversion of Candida albicans from commensalism to pathogenism. Arch Dermatol. 1971 Mar;103(3):311–319. [PubMed] [Google Scholar]
- Strong D. M., Ahmed A. A., Thurman G. B., Sell K. W. In vitro stimulation of murine spleen cells using a microculture system and a multiple automated sample harvester. J Immunol Methods. 1973 Apr;2(3):279–291. doi: 10.1016/0022-1759(73)90054-9. [DOI] [PubMed] [Google Scholar]
- Sweet C. E., Kaufman L. Application of agglutinins for the rapid and accurate identification of medically important Candida species. Appl Microbiol. 1970 May;19(5):830–836. doi: 10.1128/am.19.5.830-836.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Higgs J. M., Wells R. S., Yamamura M., Hobbs J. R., Holt P. J. Immune abnormalities associated with chronic mucocutaneous candidiasis. Cell Immunol. 1973 Mar;6(3):348–361. doi: 10.1016/0008-8749(73)90035-x. [DOI] [PubMed] [Google Scholar]
- WINNER H. I. A study of Candida albicans agglutinins in human sera. J Hyg (Lond) 1955 Dec;53(4):509–512. doi: 10.1017/s0022172400001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Geelhoed G. W., Levine A. S. Fungemia with compromised host resistance. A study of 70 cases. Ann Intern Med. 1974 May;80(5):605–612. doi: 10.7326/0003-4819-80-5-605. [DOI] [PubMed] [Google Scholar]


