Abstract
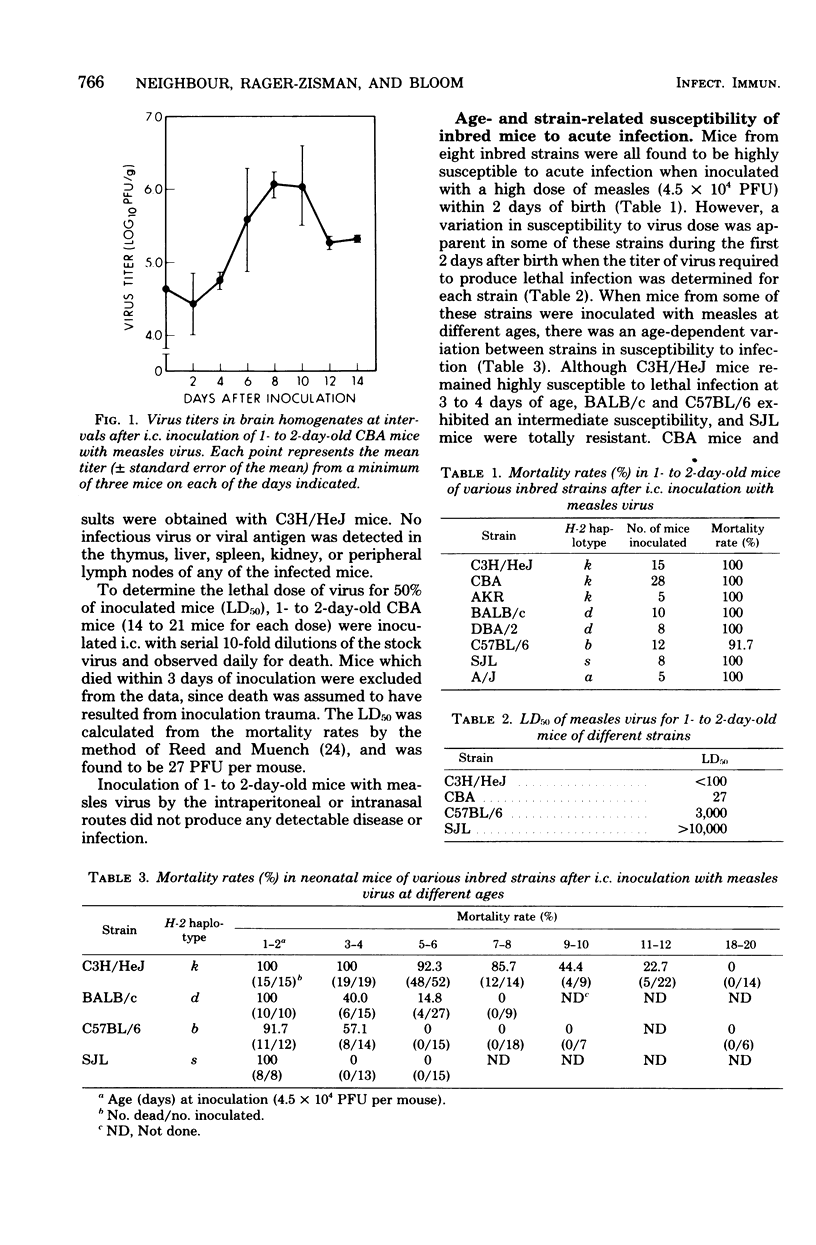
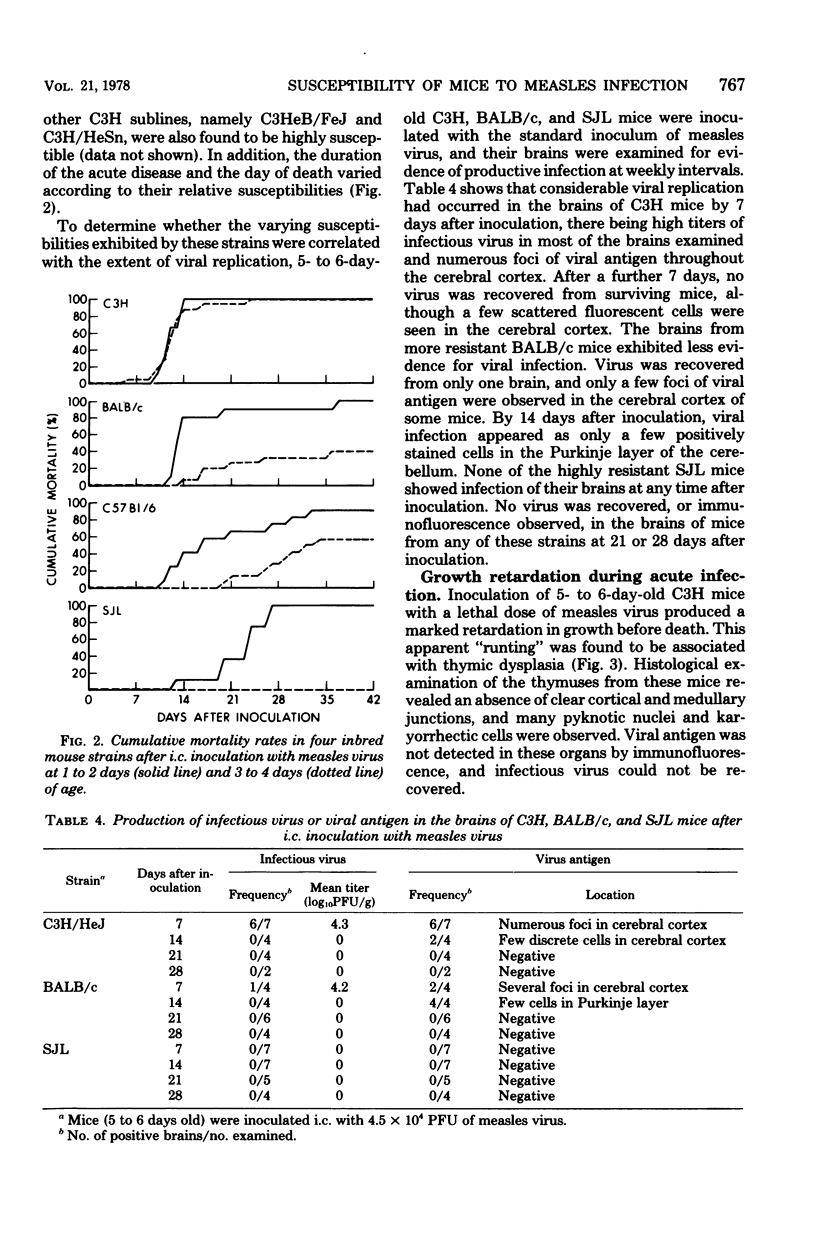
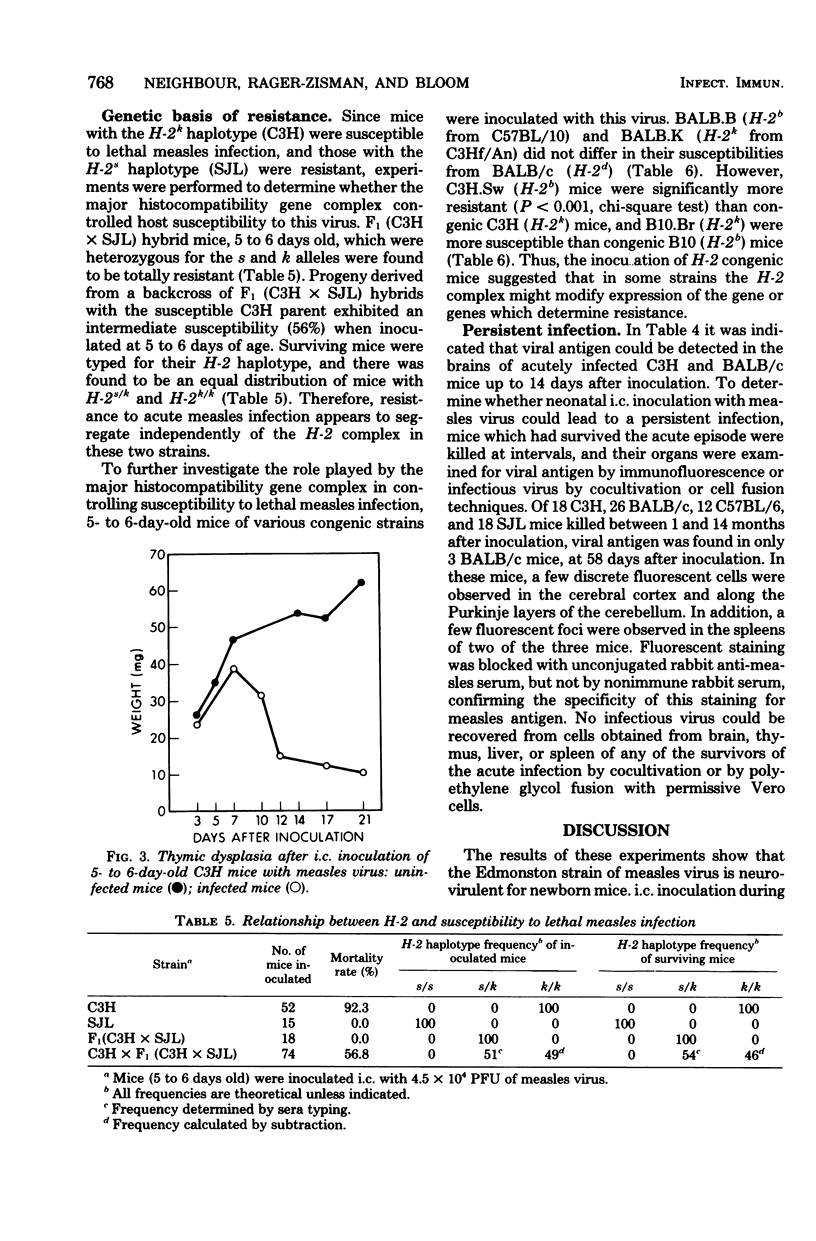
Intracerebral inoculation of neonatal mice with the Edmonston strain of measles virus produced an acute, lethal encephalitis and thymic dysplasia in susceptible mice. There was an age-related development of resistance to infection. This resistance was strain-dependent and appeared to be associated with the extent of virus growth in the brain. Studies on the genetic basis for susceptibility, using hybrid and backcross mice, revealed that the principal determinant of host resistance to acute infection was a dominant gene or genes which segregated independently of the H-2 complex. A small number of survivors of the acute infection showed persistence of measles virus antigens in the cerebellum and spleen for up to 2 months after inoculation. However, the low frequency of this persistence indicated that, at this time, intracerebral inoculation of neonatal mice with the Edmonston strain of measles virus constitutes a difficult model for the study of persistant measles infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. M., IMAGAWA D. T. Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med. 1962 Dec;111:562–566. doi: 10.3181/00379727-111-27855. [DOI] [PubMed] [Google Scholar]
- Black F. L. The association between measles and multiple sclerosis. Prog Med Virol. 1975;21:158–164. [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. II. The neuropathology of acute and chronic infections. Exp Mol Pathol. 1973 Jun;18(3):345–356. doi: 10.1016/0014-4800(73)90030-0. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatability complex. J Exp Med. 1974 Dec 1;140(6):1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Fuccillo D. A., Kurent J. E., Sever J. L. Slow virus diseases. Annu Rev Microbiol. 1974;28(0):231–234. doi: 10.1146/annurev.mi.28.100174.001311. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Mullinix J., Narayan O., Johnson R. T. Age dependence of viral expression: comparative pathogenesis of two rodent-adapted strains of measles virus in mice. Infect Immun. 1974 Apr;9(4):690–695. doi: 10.1128/iai.9.4.690-695.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales A. A procedure for the fusion of cells in suspension by means of polyethylene glycol. Somatic Cell Genet. 1977 Mar;3(2):227–230. doi: 10.1007/BF01551817. [DOI] [PubMed] [Google Scholar]
- Hall W. W., ter Meulen V. RNA homology between subacute sclerosing panencephalitis and measles viruses. Nature. 1976 Dec 2;264(5585):474–477. doi: 10.1038/264474a0. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Experimental measles encephalitis: a genetic analysis. Infect Immun. 1975 Oct;12(4):785–790. doi: 10.1128/iai.12.4.785-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Jersild C., Fog T., Hansen G. S., Thomsen M., Svejgaard A., Dupont B. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet. 1973 Dec 1;2(7840):1221–1225. doi: 10.1016/s0140-6736(73)90970-7. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. The possible viral etiology of multiple sclerosis. Adv Neurol. 1975;13:1–46. [PubMed] [Google Scholar]
- LINDENMANN J. INHERITANCE OF RESISTANCE TO INFLUENZA VIRUS IN MICE. Proc Soc Exp Biol Med. 1964 Jun;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- Lilly F. The role of genetics in Gross virus leukemogenesis. Bibl Haematol. 1970;(36):213–220. doi: 10.1159/000391710. [DOI] [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Oldstone B. A., Pincus T. Histocompatibility-linked genetic control of specific immune responses to viral infection. Transplant Rev. 1974;19(0):209–225. doi: 10.1111/j.1600-065x.1974.tb00133.x. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Histocompatibility studies and multiple sclerosis. Arch Neurol. 1976 Jun;33(6):395–398. doi: 10.1001/archneur.1976.00500060001001. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J., Mitchell G. F., McDevitt H. O. Histocompatibility-linked genetic control of disease susceptibility. Murine lymphocytic choriomeningitis virus infection. J Exp Med. 1973 May 1;137(5):1201–1212. doi: 10.1084/jem.137.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertschuk L. P., Cook A. W., Gupta J. Measles antigen in multiple sclerosis: identification in the jejunum by immunofluorescence. Life Sci. 1976 Nov 15;19(10):1603–1608. doi: 10.1016/0024-3205(76)90107-7. [DOI] [PubMed] [Google Scholar]
- Prasad I., Broome J. D., Pertschuk L. P., Gupta J., Cook A. W. Recovery of Paramyxovirus from the jejunum of patients with multiple sclerosis. Lancet. 1977 May 28;1(8022):1117–1119. doi: 10.1016/s0140-6736(77)92381-9. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Merigan T. C. A useful quantitative semimicromethod for viral plaque assay. Proc Soc Exp Biol Med. 1973 Apr;142(4):1174–1179. doi: 10.3181/00379727-142-37202. [DOI] [PubMed] [Google Scholar]
- Steeves R., Lilly F. Interactions between host and viral genomes in mouse leukemia. Annu Rev Genet. 1977;11:277–296. doi: 10.1146/annurev.ge.11.120177.001425. [DOI] [PubMed] [Google Scholar]
- Utermohlen V., Farmer J., Kornbluth J., Kornstein M. The relationship between direct migration inhibition with measles antigen and E rosettes in normals and patients with multiple sclerosis. Clin Immunol Immunopathol. 1978 Jan;9(1):63–66. doi: 10.1016/0090-1229(78)90121-6. [DOI] [PubMed] [Google Scholar]
- Utermohlen V., Zabriskie J. B. A suppression of cellular immunity in patients with multiple sclerosis. J Exp Med. 1973 Dec 1;138(6):1591–1596. doi: 10.1084/jem.138.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971 Dec;107(6):1593–1598. [PubMed] [Google Scholar]
- Wechsler S. L., Fields B. N. Differences between the intracellular polypeptides of measles and subacute sclerosing panencephalitis virus. Nature. 1978 Mar 30;272(5652):458–460. doi: 10.1038/272458a0. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K., Uchida N., Katow S., Sato T. A., Kobune K. Growth of measles virus in nervous tissues. IV. Neurovirulence of wild measles and SSPE viruses in monkeys. Jpn J Med Sci Biol. 1976 Aug;29(4):177–186. doi: 10.7883/yoken1952.29.177. [DOI] [PubMed] [Google Scholar]


