Abstract
The superiority of the pediatric protocol for adolescents with acute lymphoblastic leukemia (ALL) has already been demonstrated, however, its efficacy in young adults remains unclear. The ALL202-U protocol was conducted to examine the efficacy and feasibility of a pediatric protocol in adolescents and young adults (AYAs) with BCR–ABL-negative ALL. Patients aged 15–24 years (n=139) were treated with the same protocol used for pediatric B-ALL. The primary objective of this study was to assess the disease-free survival (DFS) rate and its secondary aims were to assess toxicity, the complete remission (CR) rate and the overall survival (OS) rate. The CR rate was 94%. The 5-year DFS and OS rates were 67% (95% confidence interval (CI) 58–75%) and 73% (95% CI 64–80%), respectively. Severe adverse events were observed at a frequency that was similar to or lower than that in children treated with the same protocol. Only insufficient maintenance therapy significantly worsened the DFS (hazard ratio 5.60, P<0.001). These results indicate that this protocol may be a feasible and highly effective treatment for AYA with BCR–ABL-negative ALL.
Introduction
Acute lymphoblastic leukemia (ALL) has been very successfully treated in children, however, the prognosis of patients markedly deteriorates from the onset of adolescence to adulthood. A period analysis of ALL patients between 2000 and 2004 showed 5-year relative survival rates of 80.7% in patients aged 10–14 years and 44.8% in patients aged 20–29 years.1,2 Retrospective studies that focused on patients aged 15–21 years reported that adolescents and young adults (AYAs) treated with adult ALL protocols had poorer outcomes than similarly aged patients treated with pediatric protocols.3, 4, 5, 6 However, these studies compared patients treated with a pediatric protocol by pediatricians to those treated with an adult protocol by physicians; the former were adolescents with a median age of 16 or 17 and the latter were AYAs with a median age 19 or older. These findings were similar to those of recently reported prospective studies by pediatric groups.7,8 The reason of the efficacy of pediatric protocols on young adults and how the difference in age between these patient groups is responsible for the observed differences in survival have yet to be determined, and can only be clarified by examining patients treated with pediatric protocols in an adult study group. Several prospective clinical trials using pediatric regimens for adults are currently ongoing. These studies have been divided into two types based on their regimens and patients: a pediatric-inspired protocol with dose reductions in a pediatric protocol for adults up to 60 years, and an unmodified pediatric protocol for AYA up to 30 years. A few of the former studies have already been completed, and the results obtained revealed marked improvements in the survival rates of ALL patients up to 60 years old.9,10 However, the possibility that AYA may achieve better survival rates with the original pediatric protocol was not investigated in these studies. The feasibility and efficacy of an unmodified pediatric protocol for AYA should be examined. One previous study reported marked improvements in survival rates;11 however, only standard-risk ALL were treated. Therefore, the efficacy of this protocol in young adults with high-risk ALL remains unknown.
The Japan Adult Leukemia Study Group (JALSG) conducted a phase 2 trial in which patients aged 15–24 years with BCR–ABL-negative ALL were treated with the same protocol developed for children with ALL by the Japan Association of Childhood Leukemia Study (JACLS). We analyzed the outcomes and prognostic factors of the 139 AYA patients treated in this trial.
Patients and methods
Patients and eligibility criteria
The JALSG ALL202-U (ALL202-U) study is a prospective nonrandomized phase 2 trial, a part of the JALSG ALL202 (ALL202) study conducted by JALSG and was registered at UMIN-CTR (ID: C000000064). Eligibility criteria were common with the ALL202 study.12 The protocol was approved by the Institutional Review Board of each hospital. Written informed consent was obtained from all patients before registration in accordance with the Declaration of Helsinki. Guardians also gave written informed consent when patients were under 20 years old. The study was initiated in August 2002 and closed for patient inclusion in October 2009.
Diagnostic procedure
ALL was diagnosed according to the French–American–British classification13 using morphology, cytochemistry and immunophenotyping studies at each institution. Mature B-cell ALL was excluded. Immunophenotyping and cytogenetic studies were performed as described previously.14 The multiplex reverse transcription-PCR test was described previously.12
Study design and treatment
The study design of ALL202 has previously been described in detail.12 Patients were treated differently according to age and the BCR–ABL diagnosis results. Patients aged 15–24 years and negative for BCR–ABL were treated with the same pediatric regimen as the ALL202-U study. The protocol was conducted for high-risk pediatric B-ALL in the ALL-02 study by the JACLS and designated as ALL-02-HR.15 The study was initiated in April 2002, closed for patient inclusion in May 2008 and the results are awaited. The toxicity data of this study, which have been referred later, were obtained by analysis in May 2011.
The treatment schedule for ALL202-U is shown in Table 1. Patients underwent a 7-day prephase therapy with prednisolone (PSL) and a single intrathecal injection of methotrexate (MTX) after registration. Responsiveness to PSL was judged on day 8, and BCR–ABL-negative patients continued the protocol study. Patients who achieved complete remission (CR) after induction therapy received consolidation therapy, sanctuary therapy, reinduction therapy and reconsolidation therapy. Patients who did not achieve CR after induction therapy received consolidation therapy. If CR was not achieved with this therapy, protocol therapy was terminated as induction failure.
Table 1. JALSG-ALL202-U schedule.
| Phases/drugs | Route | Doses | Days |
|---|---|---|---|
| Induction therapy (weeks 1–5) | |||
| Methotrexate | IT | 12 mg/body | 1 |
| Prednisolone | PO/IV | 60 mg/m2 | 1–7 |
| Dexamethasone | IV | 10 mg/m2 | 8–14 |
| Vincristine | IV | 1.5 mg/m2 a | 8, 15, 22, 29 |
| THP-adriamycin | IV | 25 mg/m2 | 8, 9 |
| Cyclophosphamide | IV | 1200 mg/m2 | 10 |
| L-asparaginase | IV/IM | 6000 U/m2 | 15, 17, 19, 21, 23, 25, 27, 29 |
| Prednisolone | PO | 40 mg/m2 | 15–28 |
| IT-tripleb | IT | 8, 22c | |
| Consolidation thrapy (weeks 6–9) | |||
| Cyclophosphamide | IV | 750 mg/m2 | 1, 8 |
| THP-adriamycin | IV | 25 mg/m2 | 1, 2 |
| Cytarabine | IV | 75 mg/body | 1–6, 8–13d |
| Mercaptopurine | PO | 50 mg/m2 | 1–14 |
| IT-tripleb | IT | 1, 8 | |
| Sanctuary therapy (weeks 10–11) | |||
| Methotrexatee | IV (24 h) | 3 g/m2 | 1, 8 |
| IT-tripleb | IT | 2, 9 | |
| Reinduction therapy (weeks 12–15) | |||
| Vincristine | IV | 1.5 mg/m2 a | 1, 8, 15 |
| THP-adriamycin | IV | 25 mg/m2 | 1, 8 |
| Cyclophosphamide | IV | 500 mg/m2 | 1, 8 |
| L-asparaginase | IM | 6000 U/m2 | 1, 3, 5, 8, 10, 12 |
| Prednisolone | PO | 40 mg/m2 | 1–14 |
| IT-tripleb | IT | 1 | |
| Reconsolidation therapy (weeks 16–19) | |||
| Same as consolidation therapy | |||
| Maintenance therapy 1-A (weeks 20–25) for CNS-invasion-negative cases | |||
| Methotrexate | IV | 150 mg/m2 | 1, 15, 29 |
| Mercaptopurine | PO | 50 mg/m2 f | 1–28 |
| IT-tripleb | IT | 29 | |
| Maintenance therapy 1-B (weeks 20–25) for CNS-invasion-positive cases | |||
| Cranial irradiation | 1.5 Gry × 8 | 1–12g | |
| Methotrexate | IV | 150 mg/m2 | 29 |
| Mercaptopurine | PO | 50 mg/m2 f | 1–28 |
| IT-tripleb | IT | 1, 8 | |
| Maintenance therapy 2 (weeks 26–29, 46–49, 66–69, 86–89) | |||
| Vincristine | IV | 1.5 mg/m2 a | 1, 8, 15 |
| Cyclophosphamide | IV | 600 mg/m2 | 8 |
| L-asparaginase | IM | 10000 U/m2 | 1, 8, 15 |
| Prednisolone | PO | 40 mg/m2 | 1–14 |
| Maintenance therapy 3 (weeks 30–35, 40–45, 50–55, 60–65, 70–75, 80–85, 90–95) | |||
| Methotrexate | IV | 150 mg/m2 | 1, 15, 29 |
| Mercaptopurine | PO | 50 mg/m2 f | 1–28 |
| IT-tripleb | IT | 29h,i | |
| Maintenance therapy 4 (weeks 36–39, 56–59, 76–79, 96–98) | |||
| Vincristine | IV | 1.5 mg/m2 a | 1, 8, 15 |
| THP-adriamycin | IV | 25 mg/m2 | 8 |
| L-asparaginase | IM | 10 000 U/m2 | 1, 8, 15 |
| Prednisolone | PO | 40 mg/m2 | 1–14 |
Abbreviations: CNS, central nervous system; JALSG, Japan Adult Leukemia Study Group; IM, intramuscularly; IT, intrathecally; IV, intravenously; PO, per os; WBC, while blood cell.
Maximum dose was 2 mg per body.
IT-triple consisted of methotrexate 12 mg, cytarabine 30 mg and hydrocortisone 25 mg.
On days 8, 11, 15, and 22, when CNS invasion was positive.
Administration was stopped, when neutrophil count went down to 0/l.
With folinic acid rescue (15 mg/m2, IV, six times every 6 h), beginning 42 h after the start of methotrexate infusion.
Dose should be adjusted to keep WBC count from 2000 to 3000/ul.
Eight times during this period.
For CNS-invasion-negative cases.
Not on weeks 74 and 94.
Allo-stem cell transplantation (SCT) was recommended for patients with t(4;11) who achieved CR during their first CR if a human leukocyte antigen-matched sibling was available, and allo-SCT from an alternative donor was allowed. An indication for SCT was decided for patients with other types according to institutional discretion. Each institution decided the preparative and post-transplant regimens for SCT according to its own discretion.
Detailed rules for treatment
Every therapy had a planned therapy duration. New therapy was started on the planned day if neutrophil and platelet counts had reached ⩾0.5 × 109/l and ⩾50 × 109/l, respectively, and patients had no significant infection at that time. Therapies could be started earlier if patients fulfilled the above conditions. Delays within 3 days for social reasons and 4 weeks because of complications were allowed. Folic acid rescue in sanctuary therapy was increased to every 3 h when the blood concentration of MTX was ⩾1.0 μmol/l 48 h after its administration or ⩾0.2 μmol/l after 72 h, and was continued until the MTX concentration fell to <0.1 μmol/l. When the MTX concentration was ⩾0.1 μmol/l and <0.2 μmol after 72 h, folic acid rescue was added only four times every 6 h. Maintenance therapy consisted of 16 courses of therapy. The dose of 6-MP was adjusted to maintain the white blood cell (WBC) count at 2–3 × 109/l. Central nervous system (CNS) prophylaxis included the administration of 14 courses of intrathecal therapy of MTX, cytarabine and hydrocortisone and a single intrathecal injection of MTX.
Evaluation of patients
CR was defined as the presence of all of the following: <5% blasts in bone marrow, no leukemic blasts in peripheral blood, recovery of peripheral blood values to neutrophil counts of at least 1.0 × 109/l and platelet counts of at least 100 × 109/l, and no evidence of extramedullary leukemia. Relapse was defined as the presence of at least one of the following: recurrence of>10% leukemic cells in bone marrow or any leukemic cells in peripheral blood or extramedullary sites. Toxicity was evaluated based on the National Cancer Institute Common Toxicity Criteria (NCI-CTC) Version 2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf). Corticosteroid sensitivity was defined as a peripheral blood blast cell count <1.5 × 109/l after the 7-day corticosteroid prephase.
Sample size estimation and statistical analysis
This study was designed as phase 2 and the sample size was determined before the study. We set an expected 5-year disease-free survival (DFS) rate of 50%, and estimated that 96 patients were required to achieve a 95% confidence interval (CI) of narrower than ±10%. Considering potential dropout because of ineligibility or loss to follow-up, we finally used 120 as the required number of patients.
The primary objective of this study was to assess DFS rate, and the secondary aims were to assess toxicity, the CR rate and the overall survival (OS) rate. An exploratory evaluation of potential prognostic factors was also conducted. We defined DFS as the time from the date of achieving CR to relapse, death or the last visit, and OS as the time from the first day of therapy to death or the last visit. Patients undergoing SCT were not censored at the time of transplantation and were evaluated with the inclusion of a post-transplantation period. The results for Ph-negative ALL patients under 25 years old in the JALSG ALL97 study (ALL97-U) were used as a reference. The treatment schedule for ALL97 has been reported previously14 and was shown in Supplementary Table 1. The Χ2 test was used to statistically analyze characteristic differences between patient groups. The Kaplan–Meier product limit method was performed to estimate DFS and OS. Patients were divided into two groups in some analyses. Patients whose WBC counts were <30 × 109/l and karyotype risks that were standard or intermediate in the modified MRC UKALLXII/ECOG E2993ALL cytogenetic classification16 were defined as the standard-risk group, and others were defined as the high-risk group. The DFS rates of each group were analyzed separately. To compare DFS and OS rates, the log-rank test was used for univariate analysis, and a Cox proportional hazard model for uni- and multivariate analyses. To evaluate maintenance therapy insufficiency, we treated the termination of maintenance therapy as a time-varying covariate. Stata SE 11.2 (Stata Co., College Station, TX, USA) was used for all statistical analyses.
Results
Patient entry and characteristics
Between August 2002 and October 2009, 150 patients from 59 hospitals participating in the JALSG were enrolled in this study. Eleven patients were excluded because two had been misdiagnosed (one with acute myeloid leukemia and one with BCR–ABL-positive ALL), four had dropped out before starting the treatment, four had been registered after prephase therapy and one was registered before protocol approval by the Institutional Review Board. Therefore, we here reported the outcomes of 139 eligible patients. The diagnosis of BCR–ABL negativity was based on the Multiplex RQ-PCR assay (n=124), BCR–ABL RQ-PCR assay (n=1), fluorescent in situ hybridization analysis (n=7) and chromosome karyotype assay (n=7). The pretreatment characteristics of ALL202-U and ALL97-U were summarized in Table 2. The median age was 19 years and there were 78 men (56%) and 61 women. Cytogenetic evaluations were performed in all 139 patients, and revealed that all were Ph-negative. Results were classified according to the modified MRC UKALLXII/ECOG E2993ALL cytogenetic subgroups:16 the very high-risk group (n=15) included t(4;11), complex karyotype, defined as >5 abnormalities without known translocations or low hypodiploidy/near triploidy; the high-risk group (n=8) included other MLL translocations, monosomy 7 with <5 abnormalities or t(1;19); the intermediate-risk group (n=110) included a normal karyotype or other miscellaneous abnormal karyotypes; the standard-risk group (n=2) included high hyperdiploidy. The multiplex RQ-PCR assay was performed for 124 patients. Twelve sets of primers were used to detect WT1, MDR1, and nine distinct fusion gene transcripts, namely, major and minor BCR–ABL, TEL-AML1, E2A-PBX1, MLL-AF4, MLL-AF6, MLL-AF9, MLL-ENL, SIL-TAL1 and GAPDH as an internal control. One hundred eight samples were analyzed by the full set of primers, eight samples by primers not including MDR1 and eight samples by primers not including MDR1, MLL-ENL and SIL-TAL1. Six patients were positive for E2A-PBX1, two for TEL-AML1, one for MLL-AF4 and one for MLL-ENL. ALL patients were negative for MLL-AF6 and MLL-AF9.
Table 2. Patient characteristics.
| Characteristics |
ALL202-U (n=139) |
ALL97a (n=104) |
P-value |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Sex | |||
| Male | 78 (56) | 58 (56) | |
| Female | 61 (44) | 46 (44) | 0.957 |
| Age | |||
| Median | 19 | 19 | |
| Age <20 | 83 (60) | 54 (52) | |
| Age ⩾20 | 56 (40) | 50 (48) | 0.226 |
| PS | |||
| 0–1 | 128 (92) | 93 (89) | |
| 2–4 | 11 (8) | 11 (11) | 0.474 |
| WBC count (/μl) | |||
| Median | 10 500 | 11 480 | |
| WBC <50 000 | 104 (75) | 79 (76) | |
| WBC ⩾50 000 | 35 (25) | 25 (24) | 0.838 |
| Serum LDH level | |||
| Normal | 20 (14) | 14 (13) | |
| Elevated | 119 (86) | 90 (87) | 0.415 |
| Phenotype | |||
| CD19+, CD10- | 18 (13) | 20 (19) | |
| CD10+ | 89 (64) | 69 (66) | |
| CD19−, CD7+ | 31 (22) | 14 (14) | 0.591b |
| Unknown | 1 (1) | 1 (1) | |
| Karyotypec | |||
| Standard risk | 2 (1) | 5 (5) | |
| Intermediate risk | 110 (79) | 74 (71) | |
| High risk | 11 (8) | 7 (7) | |
| Very high risk | 15 (11) | 7 (7) | 0.322b |
| Unknown | 1 (1) | 11 (10) | |
| Chimera mRNA | |||
| E2A-PBX1 | 6 (5) | ||
| SIL-TAL1 | 4 (3) | ||
| TEL-AML1 | 2 (2) | ||
| MLL-AF4 | 1 (1) | ||
| MLL-ENL | 1 (1) | ||
| CNS involvement | |||
| Negative | 128 (95) | 103 (99) | |
| Positive | 7 (5) | 1 (1) | 0.072 |
Abbreviations: CNS, central nervous system; LDH, lactic acid dehydrogenase; PS, performance status; WBC, white blood cell.
Ph-negative patients under 25 years were extracted.
Analyzed excluding unknown cases.
Modified MRC UKALLXII/ECOG E2993ALL cytogenetic subgroups.
Response to induction therapy
The results of therapy are summarized in Supplementary Table 2. A total of 130 (94% (95% CI 88–97%)) of 139 evaluated patients achieved CR: 124 after the first treatment and 6 after the second course. Four patients died of sepsis during the first induction therapy before their remission status could be ascertained, and these were the only deaths that occurred during induction therapy. Three patients failed to achieve CR after two courses of therapy. Two patients dropped out of the study without starting the second therapy, because the first therapy failed to achieve CR. These results were markedly better than ALL97-U. The CR rate was 84% (95% CI 75–90%) and 12 patients died during induction therapy in ALL97-U.
Survival
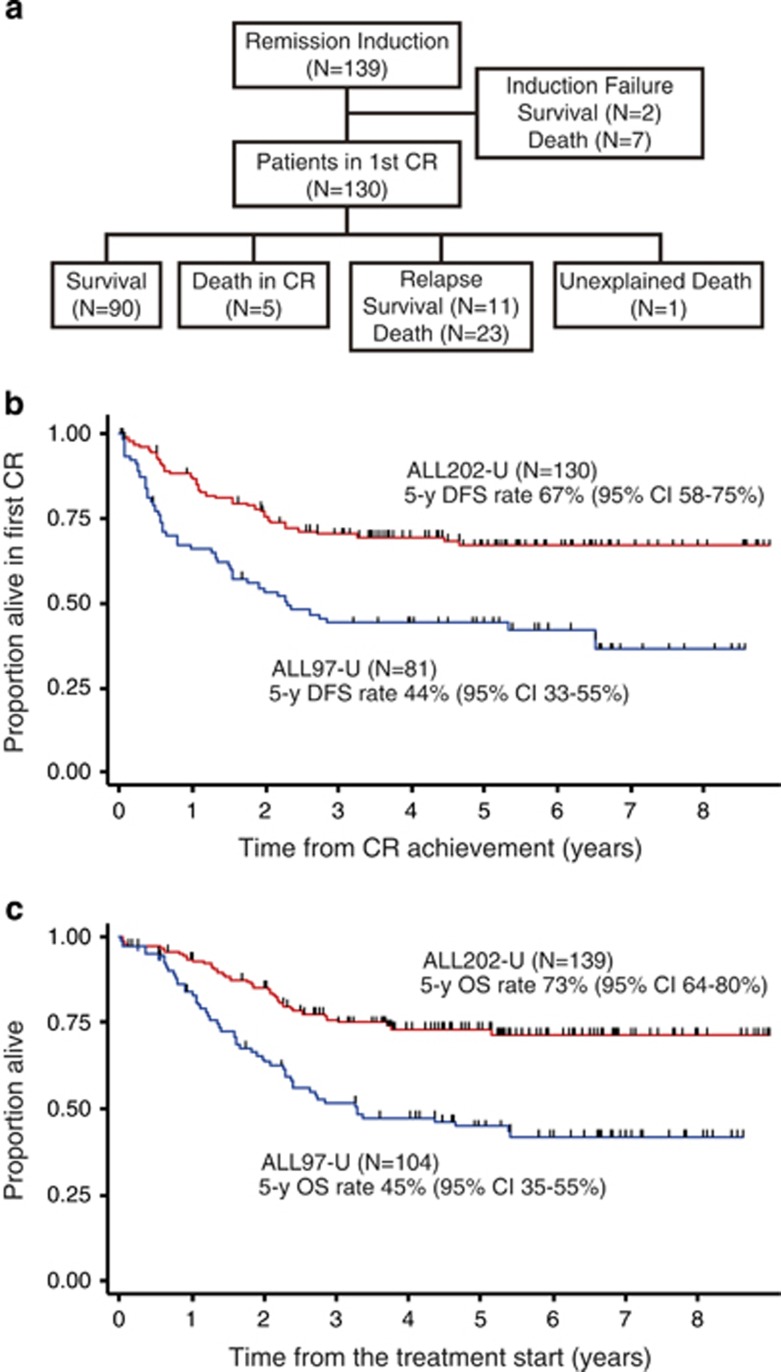
Nine out of 139 eligible patients did not achieve CR and 7 of them died. Of the 130 CR patients, 5 patients died in remission, 1 died for an unknown reason and 34 patients relapsed; 19 of them received SCT and 23 relapsed patients died. A total of 36 patients died (Figure 1a). The estimated 5-year DFS rate was 67% (95% CI 58–75%, Figure 1b) and the estimated probability of the OS rate at 5 years was 73% (95% CI 64–80% Figure 1c). Both the DFS rate and OS rate were markedly better than those of ALL97-U patients (44 and 45%, respectively; Figures 1b and c).
Figure 1.
Comparison of DFS and OS rates. (a) Patient flow chart. (b) Comparison of DFS rates between ALL202-U (red line) and ALL97-U (blue line). The median follow-up times were 5.1 and 5.2 years, respectively. (c) Comparison of OS rates between ALL202-U (red line) and ALL97-U (blue line). The median follow-up times were 5.1 and 5.8 years, respectively.
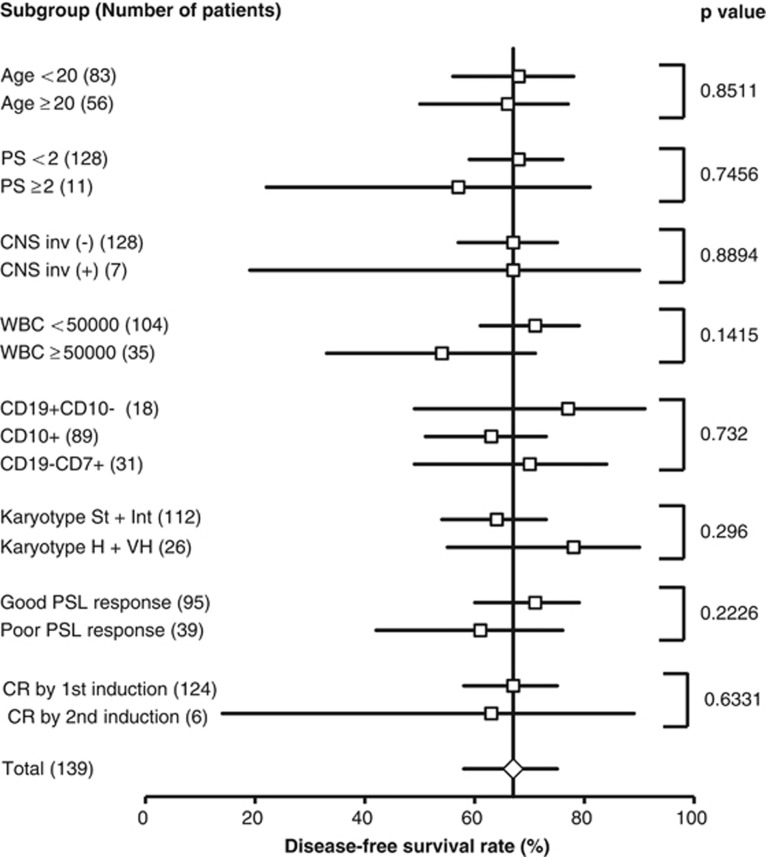
The results of univariate analysis on the effects of clinical and biological features on the DFS rate are summarized in Figure 2 as a forest plot. Age, performance status, CNS involvement, WBC counts, immunophenotype, cytogenetics, PSL response and CR achievement by the second induction therapy did not correlate with DFS.
Figure 2.
Forest plot of subgroup analysis for DFS rates. 5-year DFS rate of each subgroup was calculated and compared by the log-rank test. Patients undergoing transplantation were not censored. The 5-year DFS rate with 95% CIs are plotted and P-values of the log-rank test are shown. Numbers following subgroup names indicate the number of cases in the groups.
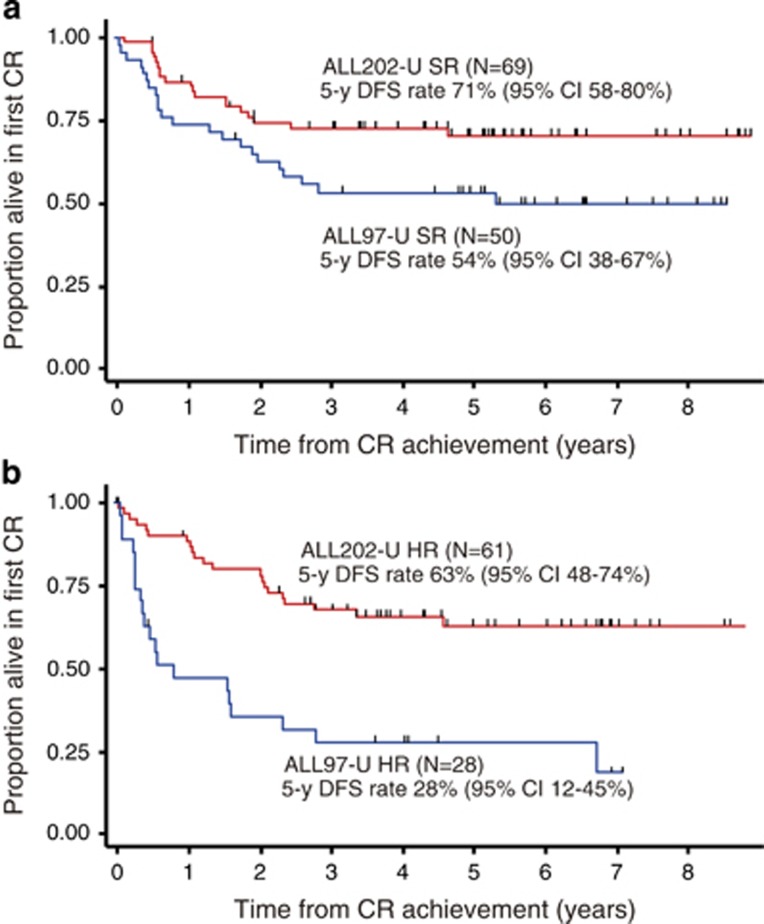
We stratified patients with widely accepted risk factors, WBC counts and karyotypes as described in the Patients and Methods section, and analyzed survival in each group. Sixty-nine and 61 patients in ALL202-U were classified into the standard-risk group and high-risk group, respectively, and 50 and 28 patients in ALL97-U were classified in a similar manner. The DFS rate of ALL202-U patients was markedly better than that of ALL97-U patients both in the standard-risk group (71% vs 54%) and high-risk group (63% vs 28% Figures 3a and b). As a result, no significant difference was observed in the DFS rate between the standard-risk and high-risk groups in ALL202-U (71% vs 63%, P=0.4291; compare red lines in Figures 3a and b), however, it was significant in ALL97-U (54% vs 28%, P=0.0053; compare blue lines in Figures 3a and b).
Figure 3.
Comparison of the DFS rate in each risk group. (a) Comparison between ALL202-U standard-risk (SR) patients (red line) and ALL97-U SR patients (blue line). (b) Comparison between ALL202-U high-risk (HR) patients (red line) and ALL97-U HR patients (blue line).
Some patient groups with possible poor prognostic factors, such as severe leukocytosis, pro-B and T-cell phenotypes, and poor PSL responses, contained more patients who received SCT in the first remission (Supplementary Table 3), which suggested that good survival outcome of ALL202-U was the result of the rescue of high-risk patients by SCT, however, no significant difference was observed in the DFS rate between patients that received SCT and those who did not, even in the high-risk group (Supplementary Figures 1A and B). These results suggested that the effect of the possible rescue of high-risk patients by SCT, if any, was not marked.
Toxicity
A full assessment of toxicity was performed in 1688 courses of chemotherapy (139 induction therapies, 126 consolidation therapies, 113 sanctuary therapies, 102 reinduction therapies, 98 reconsolidation therapies and 1110 maintenance therapies). Ninety-nine percent of patients developed grade 4 neutropenia during induction therapy, however, it was difficult to distinguish this from hematopoietic disorders by leukemia. The grade 3–4 adverse events observed during induction therapy were as follows: febrile neutropenia, sepsis and other infections occurred in 46.5%, 15% and 4.4% of patients, respectively. Elevated alanine aminotransferase levels, pancreatitis and ileus were observed in 27.8%, 6.6% and 3.6%, respectively. Eighteen (13.2%) and 10 (7.2%) patients developed disseminated intravascular coagulopathy and gastrointestinal bleeding, respectively. Hyperglycemia, neuropathy and tumor lysis syndrome occurred in 4.4%, 3.6% and 3.6%, respectively. Diarrhea, heart disease, creatinine elevations and brain bleeding were observed in <1% of patients. Severe adverse events such as neutropenia, thrombocytopenia, febrile neutropenia, sepsis, hepatic toxicity, pancreatitis and neuropathy occurred frequently during post-remission therapy. These have been summarized in Table 3. Toxicity was evaluated in the ALL97 study with the toxicity grading criteria of the Japan Clinical Oncology Group (JCOG). ALL202-U results were compared with those of ALL97-U in the points where the criteria coincided between NCI-CTC version 2 and the JCOG (Table 3). Sepsis, hepatic toxicity and neuropathy were more frequent in ALL202-U, although no patient died from the adverse events associated with chemotherapy during post-remission therapy in this study. In the pediatric study, JACLS ALL-02, patients in the high-risk group were treated with the ALL-02 HR protocol, which was the same as JALSG ALL202-U; 136 patients aged 10–18 years (90% patients were <15 years old) were treated with ALL-02 HR. Severe adverse events, except for pancreatitis, occurred more frequently in pediatric patients (Table 3).
Table 3. Comparison of adverse effect.
|
ALL202-U vs ALL97 | |||||
|---|---|---|---|---|---|
| Therapy | G4 neutropenia (%) | G3-G4 thrombocytopenia (%) | Sepsis (%) | G3-G4 hepatic toxicity (%) | G3-G4 neuropathy (%) |
| ALL202-U (age 15–24) | |||||
| Induction | 15 | 27.8 | 3.6 | ||
| Consolidation | 99.2 | 97.8 | 7.4 | 13.5 | 2.4 |
| Sancturary | 12 | 19.7 | 2.6 | 13.2 | 1.8 |
| Reinduction | 65 | 42.6 | 3.9 | 16.7 | 0 |
| Reconsolidation | 99 | 100 | 9.1 | 5.1 | 4 |
| ALL97 (age 15–24) | |||||
| Induction | 3.8 | 11.2 | 0 | ||
| C1 | 73.7 | 10.5 | 0 | 4.2 | 0 |
| C2 | 61.7 | 9.9 | 0 | 0 | 0 |
| C3 | 64.6 | 3.8 | 0 | 0 | 0 |
| C4 | 97.1 | 97.1 | 0 | 0 | 0 |
| C5 | 41.9 | 1.6 | 0 | 0 | 0 |
| C6 | 58.9 | 25 | 0 | 0 | 0 |
| C7 | 86.8 | 9.4 | 0 | 0 | 0 |
| C8 | 98 | 100 | 2.2 | 0 | 0 |
| AYA vs pediatrics | |||||
|---|---|---|---|---|---|
| Therapy | Febrile neutropenia (%) | G3-G4 pancreatitis (%) | G4 hepatic toxicity (%) | G3-G4 neuropathy (%) | |
| ALL202-U (age 15–24) | |||||
| Induction | 46.5 | 6.6 | 1.0 | 3.6 | |
| Consolidation | 44.4 | 0.0 | 0.0 | 2.4 | |
| Sancturary | 9.7 | 0.0 | 0.0 | 1.8 | |
| Reinduction | 25.5 | 5.8 | 1.0 | 0.0 | |
| Reconsolidation | 55.6 | 0.0 | 0.0 | 4.0 | |
| Maintenance | 0.8 | 0.3 | 0.3 | 0.2 | |
| ALL-02-HR (age 10–18) | |||||
| Induction | 63.9 | 5.0 | 3.4 | 9.3 | |
| Consolidation | 58.0 | 1.1 | 3.5 | 6.5 | |
| Sancturary | 37.8 | 0.0 | 0.0 | 13.3 | |
| Reinduction | 51.8 | 1.1 | 1.1 | 3.3 | |
| Reconsolidation | 73.7 | 0.0 | 0.0 | 0.0 | |
| Maintenance | 8.5 | 0.3 | 0.6 | 0.2 | |
Abbreviation: AYA, adolescent and young adult.
Protocol adherence
Therapies were delayed in many patients, and this was attributed to the adverse events associated with chemotherapy, however, some patients could proceed to the next therapy earlier than planned. The median delays from the planned schedule were 7 (range 0 to 171), 7 (range −9 to 35), 9 (range 0 to 36), 6 (range −8 to 70) and 19 (range −5 to 62) days in induction, consolidation, sanctuary, reinduction and reconsolidation therapy, respectively. As for the 57 patients who completed maintenance therapy, the median duration of maintenance therapy was 633 (range 553–881) days, which was 80 (range 0–328) days more than the planned schedule. No patients could complete the whole therapy without delays.
L-asparaginase dose reductions were required for 48 (35%), 18 (18%) and 38 (47%) patients because of its adverse events in induction, reinduction and maintenance therapy, respectively. Seventeen (30%) patients could complete the whole therapy without dose reductions in any drugs.
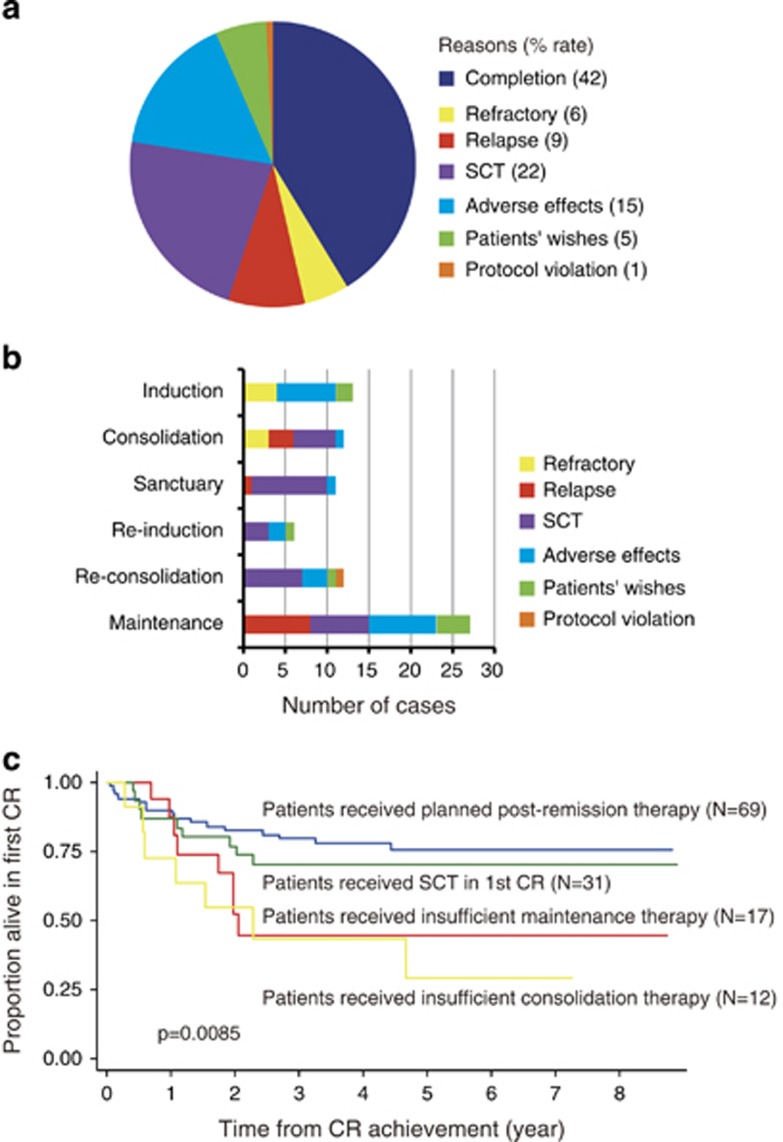
Fifty-seven (41%) patients could complete the whole therapy and 81 (59%) dropped out of the protocol therapy. The reasons, frequencies and periods of protocol therapy terminations have been summarized in Figures 4a and b. Seven (6%) patients were primary refractory, including early death, and 12 (9%) relapsed. Thirty-one (22%) patients dropped out of the study in the first remission to receive SCT. Twenty-two (16%) patients terminated protocol therapy because of severe adverse events. Eight (6%) patients dropped out of the study for their own reasons. One (1%) patient received the same maintenance therapy as ALL97 because of a doctor's mistake. This case was treated as a dropout because of a protocol violation. A significantly large number of patients dropped out after reconsolidation therapy for reasons other than relapse and SCT, and subsequently received no or insufficient maintenance therapy. In order to analyze the effects of insufficient maintenance therapy on survival, patients who achieved CR were divided into four groups: patients who did not drop out for reasons other than relapse (patients received planned post-remission therapy), those who dropped out because of SCT (patients received SCT in first CR) and those who dropped out for reasons other than relapse and SCT before and after the completion of drug administration in reconsolidation therapy (patients received insufficient consolidation therapy and patients received insufficient maintenance therapy, respectively). The estimated 5-year DFS rates of these groups were 76% (95% CI 63–84%), 70% (95% CI 50–83%), 29% (95% CI 5–59%) and 45% (95% CI 19–68%), respectively (Figure 4c). The DFS rate of patients who received insufficient maintenance therapy was compared with others using a proportional hazard model with time-varying covariates. The hazard ratio of insufficient maintenance therapy was 5.59 (95% CI 2.52–12.41, P<0.001) in univariate analysis and 5.60 (95% CI 2.36–13.26, P<0.001) in multivariate analysis (Table 4).
Figure 4.
Analysis of protocol therapy termination. (a) The reasons for and frequencies of protocol therapy termination. (b) The periods of and reasons for protocol therapy termination. (c) The effect of therapy insufficiency on the DFS rate. DFS rates were compared among groups of patients who received planned post-remission therapy (blue line), those who received SCT in first CR (green line), those who received insufficient consolidation therapy (yellow line) and those who received insufficient maintenance therapy (red line).
Table 4. Multivariate analysis of the effect of biological and clinical features on DFS.
| Parameters | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Insufficient maintenance therapy | 5.60 (2.36–13.26) | <0.001 |
| Age ⩾20 | 1.25 (0.62–2.52) | 0.531 |
| PS ⩾2 | 1.28 (0.42–3.91) | 0.662 |
| CNS involvement (+) | 0.93 (0.19–4.50) | 0.927 |
| WBC ⩾50000 | 1.63 (0.77–3.43) | 0.195 |
| Karyotype high + very high | 0.72 (0.27–1.92) | 0.516 |
| B-cell phenotype | 1.36 (0.58–3.21) | 0.484 |
| Poor PSL response | 1.52 (0.71–3.27) | 0.284 |
| CR by 2nd induction | 1.64 (0.34–7.98) | 0.538 |
| SCT in 1st remission | 1.01 (0.43–2.37) | 0.980 |
Abbreviations: CI, confidence interval; CNS, central nervous system; CR, complete remission; DFS, disease-free survival; PS, performance status; PSL, prednisolone; SCT, stem cell transplantation; WBC, white blood cell.
Discussion
The results of this prospective study indicate that the pediatric protocol, ALL202-U, enabled markedly better survival rates to be achieved by AYA with ALL than the conventional adult protocol, ALL97. The OS rate reported here was similar to those reported in previous retrospective studies: 78% (age 15–20),3 71% (age 15–17),4 79% (age 15–18)6 and 67% (age 16–20). 5 It was also similar to previous prospective studies by two pediatric groups (81% in those aged 15–18 years and 78% in those aged 16–21 years)7,8 and an adult group (6-year OS rate for standard-risk ALL patients: 69% in those aged 15–30 years).11 Patients who received the pediatric protocol treatment were mainly adolescents and >80% were 18 years and under in all retrospective studies and prospective studies by pediatric groups.7,8 Regardless of the prospective or retrospective design, comparisons with pediatric group studies could not conclude the efficacy of the pediatric protocol in young adults, however, the only study of an adult group included only standard-risk ALL patients (WBC count ⩽30 × 109/l, and absence of t(9;22), t(1;19), t(4;11) or any other 11q23 rearrangements).11 Ours is the only study on whole Ph-negative AYA ALL that used an unmodified pediatric protocol. The results obtained in our study demonstrated for the first time that a pediatric protocol was feasible and could also markedly improve survival in Ph-negative high-risk young adult ALL patients.
Concerning the key difference between pediatric protocols and adult protocols, pediatric protocols use more non-myelosuppressive drugs, such as glucocorticoids and L-asparaginase and fewer myelosuppressive drugs, such as anthracycline,5 which was applied to the comparison between ALL202-U and ALL97. Many differences existed between these two protocols, such as the cumulative doses and dose intensities of each drug, treatment durations and prophylaxis of CNS involvement; therefore, we cannot identify the key difference responsible for the different treatment outcomes by comparing the whole protocols. However, this comparison becomes simple by focusing on induction therapy. The treatment schedules were similar between the protocols, except that ALL202-U had three treatments with intrathecal injections and prephase therapy of PSL for 7 days. Therefore, marked differences were observed in the cumulative doses. The main difference noted was that ALL202-U used more L-asparaginase (48 000 vs 18 000 U/m2) and glucocorticoids (980 mg/m2 PSL and 70 mg/m2 dexamethasone vs 840 mg/m2 PSL) and less anthracycline (50 mg/m2 THP-adriamycin vs 135 mg/m2 daunorubicin). Owing to the upper limit of the dose (2 mg per body), the planned cumulative doses of vincristine were almost identical (8.0 vs 7.8 mg per body). Therefore, these differences have been implicated in the marked difference observed in the CR rate of the first induction therapy (89.2% vs 76.0%), which suggests that the increased doses of L-asparaginase and glucocorticoids partly contributed to the improved survival in the ALL202-U study.
The indication for allo-SCT in the first remission remains a controversial issue in ALL. SCT is currently recommended in Japan in the first remission for high-risk ALL, defined by WBC >30 × 109/l, a high-risk karyotype such as t(9;22), t(4;11), t(1;19), and +8, age ⩾30, or late CR achievement. Therefore, it was unavoidable that unignorable number of patients received allo-SCT, which made the interpretation of the results of this study difficult, however, SCT did not affect the DFS rate in multivariate analysis (hazard ratio 1.01; Table 3) and did not improve the DFS rate of ALL202-U patients, even in the high-risk group (P=0.9394; Supplementary Figures 1A and B). Therefore, the superiority of ALL202-U to historical control was not impaired. Based on the good outcomes observed in this study, SCT will no longer be recommended in the first remission for this type of high-risk ALL of AYA if patients are treated by this protocol. The indication for SCT in the first remission for ALL of AYA should be similar to that for pediatric patients. Children with Ph-negative ALL in Japan are recommended to receive SCT in the first remission if they are positive for the chromosome 11q23 abnormality, show a poor PSL response, or achieve CR later than 6 weeks from the treatment start.
A poor PSL response was previously shown to be a stronger prognostic factor than age and the WBC count in pediatric B-ALL.17,18 The JACLS ALL-02 study and other pediatric studies used the PSL response for risk stratification. In our study, a poor PSL response did not significantly worsen the prognosis of patients (Figure 2). Patients with a poor PSL response received SCT during the first remission more frequently than those with a good PSL response (33% vs 22% Supplementary Table 3), and 22% of patients had T-ALL in this study (Table 2). These results should be considered, however, a poor PSL response was not a significant risk factor in multivariate analysis (Table 4). The prognostic impact of the PSL response should be investigated in a larger number of patients. Considering the rarity of ALL in AYA, this should be examined in patients with a wider age range. Among other patient characteristics and therapy responses, the risks of late CR achievement and presence of t(4;11), other 11q23 rearrangements and t(1;19) could not be determined in this study because of the small number of patients (6, 1, 1 and 6, respectively) and high frequency of receiving SCT (50%, 100%, 0% and 67%, respectively).
The toxicity of the ALL202-U protocol appeared to be high because severe adverse events occurred more frequently in this study, however, the death rate during induction therapy was lower in this study than ALL97 (3% vs 12% Supplementary Table 2), and this may have been because patients achieved CR more frequently and quickly in this study. In addition, no chemotherapy-related deaths were observed during post-remission therapy in this study. These results indicate the tolerability of this protocol by AYA. Children treated with the same protocol in the JACLS ALL-02 study exhibited severe adverse events more frequently than AYA in this study. Our results indicated that AYA tolerated chemotherapy better than children, except for L-asparaginase-induced pancreatitis. These results suggested that ALL202-U was feasible as a treatment for AYA with BCR–ABL-negative ALL.
Adherence to the protocol was not good in this study, and this was mainly due to the high toxicity of this treatment. Protocol therapy was frequently terminated because of adverse events and the patients' wishes. Such therapy terminations were the most frequent during maintenance therapy (Figure 4b), although the frequency of severe adverse events was markedly less during maintenance therapy than all other post-remission therapies (Table 3). This result suggested maintenance therapy may have been terminated because of less severe adverse events. Another reason is the difficulty in maintaining motivation for therapy in AYA against their psychosocial conditions. Our results clearly showed the significant importance of completing maintenance therapy. This information will help to maintain motivation for therapy, and may lead to further improvements in the outcomes of patients.
Taken together, ALL202-U caused high, but acceptable toxicity and led to a markedly better outcome than the previous study and is thought to be a feasible and highly effective treatment for AYA with BCR–ABL-negative ALL, including high-risk cases.
Acknowledgments
We thank Masayuki Towatari MD, PhD, Itsuro Jinnai MD, PhD, Daisuke Imanishi MD, PhD and all physicians and staff at the participating centers. We also thank Manami Kira and Yuko Makino for their secretarial assistance. In addition to the authors, the investigators listed in the Appendix are acknowledged for contributing to this trial. This work was supported in part by MHLW KAKENHI (grant numbers: H23-Ganrinsho-Ippan-004, H19-Seibutsushigen-Ippan-011 and H17-Ganrinsho-Ippan-002), the National Cancer Center Research and Development Fund (23-A-23) and a grant from the Nonprofit Organization for Support Japan Adult Leukemia Study Group (NPO-JALSG).
Consultancy: NU (Pfizer) and TN (Pfizer). Honoraria: OS (Kyowahakko-Kirin); HH (Kyowahakko-Kirin, Nippon Shinyaku); YA (Kyowahakko-Kirin, Shionogi); NU (Kyowahakko-Kirin, Nippon Shinyaku, Shionogi, Pfizer); YM (Kyowahakko-Kirin, Nippon Shinyaku, Pfizer); YK (Nippon Shinyaku); TN (Kyowahakko-Kirin, Nippon Shinyaku, Shionogi). Research funding: JM (Kyowahakko-Kirin, Shionogi, Pfizer); NU (Kyowahakko-Kirin, Nippon Shinyaku, Shionogi, Pfizer, Meiji Seika Pharma); HK (Bristol-Myers Squibb, Chugai Pharmaceutical, Kyowahakko-Kirin, Dainippon Sumitomo Pharma, Zenyaku Kogyo and FUJIFILM Corporation); YM (Kyowahakko-Kirin, Pfizer); YK (Pfizer); TN (Kyowahakko-Kirin).
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990-2004. J Natl Cancer Inst. 2008;100:1301–1309. doi: 10.1093/jnci/djn276. [DOI] [PubMed] [Google Scholar]
- Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980 s to the early 21st century. Blood. 2009;113:1408–1411. doi: 10.1182/blood-2008-06-164863. [DOI] [PubMed] [Google Scholar]
- Boissel N, Auclerc MF, Lheritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48:254–261. doi: 10.1002/pbc.20749. [DOI] [PubMed] [Google Scholar]
- Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bont JM, Holt B, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–2035. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- Barry E, DeAngelo DJ, Neuberg D, Stevenson K, Loh ML, Asselin BL, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25:813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- Nachman JB, La MK, Hunger SP, Heerema NA, Gaynon PS, Hastings C, et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children's oncology group. J Clin Oncol. 2009;27:5189–5194. doi: 10.1200/JCO.2008.20.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- Storring JM, Minden MD, Kao S, Gupta V, Schuh AC, Schimmer AD, et al. Treatment of adults with BCR-ABL negative acute lymphoblastic leukaemia with a modified paediatric regimen. Br J Haematol. 2009;146:76–85. doi: 10.1111/j.1365-2141.2009.07712.x. [DOI] [PubMed] [Google Scholar]
- Ribera JM, Oriol A, Sanz MA, Tormo M, Fernandez-Abellan P, del Potro E, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Espanol de Tratamiento en Hematologia pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843–1849. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]
- Towatari M, Yanada M, Usui N, Takeuchi J, Sugiura I, Takeuchi M, et al. Combination of intensive chemotherapy and imatinib can rapidly induce high-quality complete remission for a majority of patients with newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia. Blood. 2004;104:3507–3512. doi: 10.1182/blood-2004-04-1389. [DOI] [PubMed] [Google Scholar]
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Jinnai I, Sakura T, Tsuzuki M, Maeda Y, Usui N, Kato M, et al. Intensified consolidation therapy with dose-escalated doxorubicin did not improve the prognosis of adults with acute lymphoblastic leukemia: the JALSG-ALL97 study. Int J Hematol. 2010;92:490–502. doi: 10.1007/s12185-010-0672-z. [DOI] [PubMed] [Google Scholar]
- Hasegawa D, Hara J, Suenobu S, Takahashi Y, Sato A, Suzuki N, et al. Successful Abolition of prophylactic cranial irradiation in children with non-T acute lymphoblastic leukemia (ALL) in the Japan Association of Childhood Leukemia Study (JACLS) ALL-02 Trial. ASH Annual Meeting Abstracts. 2011;118:1506. [Google Scholar]
- Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood. 2008;111:2563–2572. doi: 10.1182/blood-2007-10-116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrappe M, Reiter A, Ludwig WD, Harbott J, Zimmermann M, Hiddemann W, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. J Clin Oncol. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.