Abstract
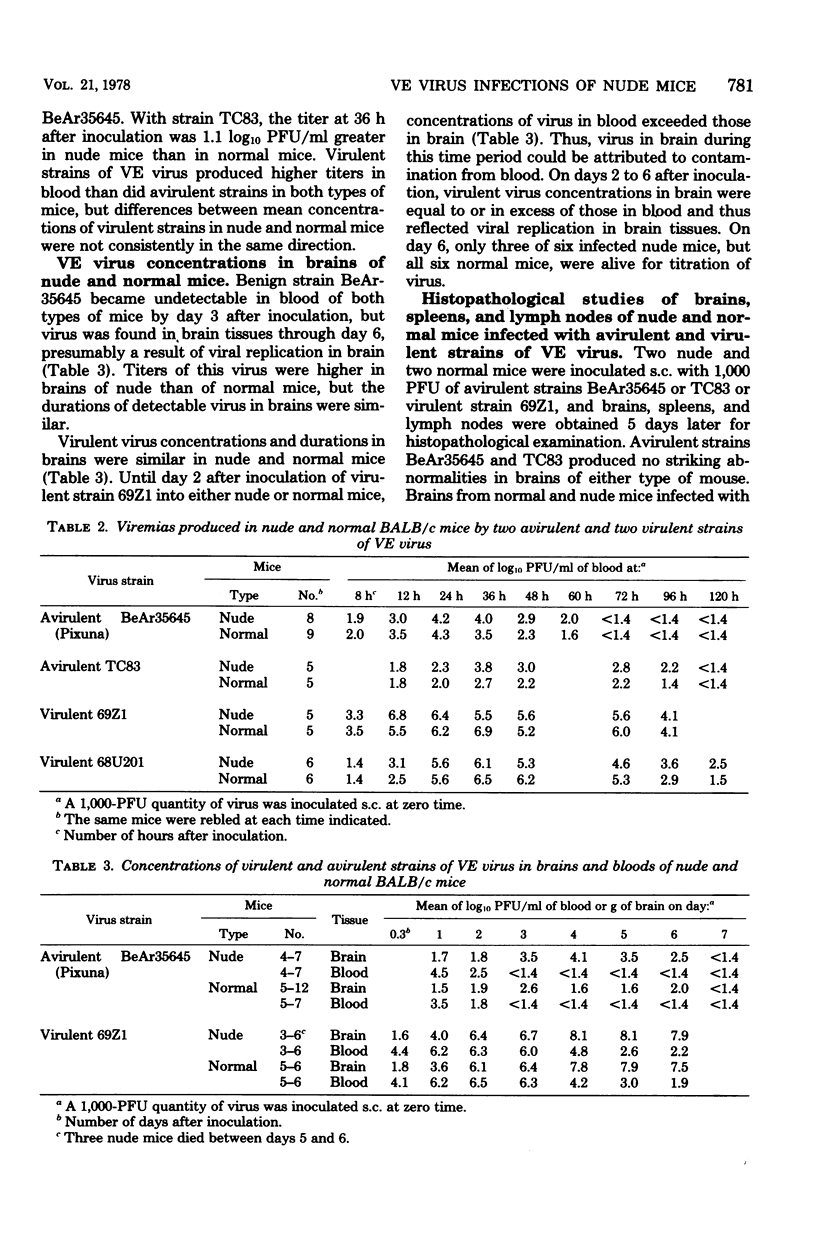
Two strains of Venezuelan encephalitis virus that are avirulent for normal BALB/c mice inoculated subcutaneously were also avirulent for infected congenitally athymic (nude) mice of the same strain. Viremias were of similar magnitudes and durations in normal and nude mice. Brain concentrations were higher in nude mice with the one avirulent strain tested, although the periods of detectable virus in brains were similar. No lesions were found in brains, spleens, or lymph nodes by ordinary histopathological examination. Viral neutralizing antibody titers in plasmas at 1 to 3 weeks after infection were lower and more transient in nude than in normal mice, and implantations of thymic tissues into nude mice partially restored their neutralizing antibody responses. Concentrations of spleen cells producing antibodies that lysed sheep erythrocytes 4 days after inoculation of erythrocytes and avirulent virus into nude mice were above the levels of uninfected nude mice. These concentrations were similar in infected and uninfected normal mice. In contrast, two mouse-virulent strains of Venezuelan encephalitis virus killed nude mice faster than normal mice after subcutaneous inoculation. Yet concentrations and durations of virus in bloods and brains were not consistently different between nude and normal mice. There were perivascular monocytes in brains and slight architectural alterations of spleens and lymph nodes. Concentrations of spleen cells producing antibodies hemolytic for sheep erythrocytes 4 days after inoculation with erythrocytes were depressed in nude and normal mice by infection with virulent strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGE T. O., GLEISER C. A., GOCHENOUR W. S., Jr, MIESSE M. L., TIGERTT W. D. Studies on the virus of Venezuelan equine encephalomyelitis. II. Modification by specific immune serum of response of central nervous system of mice. J Immunol. 1961 Nov;87:509–517. [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- Croy B. A., Osoba D. Nude mice--a model system for studying the cellular basis of the humoral immune response. Cell Immunol. 1973 Nov;9(2):306–318. doi: 10.1016/0008-8749(73)90082-8. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., KELLY W. D., ROTSTEIN J., VARCO R. L. Immunological deficiency diseases. Agammaglobulinemia, hypogammaglobulinemia, Hodgkin's disease and sarcoidosis. Prog Allergy. 1962;6:187–319. [PubMed] [Google Scholar]
- Hapel A. J. The protective role of thymus-derived lymphocytes in arbovirus-induced meningoencephalitis. Scand J Immunol. 1975;4(3):267–278. doi: 10.1111/j.1365-3083.1975.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Craig C. P., Trevino G. S., Dougherty S. F., Mergenhagen S. E. Enhanced humoral immunity in mice infected with attenuated Venezuelan equine encephalitis virus. J Immunol. 1969 Oct;103(4):699–707. [PubMed] [Google Scholar]
- Hrusková J., Rychterová V., Kliment V. The influence of infection with Venezuelan equine encephalomyelitis virus on antibody response against sheep erythrocytes. I. Experiments on mice. Acta Virol. 1972 Mar;16(2):115–124. [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- MCKINNEY R. W., BERGE T. O., SAWYER W. D., TIGERTT W. D., CROZIER D. USE OF AN ATTENUATED STRAIN OF VENEZUELAN EQUINE ENCEPHALOMYELITIS VIRUS FOR IMMUNIZATION IN MAN. Am J Trop Med Hyg. 1963 Jul;12:597–603. doi: 10.4269/ajtmh.1963.12.597. [DOI] [PubMed] [Google Scholar]
- Manning D. D., Reed N. D., Shaffer C. F. Maintenance of skin xenografts of widely divergent phylogenetic origin of congenitally athymic (nude) mice. J Exp Med. 1973 Aug 1;138(2):488–494. doi: 10.1084/jem.138.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R., Kimoto K., Takeya K. The role of the thymus in antibody production and in resistance to Japanese encephalitis virus infection. Arch Gesamte Virusforsch. 1970;29(1):32–38. doi: 10.1007/BF01253877. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression and experimental virus infection of the nervous system. Adv Virus Res. 1970;16:397–448. doi: 10.1016/S0065-3527(08)60028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennycuik P. R. Unresponsiveness of nude mice to skin allografts. Transplantation. 1971 Apr;11(4):417–418. doi: 10.1097/00007890-197104000-00012. [DOI] [PubMed] [Google Scholar]
- SCHERER W. F. INAPPARENT VIRAL INFECTION OF CELLS IN VITRO. I. CONVERSION OF INAPPARENT TO APPARENT INFECTION BY ENVIRONMENTAL ALTERATION OF CHICKEN EMBRYONIC CELLS IN CULTURES INOCULATED WITH JAPANESE ENCEPHALITIS VIRUS. Am J Pathol. 1964 Sep;45:393–411. [PMC free article] [PubMed] [Google Scholar]
- Scherer W. F., Dickerman R. W., Ordonez J. V. Discovery and geographic distribution of Venezuelan encephalitis virus in Guatemala, Honduras, and British Honduras during 1965-68, and its possible movement to Central America and México. Am J Trop Med Hyg. 1970 Jul;19(4):703–711. doi: 10.4269/ajtmh.1970.19.703. [DOI] [PubMed] [Google Scholar]
- Scherer W. F., Ellsworth C. A., Ventura A. K. Studies of viral virulence. II. Growth and adsorption curves of virulent and attenuated strains of Venezuelan encephalitis virus in cultured cells. Am J Pathol. 1971 Feb;62(2):211–219. [PMC free article] [PubMed] [Google Scholar]
- Scherer W. F., Ordonez J. V., Jahrling P. B., Pancake B. A., Dickerman R. W. Observations of equines, humans and domestic and wild vertebrates during the 1969 equine epizootic and epidemic of Venezuelan encephalitis in Guatemala. Am J Epidemiol. 1972 Mar;95(3):255–266. doi: 10.1093/oxfordjournals.aje.a121393. [DOI] [PubMed] [Google Scholar]
- Staab E. V., Normann S. J., Craig C. P. Alterations in reticuloendothelial function by infection with attenuated Venezuelan equine encephalitis (VEE) virus. J Reticuloendothel Soc. 1970 Oct;8(4):342–348. [PubMed] [Google Scholar]
- Sussdorf D. H., McCann R. L. Serum concentrations of four immunoglobulins in non-immunized and immunized athymic ('nude') mice. Immunochemistry. 1975 Jul;12(6-7):607–609. doi: 10.1016/0019-2791(75)90094-4. [DOI] [PubMed] [Google Scholar]
- Woodman D. R., McManus A. T., Eddy G. A. Extension of the mean time to death of mice with a lethal infection of Venezuelan equine encephalomyelitis virus by antithymocyte serum treatment. Infect Immun. 1975 Nov;12(5):1006–1011. doi: 10.1128/iai.12.5.1006-1011.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


