Abstract
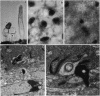
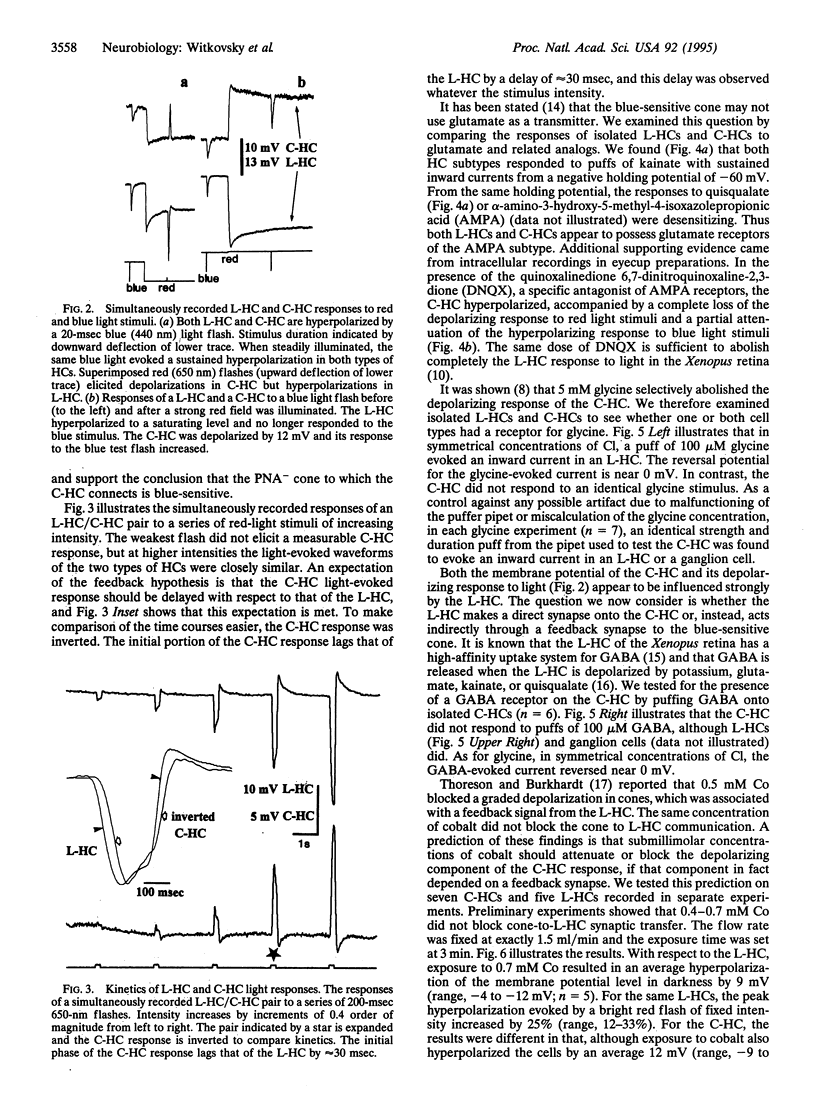
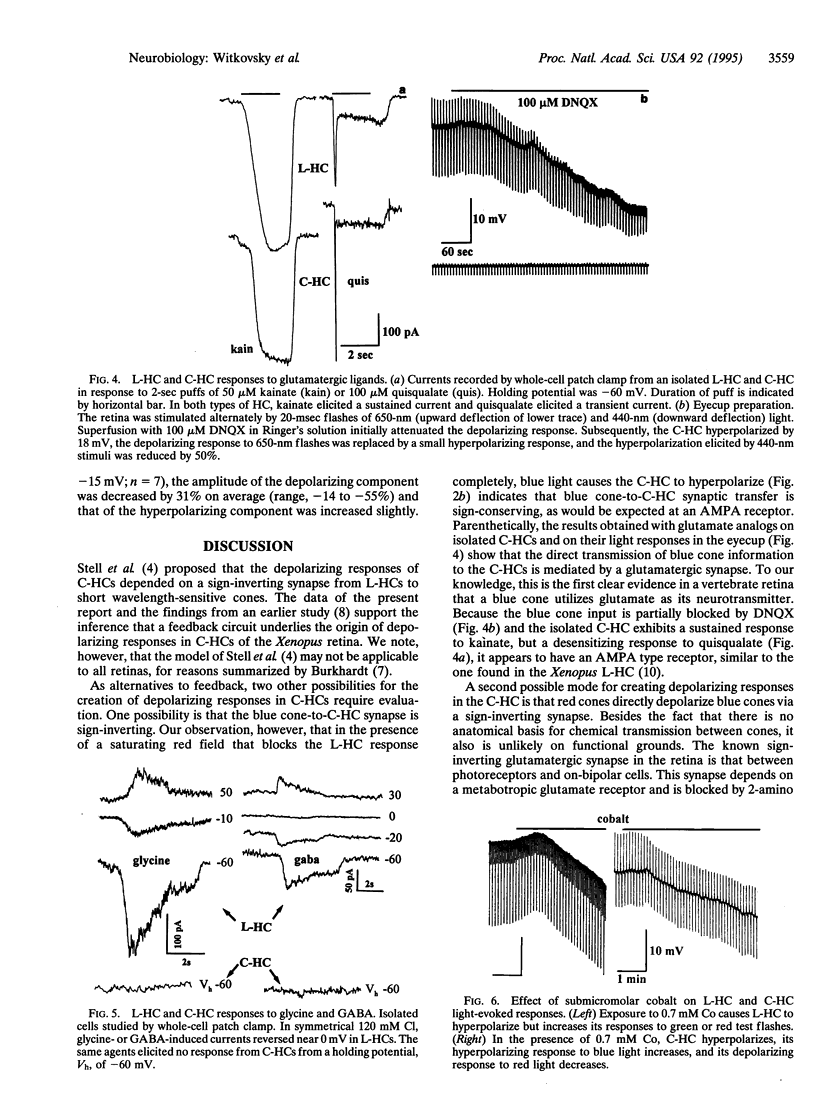
It has been proposed that the depolarizing responses of chromaticity horizontal cells (C-HCs) to red light depend on a feedback signal from luminosity horizontal cells (L-HCs) to short-wavelength-sensitive cones in the retinas of lower vertebrates. In this regard we studied the C-HCs of the Xenopus retina. C-HCs and L-HCs were identified by physiological criteria and then injected with neurobiotin. The retina then was incubated with peanut agglutinin, which stains red-but not blue-sensitive cones. Electron microscopic examination revealed that L-HCs contact all cone classes, whereas C-HCs contact only blue-sensitive cones. Simultaneous recordings from C-HC/L-HC pairs established that when the L-HC was saturated by a steady bright red light, C-HCs alone responded to a superimposed blue stimulus. In response to red test flashes, the C-HC response was delayed by approximately 30 msec with respect to the L-HC response. Isolated HCs of both subtypes were examined by whole-cell patch clamp. Both responded to kainate with sustained inward currents and to quisqualate or alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) with desensitizing currents from a negative holding potential; i.e., both have AMPA-type glutamate receptors. gamma-Aminobutyric acid or glycine opened a chloride channel in the L-HC, whereas the C-HC was unresponsive to either inhibitory amino acid. Since glycine has been shown to abolish selectively the depolarizing response of the C-HC, this finding and other pharmacological data strongly implicate the L-HC in the underlying circuit. Moreover, because the C-HC does not respond to gamma-aminobutyric acid, the neurotransmitter of the L-HC, by elimination, a feedback synapse from L-HC to blue cone is the most plausible mechanism for the creation of depolarizing responses in C-HCs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopian A., Witkovsky P. Modulation of transient outward potassium current by GTP, calcium, and glutamate in horizontal cells of the Xenopus retina. J Neurophysiol. 1994 May;71(5):1661–1671. doi: 10.1152/jn.1994.71.5.1661. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. Synaptic feedback, depolarization, and color opponency in cone photoreceptors. Vis Neurosci. 1993 Nov-Dec;10(6):981–989. doi: 10.1017/s0952523800010087. [DOI] [PubMed] [Google Scholar]
- Cunningham J. R., Neal M. J., Stone S., Witkovsky P. GABA release from Xenopus retina does not correlate with horizontal cell membrane potential. Neuroscience. 1988 Jan;24(1):39–48. doi: 10.1016/0306-4522(88)90309-0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Piccolino M. Sustained feedback effects of L-horizontal cells on turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):465–480. doi: 10.1098/rspb.1980.0008. [DOI] [PubMed] [Google Scholar]
- Gottesman J., Burkhardt D. A. Response properties of C-type horizontal cells in the retina of the bowfin. Vision Res. 1987;27(2):179–189. doi: 10.1016/0042-6989(87)90180-5. [DOI] [PubMed] [Google Scholar]
- Hollyfield J. G., Rayborn M. E., Sarthy P. V., Lam D. M. The emergence, localization and maturation of neurotransmitter systems during development of the retina in Xenopus laevis. I. Gamma aminobutyric acid. J Comp Neurol. 1979 Dec 15;188(4):587–598. doi: 10.1002/cne.901880406. [DOI] [PubMed] [Google Scholar]
- Krizaj D., Akopian A., Witkovsky P. The effects of L-glutamate, AMPA, quisqualate, and kainate on retinal horizontal cells depend on adaptational state: implications for rod-cone interactions. J Neurosci. 1994 Sep;14(9):5661–5671. doi: 10.1523/JNEUROSCI.14-09-05661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Liu W. L., Kalloniatis M., Raiguel S. F., van Haesendonck E. Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci. 1990 Dec;10(12):4006–4034. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T., Kouyama N. Electron microscopic study of synaptic contacts between photoreceptors and HRP-filled horizontal cells in the turtle retina. J Comp Neurol. 1986 Aug 8;250(2):141–156. doi: 10.1002/cne.902500202. [DOI] [PubMed] [Google Scholar]
- Perry R. J., McNaughton P. A. Response properties of cones from the retina of the tiger salamander. J Physiol. 1991 Feb;433:561–587. doi: 10.1113/jphysiol.1991.sp018444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell W. K., Lightfood D. O., Wheeler T. G., Leeper H. F. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975 Dec 5;190(4218):989–990. doi: 10.1126/science.1188380. [DOI] [PubMed] [Google Scholar]
- Stone S., Witkovsky P., Schütte M. A chromatic horizontal cell in the Xenopus retina: intracellular staining and synaptic pharmacology. J Neurophysiol. 1990 Dec;64(6):1683–1694. doi: 10.1152/jn.1990.64.6.1683. [DOI] [PubMed] [Google Scholar]
- Stone S., Witkovsky P. The actions of gamma-aminobutyric acid, glycine and their antagonists upon horizontal cells of the Xenopus retina. J Physiol. 1984 Aug;353:249–264. doi: 10.1113/jphysiol.1984.sp015334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. gamma-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7961–7964. doi: 10.1073/pnas.81.24.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Kaneko A. gamma-Aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3501–3505. doi: 10.1073/pnas.84.10.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson W. B., Burkhardt D. A. Ionic influences on the prolonged depolarization of turtle cones in situ. J Neurophysiol. 1991 Jan;65(1):96–110. doi: 10.1152/jn.1991.65.1.96. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kleinschmidt J., Sun P., Witkovsky P. Identification of cone classes in Xenopus retina by immunocytochemistry and staining with lectins and vital dyes. Vis Neurosci. 1994 Nov-Dec;11(6):1185–1192. doi: 10.1017/s0952523800006982. [DOI] [PubMed] [Google Scholar]