Abstract
Introduction
Obesity is a chronic disease and a major global health challenge. Apart from bariatric surgery, which is costly and not without risk, there are currently no successful long-term treatment options for obesity. The history of pharmacological agents for obesity has been turbulent with many examples of drugs being removed from the market due to significant side effects. Orlistat and sibutramine (the latest drugs on the market) provide only modest weight loss and are both associated with high attrition rates due to intolerable side effects. Furthermore, sibutramine was recently withdrawn from the market. There is a need for the development of safe and efficacious drug treatments for obesity.
Areas covered
The history of leptin therapy as an orphan drug, leptin-replacement therapy as a treatment for obesity, preclinical studies showing the efficacy of leptin/amylin combination and finally, the very promising early clinical findings using pramlintide/meteleptin combination therapy in overweight to obese individuals.
Expert opinion
Combination pharmacological therapy, such as pramlintide/ metreleptin, for the treatment of obesity is very promising and is supported by encouraging weight loss results and improvement in metabolic makers in early-phase clinical studies. However the latest randomized clinical trial on pramlintide/metreleptin was recently stopped due to safety concerns.
Keywords: amylin, leptin, metreleptin, obesity, pramlitide
1. Introduction
In the pharmacological development of new drugs for the treatment of obesity, the safety of the product is paramount and must be considered by weighing up the risks to the individual from obesity versus possible problems that may occur with drug treatment. Unfortunately, the history of the pharmacological treatment of obesity has been turbulent largely due to safety concerns [1,2]. Obesity is a chronic disease that has many causes, all leading to a persistent imbalance between energy intake and energy expenditure. The resulting excess adipose tissue has been linked to higher risk of developing many diseases including type 2 diabetes and cardiovascular disease, some types of cancers, disabilities, pulmonary and gastrointestinal tract complications as well as depression [3]. As expected from such a complex disease resulting from an interaction between the environment and a myriad of susceptibility genes, cure is rare and treatment is thus aimed at palliation. Whatever the primary site of action for a pharmacological agent may be, the net effect must be a reduction in food intake and/or an increase in energy expenditure.
Single agents have been very deceiving in general and physicians are now left with almost no long-term pharmacological tools to treat the disease. However, since the popular success of Fen-Phen, a combination of fenfluramine and phentermine, in the 1980s [4] it became clear that a combination of drugs would be necessary to treat obesity successfully despite the fact that fenfluramine was withdrawn from the market in 1996 because of fatal pulmonary hypertension. Given the poor long-term success with dietary interventions and the modest efficacy and lingering safety concerns associated with anorectic small-molecules, there is an urgent need for new approaches that translate scientific advances into innovative therapies which offer durable, clinically meaningful weight loss with minimal side effects. Such goals have lead many pharmaceutical and biotech companies to initiate randomized double-blind clinical trials using combinations of existing drugs for the treatment of obesity. The potential use of a rational and promising combinatorial neurohormonal approach using peptide hormones which have physiological roles in the regulation of food intake and body weight, and potentially devoid of off-target toxicities was recently proposed.
Leptin, a neurohormone that is predominantly secreted by adipocytes and primarily binds to receptors in the hypothalamus, plays a key role in regulating long-term energy homeostasis in rodents and humans. Leptin receptors are also widely expressed throughout the body with extrahypothalamic effects on skeletal muscle, adipose tissue, the liver and pancreas [5-7] and leptin itself also plays an essential role in the initiation of puberty. Amylin, a neuroendocrine peptide hormone that is co-secreted with insulin from pancreatic β-cells and binds to receptors in the hindbrain, contributes to short-term energy regulation [8]. In a comprehensive series of preclinical studies, scientists at Amylin Pharmaceuticals showed that combination treatment with amylin and leptin leads to marked, synergistic reductions in food intake (up to 45%) and body weight (up to 15%) in otherwise leptin-resistant DIO rats [9,10]. In this drug evaluation, we review the use of leptin as an orphan drug, its use in a randomized clinical trial for obesity treatment, the concept of leptin replacement, preclinical studies on leptin/amylin combination and recent clinical studies on this combination. Despite very promising initial data, the development of pramlin-tide/metreleptin combination therapy has been recently stopped due to potential safety concerns (Box 1).
Box 1. Drug Summary.
| Drug name | Pramlintide acetate | Metreleptin (Recombinant-methionyl human leptin) |
| Phase | FDA approved, March 2005 | In Phase III |
| Indication | Type 1 diabetes: Maintenance dose of 30 or 60
(as tolerated) titrated from a dose of 15 mg Insulin-Using Type 2 diabetes: Maintenance dose of 120 μg (as tolerated) titrated from a dose of 60 |
Congenital leptin deficiency, lipodystrophy, hypothalamic amenorrhea and leptin replacement therapy for weight maintenance |
| Pharmacology description | In clinical studies in patients with
insulin-using type 2 and type 1 diabetes, pramlintide administration
resulted in a reduction in mean postprandial glucose concentrations,
reduced glucose fluctuations, and reduced food intake Peak: 15 min [50] Half-life: 20 - 50 min [50,51] |
Based on results of nonclinical and clinical
studies, the mechanisms of action of metreleptin include the
following: • Correction of hyperphagia secondary to leptin deficiency and the concomitant reduction in caloric and fat intake [52,53] • Stimulation of fatty acid oxidation throughout the body and lowering of plasma, hepatic and myocellular lipid levels resulting in increased insulin sensitivity and improved glycemic control [54-61] • Improvement in insulin suppression of glucose production in the liver and increase in insulin-stimulated peripheral glucose uptake in the muscle [20,54,62] Therefore, leptin acts via multiple mechanisms to decrease triglyceride and other lipid intermediates in lipodystrophy patients, reducing their accumulation in tissues such as liver and muscle, and ameliorating severe insulin resistance, thereby improving hyperglycemia and hypertriglyceridemia Peak: 4 h [63] Half-life: 2 - 5h [63,64] |
| Route of administration | Administered subcutaneously into thigh or abdomen separate from site of insulin injection immediately before major meals (≥ 250 kcal or ≥ 30 g carbohydrate) | Administered subcutaneously into the abdomen |
| Chemical structure | Lys-Cys-Asn-Thr-Ala-Thr-Cys-Ala-Thr-Gln- Arg-Leu-Ala-Asn-Phe-Leu-Val-His-Ser-Ser- Asn-Asn-Phe-Gly-Pro-Ile-Leu-Pro-Pro-Thr- Asn-Val-Gly-Ser-Asn-Thr-Tyr-NH2 acetate (salt) with a disulfide bridge between the two Cys residues [65] |
NH2-Met-Val-Pro-Ile-Gln-Lys-Val-Gln-Asp-Asp-Thr-Lys- Thr-Leu-Ile-Lys-Thr-Ile-Val-Thr-Arg-Ile-Asn-Asp-Ile-Ser- His-Thr-Gln-Ser-Val-Ser-Ser-Lys-Gln-Lys-Val-Thr-Gly-Leu- Asp-Phe-Ile-Pro-Gly-Leu-His-Pro-Ile-Leu-Thr-Leu-Ser-Lys- Met-Asp-Gln-Thr-Leu-Ala-Val-Tyr-Gln-Gln-Ile-Leu-Thr- Ser-Met-Pro-Ser-Arg-Asn-Val-Ile-Gln-Ile-Ser-Asn-Asp- Leu-Glu-Asn-Leu-Arg-Asp-Leu-Leu-His-Val-Leu-Ala-Phe- Ser-Lys-Ser-Cys-His-Leu-Pro-Trp-Ala-Ser-Gly-Leu-Glu- Thr-Leu-Asp-Ser-Leu-Gly-Gly-Val-Leu-Glu-Ala-Ser-Gly- Tyr-Ser-Thr-Glu-Val-Val-Ala-Leu-Ser-Arg-Leu-Gln-Gly- Ser-Leu-Gln-Asp-Met-Leu-Trp-Gln-Leu-Asp-Leu-Ser-Pro- Gly-Cys-COOH |
| Pivotal trial(s) | Type 1 diabetes: [66-68] Type 2 diabetes: [69-71] |
Congenital leptin deficiency [18] Lipodystrophy: [20] Hypothalamic amenorrhea: [22] Obesity: [26,48] Weight maintenance: [38] |
2. Summary of pramlintide and metreleptin
Pramlintide acetate, a synthetic analog of amylin, is administered by subcutaneous injection before major meals to lower postprandial glucose excursions in type 1 and 2 diabetics. Amylin is a 37-amino-acid peptide hormone that is co-secreted with insulin by pancreatic β-cells. Similarly to insulin, plasma amylin concentrations rise rapidly in response to meals, peaking approximately 30 min a after meal and returning to baseline after approximately 2 h [11]. Fasting amylin levels in healthy individuals have been reported in the range of 3 -- 25 pmol/l [12]. The amylin response to meal intake is absent in type 1 diabetes mellitus (T1DM), exaggerated in obesity and diminished in type 2 diabetes mellitus (T2DM).
Human amylin is generally insoluble and has a tendency to aggregate, precluding the use of its native peptide therapeutically. To combat this, a soluble, non-aggregating, equipotent analog of human amylin, pramlintide was developed and approved by the FDA in 2005. Pramlintide is provided as an acetate salt of the synthetic 37-amino acid polypeptide, which differs in amino acid sequence from human amylin by replacement with proline at positions 25 (alanine), 28 (serine) and 29 (serine) and has a molecular mass of 3949.4 Da. Pharmokinetic studies have shown that pramlintide doses of 30 and 60 μg in patients with type 1 diabetes and 120 μg in patients with type 2 diabetes produce plasma pramlintide concentrations that approximate physiological amylin concentrations in healthy subjects. The most common side effects in pramlintide users relate to gastrointestinal events including vomiting, nausea and abdominal pain. Also, pramlintide as an adjunct treatment in patients who use meal-time insulin therapy, and co-administration of pramlintide with insulin may increase the risk of insulin-induced hypoglycemia, particularly in patients with type 1 diabetes [13]. The pharmokinetics and pharmodynamics of pramlintide have been well described in previously published reviews [14,15].
Metreleptin also known as recombinant methionyl human leptin is an analog of human hormone leptin. To express metreleptin in Escherichia coli, the sequence encoding the mature protein of human leptin (146 amino acids) was chemically synthesized utilizing optimal E. coli codons. As part of this synthesis, the nucleotides ATG (encoding methionine) were added to the 5’ end of the gene for human leptin. Therefore, the metreleptin protein encoded by this sequence is 147 amino acids in length beginning with methionine, and has a calculated molecular mass of 16,156 Da. Metreleptin treatment has largely been administered to patients with congenital leptin deficiency, lipodystrophy and hypothalamic amenorrhea.
3. Leptin as an orphan drug in humans
The first evidence that pointed to the potential use of leptin replacement therapy for obesity came in 1997 when O'Rahilly and colleagues found two severely obese children who carried a mutation in the leptin gene [16]. These two children were cousins within a highly consanguineous family of Pakistani origin who both had a homozygous frame-shift mutation involving the deletion of a single guanine nucleotide in codon 133 of the leptin gene. This resulted in very low circulating leptin levels, extreme hyperphagia and severe obesity [16]. These researchers went on to show that daily subcutaneous injections of recombinant human leptin for up to 4 years could ameliorate hyperphagia, excessive weight gain in early life and the severe obesity in these children [17]. In a case report study, daily subcutaneous recombinant leptin(0.028mg/kg) injectionswereadministeredfor 12months in a 9 year old girl with congenital leptin deficiency. At baseline, the patient weighed 94.4 kg (> 99.9th percentile for age), height was at the 91st percentile (when adjusted for bone age) and serum leptin levels were below the detection limit. After leptin treatment, the patient lost 16.4 kg (~ 78th percentile for age) with 95% of the weight loss estimated to be fat mass. Energy expenditure using whole-body indirect calorimetry was assessed at baseline and 6 and 12 months after the initiation of leptin treatment. After 6 months of leptin treatment, her total energy expenditure had decreased by 10%, but by 12 months it had returned to near baseline values. As such, the leptin-associated weight loss was largely attributed to a substantial decrease in energy intake, with the patient consuming 42% less calories (compared with baseline) during her first test meal after the initiation of leptin treatment. Serum leptin levels reached levels ranging between 25 and 40 ng/ml during a 24 h treatment period [18]. In congenital leptin-deficient subjects, losing weight in response to leptin treatment also resulted in large weight loss but in a metabolic rate similar to that predicted for the new weight and body composition [19]. This was in contrast to control obese individuals undergoing a similar weight loss by a low calorie diet who experience ‘metabolic adaptation’, a decrease in metabolic rate beyond that expected on the basis of the decreases in fat-free mass and fat mass. Although the sample size was small (n = 3), results from this study suggest that leptin may act by increasing metabolic rate [19].
Leptin replacement therapy has also proven effective in lipo-dystrophy, a disease state in which animals and humans have little white fat and develop severe diabetes, accompanied by very fatty liver, high plasma lipid levels and profound insulin resistance. In a study that treated nine congenital lipodystrophic patients with daily subcutaneous leptin (up to 0.04 mg/kg) for 4 months, serum leptin levels increased 12-fold from baseline, triglyceride levels decreased by 60% and liver volume by 28%, leading to a discontinuation or a large reduction in diabetes medication. Self reported daily caloric intake decreased significantly with therapy [20]. In another study of 10 subjects with general or Dunnigans's partial lipodistrophy, leptin replacement therapy resulted in significant reductions in steatosis and the hepatocellular ballooning injury characteristic of nonalcoholic steatohepatitis [21]. In addition, liver volume and fat assessed by MRI was significantly decreased and there were significant reductions in serum triglycerides, glucose, insulin and liver enzymes, aspartate aminotransferase and alanine aminotransferase. Importantly, in the context of congenital leptin deficiency and lipodystrophy, leptin replacement therapy has been shown to be well-tolerated with no serious adverse events [18].
Leptin replacement therapy has also proven to be a safe and effective therapy for the treatment of hypothalamic amenorrhea (HA), a disorder characterized by the cessation of menstrual cycles usually caused by chronic energy deficiency secondary to strenuous exercise and/or reduced food intake such as in patients with anorexia nervosa [22,23]. Women with HA tend to be hypoleptinemic [24,25] with a proof-of-concept study showing that 3 months of leptin replacement therapy not only normalized levels of estrogen, thyroid hormones and IGF-1 but most importantly, restored ovulatory menstruation [22]. A subsequent randomized, double-blinded placebo-controlled trial of 36 weeks of human recombinant leptin (metreleptin) replacement therapy in women with HA found similar improvements in gonadal, thyroid, growth hormone and adrenal axes but also demonstrated improvement in markers of bone metabolism, suggestive of bone formation [23].
4. Use of leptin in obesity clinical trials
In contrast, the promise of leptin as a stand-alone magic bullet for the treatment of obesity was short lived. In 1999, a randomized, controlled dose-response trial of daily subcutaneous recombinant leptin injection was performed in 54 lean and 73 obese subjects. In the initial phase of the study lasting 4 weeks, lean and obese subjects lost similar amounts of weight with leptin treatment which was statistically significant compared with baseline (p = 0.02) [26]. Obese subjects were studied for a further 20 weeks. Of the 47 patients who completed the study, the 8 receiving the highest dose of leptin lost 7.1 kg (p = 0.01 compared to baseline) while those receiving placebo lost 1.3 kg. The effects varied widely among patients, from a loss of about 15 kg to a gain of 5 kg in the group treated with the highest dose. Moreover, these doses induced skin irritation and swelling at the injection site in 62% of patients and headache in half the patients. The hope that leptin therapy would be the cure for obesity disappeared after this trial despite numerous attempts by Amgen Pharmaceuticals to pursue further studies on the hormone that many investigators thought would be a panacea for obesity treatment.
5. Leptin replacement strategy for obesity therapy
The maintenance of reduced body weight is accompanied by a metabolic response which aims to defend against further weight loss. This response is characterized by a drop in energy expenditure beyond that predicted by changes in body composition [27-29], blunted neuroendocrine functions (the thyroid and reproductive axes are suppressed, there is decreased sympathetic activity and increased parasympathetic activity) and circulating leptin levels are increased. This metabolic adaptation to caloric restriction explains, at least in part, the regain of lost body weight over time and appears to be related to the drop in leptin which occurs with weight loss [30-32]. It was proposed that rather than being a satiety signal, leptin's primary physiological role is to defend body fat stores in the face of prolonged energy deficit [33-35].
Consistent with this hypothesis, leptin replacement reverses the metabolic adaptation observed in calorie-restricted rodents [36,37] and humans [29,38]. After subjects achieved and maintained a 10% reduction in body weight by a very low calorie diet, total energy expenditure, thyroid function and sympathetic activity were significantly reduced. After five weeks of daily recombinant leptin treatment, subjects not only restored their baseline leptin levels, but also reversed this metabolic phenotype and returned to baseline values [38,39]. Moreover, leptin administration resulted in further weight reduction [39]. How ever, in a more recent study, treatment with leptin showed less pronounced effects [40]. In this randomized double-blind clinical trial, metreleptin or placebo was administered to overweight to obese subjects undergoing a moderate calorie restriction (500 kcal/day). The average weight loss achieved over the six months of moderate caloric restriction was 8.2 and 9.2% in the placebo and metrelepin groups, respectively, and both groups exhibited a significant decrease in thyroid function, namely decreased triiodothyronine and free thyroxine [40]. Significantly, there were no differences between the placebo and metreleptin-treated groups in neuroendocrine function or body composition. The authors consequently suggested that leptin replacement would only be effective when circulating leptin was low and thus indicate severe energy deprivation. Alternatively, it could be suggested that leptin deficiency induced by a moderate to severe energy restriction maintained over a certain period of time would restore central leptin sensitivity, which would then be permissive for exogenous leptin biological action.
Exogenous leptin-induced restoration of the reproductive, thyroid and adrenal axis along with an increase in markers of bone formation in women with secondary amenorrhea and hypoleptinemia (i.e., induced by chronic energy deficiency) would support the hypothesis of central leptin sensitivity restoration [22,23]. In agreement with this, metreleptin only reversed the metabolic phenotype of calorie-restricted individuals when circulating leptin was significantly decreased by 30% and maintained over several weeks [38,39], and not in individuals in whom circulating leptin was increased due to moderate caloric restriction [40]. If such a hypothesis was true, the use of metreleptin in weight management therapy would be effective only when used as an adjunct to other leptin-sensitizing treatments, thus preventing metabolic adaptation induced by caloric restriction and promoting weight maintenance.
6. Preclinical studies on leptin/ amylin combination in rodents
As described above, obesity-related leptin resistance appears to be caused by defects in leptin action in the hypothalamus [41,42].
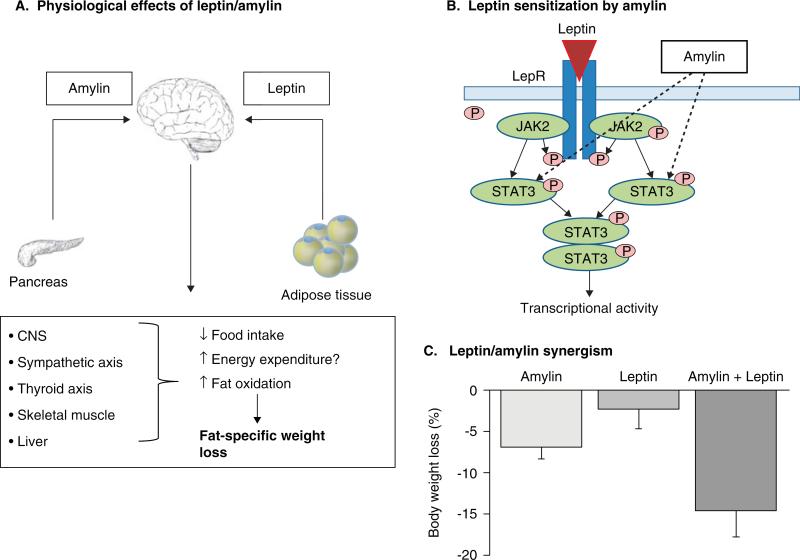
As such, combinations of leptin and pharmacological molecules able to improve or even restore central leptin sensitivity are of huge interest as pharmacological treatments for weight loss. Amylin, a peptide hormone co-secreted with insulin by pancreatic beta cells acts as a short-term satiety signal and activates multiple CNS regions to regulate both glucose and energy homeostasis. Preclinical studies of leptin/amylin combination therapy have shown promising results, largely acting by reducing food intake and increasing central leptin sensitivity (Figure 1A, B) [9,43]. Using a dose escalation response surface methodology analysis, Trevaskis et al. demonstrated in diet-induced obese mice that weight loss from the combination of leptin and amylin treatment was numerically greater than the addition of their single effect, that is, a synergism between leptin and amylin (Figure 1C). Moreover, this weight was achieved with relatively low doses of leptin [10] and prevented the metabolic phenotype characterized by a decrease in metabolic rate and bradycardia observed in pair-fed animals [10,44]. Low respiratory exchange ratios, indicative of increased fat oxidation, were observed during the weight-loss phase. Consequently, the weight loss induced under amylin--leptin treatment is fat-specific, and fat-free mass is preserved [9,44-46]. Amylin pretreatment in obese rats partially estores hypothalamic leptin signaling by increasing phosphorylation of signal transducer and activator of transcription 3 (pSTAT3). Such leptin--amylin synergy is further highlighted by diminished hypothalamic leptin-stimulated pSTAT3 signaling and reduced sensitivity to leptin-induced weight loss in amylin knockout mice [47].
Figure 1. Schematic view of the mechanism of amylin/leptin combination therapy leading to fat-specific weight loss as demonstrated in rodents.
The proposed physiological effect of amylin/leptin combination therapy is that amylin, a neuroendocrine pancreatic peptide that contributes to short-term energy regulation and leptin, a well established hormone that contributes to long-term energy regulation act synergistically in the CNS by decreasing food intake and increasing energy expenditure (A). In the arcuate nucleus, amylin increases the phosphorylation of signal transducer and activator of transcription 3 (STAT3) of the leptin signaling pathway resulting in an increase or restoration of central leptin sensitivity [9,47] (B) and therefore synergistic effects on weight loss, compared with either amylin or leptin administered alone [10] (C).
LepR: Leptin receptor.
Amylin/leptin combination therapy may also represent a strategy for weight loss maintenance when the body's biology resists further weight loss. Amylin and leptin combination therapy prevents weight regain in previously obese animals in which body weight was decreased by amylin-leptin combination treatment. This is in contrast to animals receiving leptin or amylin alone, which regained part or all the loss in body weight [46]. Additionally, amylin--leptin-treated animals exhibited an improved metabolic profile with decreased plasma insulin, total cholesterol and triglycerides, paralleled by favorable changes in liver glucose and lipid metabolism and white adipose tissue fatty acid trafficking [46]. Whereas part of such metabolic improvements could be ascribed to caloric restriction, Trevaskis et al. reported that compared with pair-fed animals, amylin--leptin-treated rats had decreased levels of stearoyl-coenzyme A desaturase-1 and fatty acid syntase and increased phosphoenolpyruvate carboxykinase (PEPCK), suggesting a direct effect of either or both amylin and leptin in improving energy substrate metabolism in peripheral tissues [10]. In addition, increased leptin receptor expression in the liver and white adipose tissue of rats treated with leptin and amylin was observed [47], indicating increased peripheral bioactivity of leptin with the leptin--amylin combination. In contrast to the CNS where leptin and amylin acts synergistically, the leptin--amylin combination appears to act in an additive manner in peripheral leptin-sensitive tissues such as preadipocytes and blood mononuclear cells in vitro and adipose tissue ex vivo [48]. In these tissues, multiple cellular pathways such as the ras-related C3 botulinum toxin substrate-alpha serine/threonine-protein kinase (AKT), AMPK and extracellular signal-regulated kinase (ERK) pathways were induced by either or both of the molecules, suggesting some of the mechanisms by which beneficial effects of the leptin--amylin combination are induced in peripheral tissues [48]. To date, studies examining the mechanism behind the synergistic effects of amylin and leptin implicate distributed hypothalamic and hindbrain sites in modulating restoration and/or augmentation of leptin responsiveness, which ultimately results in weight loss [43]. Trevaskis et al. suggests that amylin seems to enhance leptin signaling rather than vice versa with the underlying mechanisms of synergy being a result of temporal process. For example, several days of treatment were required before the weight loss curves of amylin- and amylin+leptin-treated DIO leptin resistant rats diverged [9]. It may be that amylin first alleviates leptin resistance at the level of the ventral medial hypothalamus followed by the hypothalamic arcuate nucleus, ultimately cascading to other areas of the brain with further exposure [43]. Clearly, further preclinical studies into the mechanisms and neural pathways activated by amylin-leptin are required.
7. Clinical studies on leptin--amylin combination in humans
The preclinical findings using leptin--amylin combination therapy were subsequently confirmed in a Phase II clinical study of 177 overweight to obese subjects undergoing caloric restriction (40%) concomitant with pramlintide (i.e., an amylin analog) treatment for 4 weeks before being randomized to either 20 weeks of treatment with metreleptin, pramlintide or a combination of both drugs [49]. Combination treatment with pramlintide/ metreleptin led to significantly greater weight loss from enrolment to week 20 (−13%) than treatment with pramlintide (−8.4%; p < 0.001) or metreleptin (−8.2%; p < 0.01) alone. There were trends towards improvements in fasting triglycerides, total cholesterol, low-density lipoprotein cholesterol, glycemia, insulinemia and insulin resistance. Common adverse events with these two molecules (injection site events, nausea) were mostly mild to moderate and decreased over time [49]. These results support further development of pramlintide--metreleptin as a novel, integrated neurohormonal approach to obesity pharmacotherapy.
8. Expert opinion
Obesity is a chronic disease with currently no successful long-term treatment options, apart from bariatric surgery which is both costly and not without risk. After the many failures of monotherapy, combination therapy for obesity is an encouraging step forward in the pharmacological arena. Indeed, it is no surprise that physiological mechanisms resisting weight loss such as metabolic adaptation (a drop in metabolic rate larger than predicted on the basis of loss of metabolic mass), a decrease in fat oxidation and feelings of hunger are engaged during pharmacological monotherapy. It is therefore obvious that targeting more than one mechanism will improve the chance of obtaining satisfactory weight loss. Furthermore, for both energy intake and expenditure there are redundant pathways and targeting one pathway may engage compensations in another pathway(s). Many pharmaceutical and biotech companies interested in the treatment of obesity are therefore designing clinical trials using existing compounds with different mechanisms of action on both sides of energy balance, that is, intake and expenditure.
Despite very promising weight-loss results and improvements in metabolic markers in Phase II clinical trials in overweight and mildly obese subjects, progress in the development of pramlintide/metreleptin combination therapy has since stalled, with Amylin Pharmaceuticals and Takeda Pharmaceuticals stating on 16th March 2011 that the ‘clinical study was voluntarily halted to investigate a new antibody-related laboratory finding with metreleptin treatment in two patients who participated in a previously completed clinical study of obesity’. Before further development of this promising combination of two injectable peptide/protein hormones, there needs to be full resolution of these laboratory findings. Nevertheless, the preliminary clinical studies from Vivus (Qnexa), Orexigen (Contrave) and Amylin Pharmaceuticals, prove that combination therapy is increasingly important for the management of obesity and/ or type 2 diabetes following the many failures of monotherapy. Complementary mechanisms of action of the different classes of injectable or oral agents demonstrate synergistic effects when used in combination. However, safety issues have been raised for all of the tested combinations resulting in delays before the launch of true combination therapies for obesity.
Footnotes
Declaration of interest
E Ravussin was on the Scientific Advisory Board from 2004 to 2009 for Amylin Pharmaceuticals site for RCT DFA 104. The other authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–84. doi: 10.1124/pr.59.2.2. [DOI] [PubMed] [Google Scholar]
- 2.Greenway FL, Bray GA. Combination drugs for treating obesity. Curr Diab Rep. 2010;10:108–15. doi: 10.1007/s11892-010-0096-4. [DOI] [PubMed] [Google Scholar]
- 3.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub M. Long-term weight control study: conclusions. Clin Pharmacol Ther. 1992;51:642–6. doi: 10.1038/clpt.1992.76. [DOI] [PubMed] [Google Scholar]
- 5.Shimabukuro M, Koyama K, Chen G, et al. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci USA. 1997;94:4637–41. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemecz M, Preininger K, Englisch R, et al. Acute effect of leptin on hepatic glycogenolysis and gluconeogenesis in perfused rat liver. Hepatology. 1999;29:166–72. doi: 10.1002/hep.510290110. [DOI] [PubMed] [Google Scholar]
- 7.Minokoshi Y, Kahn BB. Role of AMP-activated protein kinase in leptin-induced fatty acid oxidation in muscle. Biochem Soc Trans. 2003;31:196–201. doi: 10.1042/bst0310196. [DOI] [PubMed] [Google Scholar]
- 8.Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–71. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9•.Roth JD, Roland BL, Cole RL, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257–62. doi: 10.1073/pnas.0706473105. [This study presents key evidence from preclinical and clinical studies showing that concurrent administration of amylin and leptin elicits synergistic effects on weight loss.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trevaskis JL, Coffey T, Cole R, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679–87. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 11.Ludvik B, Kautzky-Willer A, Prager R, et al. Amylin: history and overview. Diabet Med. 1997;14(Suppl 2):S9–13. doi: 10.1002/(sici)1096-9136(199706)14:2+<s9::aid-dia397>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Juhl CB, Porksen N, Sturis J, et al. High-frequency oscillations in circulating amylin concentrations in healthy humans. Am J Physiol Endocrinol Metab. 2000;278:E484–90. doi: 10.1152/ajpendo.2000.278.3.E484. [DOI] [PubMed] [Google Scholar]
- 13. [9 August 2011]; Available from: http://online.factsandcomparisons.com.
- 14.Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother. 2011;12:1439–51. doi: 10.1517/14656566.2011.581663. [DOI] [PubMed] [Google Scholar]
- 15.Weyer C, Maggs DG, Young AA, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des. 2001;7:1353–73. doi: 10.2174/1381612013397357. [DOI] [PubMed] [Google Scholar]
- 16••.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [This study was the first to identify the leptin mutation in subjects with congenital leptin deficiency, thus showing that leptin is an important regulatory of energy balance in humans.] [DOI] [PubMed] [Google Scholar]
- 17.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 19.Galgani JE, Greenway FL, Caglayan S, et al. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab. 2010;95:851–5. doi: 10.1210/jc.2009-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–8. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 21.Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–60. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 22.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 23.Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci USA. 2011;108:6585–90. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Audi L, Mantzoros CS, Vidal-Puig A, et al. Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry. 1998;3:544–7. doi: 10.1038/sj.mp.4000418. [DOI] [PubMed] [Google Scholar]
- 25.Miller KK, Parulekar MS, Schoenfeld E, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: the effects of body composition and nutritional intake. J Clin Endocrinol Metab. 1998;83:2309–12. doi: 10.1210/jcem.83.7.4975. [DOI] [PubMed] [Google Scholar]
- 26••.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–75. doi: 10.1001/jama.282.16.1568. [This was, to our knowledge, the first and only clinical trial using leptin as a monotherapy to treat overweight and obesity.] [DOI] [PubMed] [Google Scholar]
- 27.Johannsen DL, Knuth ND, Tamboli RA, et al. Massive weight loss via bariatric surgery versus intensive lifestyle intervention: Impact on body composition, energy metabolism, and cardio-metabolic health. JAMA. 2011 [Google Scholar]
- 28••.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. doi: 10.1056/NEJM199503093321001. [This study found that the maintenance of a reduced or elevated body weight is associated with compensatory changes in energy expenditure, which oppose the maintenance of a body weight that is different from the usual weight, a phenomenen termed ‘metabolic adaptation’.] [DOI] [PubMed] [Google Scholar]
- 29.Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1286. published online July 21, 2011; doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doucet E, Imbeault P, St-Pierre S, et al. Greater than predicted decrease in energy expenditure during exercise after body weight loss in obese men. Clin Sci (Lond) 2003;105:89–95. doi: 10.1042/CS20020252. [DOI] [PubMed] [Google Scholar]
- 31.Doucet E, St PS, Almeras N, et al. Changes in energy expenditure and substrate oxidation resulting from weight loss in obese men and women: is there an important contribution of leptin? J Clin Endocrinol Metab. 2000;85:1550–6. doi: 10.1210/jcem.85.4.6500. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay A, Pelletier C, Doucet E, Imbeault P. Thermogenesis and weight loss in obese individuals: a primary association with organochlorine pollution. Int J Obes Relat Metab Disord. 2004;28:936–9. doi: 10.1038/sj.ijo.0802527. [DOI] [PubMed] [Google Scholar]
- 33.Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002;60:S15–19. doi: 10.1301/002966402320634788. [DOI] [PubMed] [Google Scholar]
- 34.Frederich RC, Lollmann B, Hamann A, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–63. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frederich RC, Hamann A, Anderson S, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–14. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 36.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 37.Ravussin Y, Gutman R, Diano S, et al. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1352–62. doi: 10.1152/ajpregu.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenbaum M, Murphy EM, Heymsfield SB, et al. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–4. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 39.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–86. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shetty GK, Matarese G, Magkos F, et al. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. Eur J Endocrinol. 2011;165:249–54. doi: 10.1530/EJE-11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286:R143–50. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 42.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–9. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 43.Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol Metab. 2010;21:473–9. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Seth R, Knight WD, Overton JM. Combined amylin-leptin treatment lowers blood pressure and adiposity in lean and obese rats. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.262. published online 21 December 2010; doi:10.1038/ijo.2010.262. [DOI] [PubMed] [Google Scholar]
- 45.Trevaskis JL, Coffey T, Cole R, et al. Amylin-mediated restoration of leptin responsiveness in diet-induced obesity: magnitude and mechanisms. Endocrinology. 2008;149:5679–87. doi: 10.1210/en.2008-0770. [DOI] [PubMed] [Google Scholar]
- 46.Trevaskis JL, Lei C, Koda JE, et al. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity (Silver Spring) 2010;18:21–6. doi: 10.1038/oby.2009.187. [DOI] [PubMed] [Google Scholar]
- 47.Turek VF, Trevaskis JL, Levin BE, et al. Mechanisms of amylin/leptin synergy in rodent models. Endocrinology. 2010;151:143–52. doi: 10.1210/en.2009-0546. [DOI] [PubMed] [Google Scholar]
- 48.Moon HS, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–56. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/ metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–43. doi: 10.1038/oby.2009.184. [This was a proof-of-concept study which evaluated the weight lowering effects of either metreleptin, pramlitide or pramlitide/metreleptin combination therapy in obese or overweight subjects.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolterman OG, Schwartz S, Corder C, et al. Effect of 14 days’ subcutaneous administration of the human amylin analogue, pramlintide (AC137), on an intravenous insulin challenge and response to a standard liquid meal in patients with IDDM. Diabetologia. 1996;39:492–9. doi: 10.1007/BF00400683. [DOI] [PubMed] [Google Scholar]
- 51.Colburn WA, Gottlieb AB, Koda J, Kolterman OG. Pharmacokinetics and pharmacodynamics of AC137 (25,28,29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J Clin Pharmacol. 1996;36:13–24. doi: 10.1002/j.1552-4604.1996.tb04147.x. [DOI] [PubMed] [Google Scholar]
- 52.McDuffie JR, Riggs PA, Calis KA, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab. 2004;89:4258–63. doi: 10.1210/jc.2003-031868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran SA, Patten N, Young JR, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53:513–19. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee Y, Yu X, Gonzales F, et al. PPARalpha is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc Natl Acad Sci USA. 2002;99:11848–53. doi: 10.1073/pnas.182420899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orci L, Cook WS, Ravazzola M, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci USA. 2004;101:2058–63. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unger RH. Leptin physiology: a second look. Regul Pept. 2000;92:87–95. doi: 10.1016/s0167-0115(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 58.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–65. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 59.Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 60.Javor ED, Cochran EK, Musso C, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54:1994–2002. doi: 10.2337/diabetes.54.7.1994. [DOI] [PubMed] [Google Scholar]
- 61.Park JY, Javor ED, Cochran EK, et al. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism. 2007;56:508–16. doi: 10.1016/j.metabol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan JL, Oral EA. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr Pract. 2010;16:310–23. doi: 10.4158/EP09154.RA. [DOI] [PubMed] [Google Scholar]
- 63.Amylin Pharmaceuticals SDCI2M2 Investigators brochure: recombinant-methionyl leptin human metreleptin for use in studies in obese subjects when administered in conjunction with pramlintide. 2010 [Google Scholar]
- 64.Chan JL, Wong SL, Mantzoros CS. Pharmacokinetics of subcutaneous recombinant methionyl human leptin administration in healthy subjects in the fed and fasting states: regulation by gender and adiposity. Clin Pharmacokinet. 2008;47:753–64. doi: 10.2165/00003088-200847110-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.SYMLINA (pramlintide acetate injection. Amylin Pharmaceuticals, Inc.; San Diego, CA: 2010. [24 August 2011]. Available from: http://documents/symlin.com/SYMLIN_PI.pdf. [Google Scholar]
- 66.Whitehouse F, Kruger DF, Fineman M, et al. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care. 2002;25:724–30. doi: 10.2337/diacare.25.4.724. [DOI] [PubMed] [Google Scholar]
- 67.Edelman S, Garg S, Frias J, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006;29:2189–95. doi: 10.2337/dc06-0042. [DOI] [PubMed] [Google Scholar]
- 68.Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21:1204–12. doi: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 69.Riddle M, Pencek R, Charenkavanich S, et al. Randomized comparison of pramlintide or mealtime insulin added to basal insulin treatment for patients with type 2 diabetes. Diabetes Care. 2009;32:1577–82. doi: 10.2337/dc09-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riddle M, Frias J, Zhang B, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care. 2007;30:2794–9. doi: 10.2337/dc07-0589. [DOI] [PubMed] [Google Scholar]
- 71.Hollander PA, Levy P, Fineman MS, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care. 2003;26:784–790. doi: 10.2337/diacare.26.3.784. [DOI] [PubMed] [Google Scholar]