Abstract
Neuronal chloride concentration [Cl−]i is an important determinant of GABAA receptor (GABAAR)-mediated inhibition and cytoplasmic volume regulation. Equilibrative cation-chloride cotransporters (CCC) move Cl− across the membrane, but accumulating evidence suggests factors other than the bulk concentrations of transported ions determine [Cl−]i. Measurement of [Cl−]i in murine brain slice preparations expressing the transgenic fluorophore Clomeleon demonstrated that cytoplasmic impermeant anions ([A]i) and polyanionic extracellular matrix glycoproteins ([A]o) constrain the local [Cl−]. CCC inhibition had modest effects on [Cl−]i and neuronal volume, but substantial changes were produced by alterations of the balance between [A]i and [A]o. Therefore, CCC are important elements of Cl− homeostasis, but local impermeant anions determine the homeostatic set-point for [Cl−], and hence, neuronal volume and the polarity of local GABAAR signaling.
The GABAAR can subserve either inhibition or excitation, because the net flux of the permeating anions HCO3− and Cl− can be reversed by modest changes in the transmembrane concentration gradient of Cl− (1, 2). NKCC1 and KCC2 are the primary cation-chloride co-transporters in neurons (3). Their unique stoichiometries result in distinct [Cl−]i equilibria: ~3 mM for KCC2 (4, 5) and ~60 mM for NKCC1 (3). The species of cotransporter expressed in the neuron is thought to determine [Cl−]i, but there is evidence that transporter expression is not the sole determinant of [Cl−]i and hence, the reversal potential for GABA (EGABA). First, CCCs transport water along with cations and Cl− as nearly isotonic saline (6, 7). Neurons express no known aquaporin water channels (8), so the CCCs have limited capacity to alter [Cl−]i independently of neuronal volume. Second, the transporter expressed may not correlate with the measured [Cl−]i (9). Third, electrophysiological and fluorometric studies demonstrate a wide distribution of [Cl−]i that rarely matches the equilibrium for either NKCC1 or KCC2 (10–15). Finally, sub-cellular differences in EGABA indicate that [Cl−]i is likely to vary within the cytoplasm of individual neurons (16, 17), a condition that cannot be efficiently maintained solely by transporters due to the high rate of dissipation of local [Cl−]i by cytoplasmic diffusion (18).
A solution is suggested by the fact that Cl− is only a minor component of the intracellular anionic milieu. The neuronal membrane is impermeable to most cytoplasmic anions, including phosphate groups associated with deoxy- and ribonucleotides, and the majority of intracellular proteins whose amino and carboxyl moieties are negatively charged at physiological pH (19). For any value of cytoplasmic volume and osmolarity, the sum of cytoplasmic impermeant anions ([A]i) and [Cl−]i must be constant ([HCO3−] is fixed by pH requirements). Thus, [Cl−]i should be constrained by [A]i. However, cotransport of Cl− and cations across a membrane that is impermeant to [A]i creates an unstable Gibbs-Donnan condition with higher intracellular vs. extracellular osmotic pressure (20). The exclusion of Na+ from the cytoplasm by Na+/K+-ATPase is thought to create a balancing Gibb-Donnan condition (7, 20), but this mechanism ignores intracellular K+, and is not supported by pharmacological inhibition of neuronal Na+/K+-ATPase (21). The sulfates on the proteoglycans of the extracellular matrix (22) comprise a more plausible impermeant extracellular ion. Manipulation of extracellular proteoglycans alter cell volume, membrane potential, and neuronal network excitability, consistent with Donnan effects (23). We investigated whether [Cl−]i is set by the local concentrations of impermeant anions on either side of the neuronal membrane.
Direct [Cl−]i measurements were performed using two-photon imaging in acute and organotypic brain slices of mice expressing Clomeleon (ratiometric fluorophore sensitive to changes in [Cl−]i (18)). In neonatal neurons from CA1 hippocampal slices (P8-9), where NKCC1 is the primary transporter expressed (24), there was a broad distribution of somatic [Cl−]i of 14.7 ± 6.5 mM (median ± SD; n = 237 neurons; 6 slices; Fig. 1A-B). In adult CA1 hippocampal slices (P32-44) where KCC2 is the primary transporter (3), the somatic [Cl−]i was also widely distributed 13.7 ± 8.9 mM (n = 227 neurons; 8 slices; Fig. 1C). Similar results were found in acute brain slices from neocortex layer IV/V and CA1 organotypic hippocampal slices (Figs. S1A,C; S2A,B; Table S1-2). Thus, most neurons have [Cl−]i not congruent with the equilibrium conditions of either KCC2 or NKCC1.
Fig. 1. [Cl−]i is not solely determined by transport activity.
A: Two-photon image of Clomeleon YFP from acute hippocampal slice CA1 (left) and neocortex layer IV/V (right). Lower panel: Detailed image of neuron outlined in right panel. B: [P8-9 hippocampal slice] Left, initial vs. new steady state [Cl−]i after 30 min NKCC1 block followed by additional 30 min block of KCC2. Lines, linear fit. Right, as left but KCC2 blocked first then KCC2/NKCC1 block. C: As B but in P32-44 CA1 neurons. D: Transporter index histogram of all neonatal hippocampal neurons (calculated as in methods and Fig. S5), 2.5 ± 5.9 (n = 237; median ± SD). Black circle indicates expected value for canonical co-operation of KCC2 and NKCC1. E: Transporter index of all recorded neonatal CA1 neurons (2.5 ± 5.9), and adult CA1 neurons (1.07 ± 5.6; n = 227) and expected values (neonatal:16.7 ± 6.5; n = 237; adult:15.7 ± 8.9; n = 227). *, P < 0.001, Mann-Whitney Rank Sum Test. F: Upper, [Cl−]i from P6-8 layer IV/V neocortical pyramidal cells (Soma: 16.0 ± 8.9 mM; Dendrite: 11.5 ± 8.3 mM; Axon: 13.9 ± 8.6; P < 0.001, Friedman test, *, P < 0.05 multiple comparison Dunn’s method). Lower, [Cl−]i from P28-30 layer IV/V neocortical pyramidal cells (Soma: 9.3 ± 6.1 mM; Dendrite: 11.4 ± 6.5 mM; Axon: 16.1 ± 7.9 mM; P < 0.001). G: Subcellular gradient differences between axon-soma (A-S) and dendrite-soma (D-S). Blockade of NKCC1 in neonates (10 µM bumetanide) and NKCC1/KCC2 in adults (100 µM bumetanide) did not alter the subcellular [Cl−]i gradients (paired neuron-process; P > 0.05; paired t-test, error lines = SD).
If [Cl−]i was primarily maintained by CCCs, the change in [Cl−]i after transport block should be the opposite of the canonical direction of the primary expressed transporter: KCC2 block should increase [Cl−]i, and NKCC1 block should decrease [Cl−]i. In neonatal CA1 slices (P8-9), blocking NKCC1 with bumetanide 10 µM did not alter [Cl−]i (15.8 ± 7.6 to 16 ± 7.3 mM, n = 105 paired cells; Fig. 1B, S3A). Addition of 10 µM of the specific KCC2 antagonist VU0240551 (25) induced a minimal decrease in [Cl−]i to 15 ± 6.9 mM (Fig. 1B; S3A), and [Cl−]i did not become passively distributed across the membrane such that ECl ~ RMP. After CCC block, [Cl−]i increased in neurons with a low initial [Cl−]i and decreased in neurons with a high initial [Cl−]i (Fig. S3A), regardless of the order of antagonist application (Fig. 1B; S3B) in adult CA1 slices (P32-44) (Fig. 1C; S3C, D), in CA1 organotypic hippocampal slices DIV6-11 (Fig. S2A-C, E-G) and in acute layer IV/V neocortical pyramidal neurons (P8-9 and P27-31; Fig. S1; Tables S1–2). If no drug was added, [Cl−]i was stable (Fig. S4C-E). In summary, the initial [Cl−]i was a better predictor of the change in [Cl−]i than was the species of blocked transporter. To quantify this, we summed the absolute values of the change in [Cl−]i during sequential block of KCC2 and NKCC1. If NKCC1 and KCC2 are working against each other in the same neuron to create a broad distribution of [Cl−]i, this sum value should be large (Fig. S5). However, this sum was nearly 0 in all neuronal populations studied (Fig. 1D-E; S2D-H). Therefore, the broad distribution of [Cl−]i cannot be explained by the net effects of oppositely-directed NKCC1 and KCC2 cotransport.
[Cl−]i varies not only between neurons but also between subcellular regions (16), which is difficult to reconcile with the high intracellular mobility of [Cl−]i (18). We measured millimolar differences in [Cl−]i at the soma vs. proximal 40 µm of the axon and proximal 200 µm of the apical dendrites of neonatal and adult neocortical neurons (sparse neocortical expression of Clomeleon permitted identification of individual processes and soma; Fig. 1F). Subcellular differences in [Cl−]i were not maintained by local CCCs because after CCC block, [Cl−]i gradients were unchanged (P > 0.05, paired t-test; Fig. 1G).
These data are not consistent with the hypothesis that [Cl−]i is determined by the species of expressed cotransporter. Rather, the data suggest that KCC2 and NKCC1 both serve as conduits for transmembrane Cl− flux whose direction is determined by other factor(s). We therefore tested the hypothesis that [A]i and [A]o determine the local [Cl−] and thus ECl and the effect of GABAA receptor activation.
Prediction 1
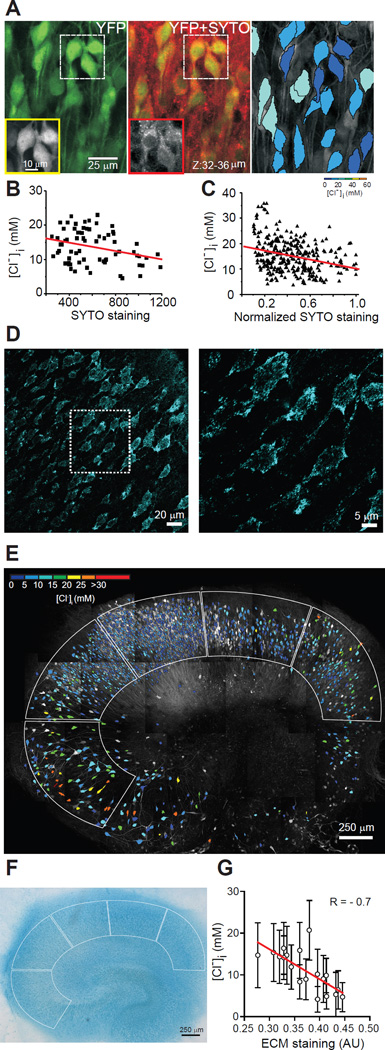
If the sum of [A]i + [Cl−]i is constant, then the broad distribution of [Cl−]i together with the large inter-cellular variance in the primary constituents of [A]i (cytoplasmic protein and nucleic acid concentrations (26)) can be used to test for the predicted reciprocal relationship between [A]i and [Cl−]i. [A]i and [Cl−]i were imaged in Clomeleon organotypic hippocampal slice cultures to facilitate the prolonged dye incubation required to estimate [A]i. CA1 pyramidal neurons demonstrated a wide range of somatic [Cl−]i of 14.8 ± 6.3 mM at DIV10-12 (n = 305 neurons; 4 slices; Fig. 2A-C). After Cl− imaging, slices were incubated for 2 hours in SYTO64 10 µM, which binds to the cytoplasmic/nuclear nucleic acids whose phosphate groups are a major component of [A]i. SYTO64 staining intensity was negatively correlated with [Cl−]i (R = −0.3; P < 0.001; Fig. 2B,C). Because nucleic acids make up approximately a third of intracellular fixed anions (27), the strength of the correlation between SYTO64 staining and [Cl−]i is what would be predicted if [A]i sets [Cl−]i.
Fig. 2. Correlation of [A]i and [A]o with neuronal [Cl−]i.
A: Left, z-stacked 2-photon image of YFP (green) from CA1 pyramidal layer in an organotypic hippocampal slice from a Clomeleon mouse. Middle, SYTO64 staining (red) of cytoplasmic/nuclear nucleic acids overlaid on the YFP image. Boxes: YFP and SYTO64 fluorescence in individual neurons. Right, Pseudo-colored neurons based on YFP/CFP ratio. B: [Cl−]i as a function of SYTO64 labeling. R = −0.29, P = 0.01, n = 70 neurons; one slice. C: [Cl−]i as a function of normalized SYTO64 labeling. R = −0.3, P < 0.001, n = 305 neurons; 4 slices. Line, linear fit. D-F: Correlation between sulfated extracellular matrix and [Cl−]i. D: Confocal images of Alcian blue staining in CA1 pyramidal layer of an organotypic hippocampal slice. Outlined area magnified in right panel. E: [Cl−]i distribution before Alcian blue staining. Borders: ROI used to calculate average [Cl−]i and Alcian blue staining intensity. F: Alcian blue staining of slice in E, transmitted light. G: Correlation between [Cl−]i and extracellular matrix (Alcian blue staining) obtained in each region of interest, 4 slices. Bars = SD; AU: arbitrary units. Line, linear fit (P < 0.001).
Prediction 2
If the sum of [A]i and [Cl−]i is constant, then increasing [A]i should decrease [Cl−]i. [A]i was increased by iso-osmotic perfusion of 20 mM gluconate, pyruvate, and D-lactate, which are weak organic acids that are transported across the cytoplasmic membrane (28). Each perfusion reduced [Cl−]i by 5–10%, consistent with a corresponding increase in [A]i due to a Nernstian distribution of the deprotonated, anionic bases at a resting membrane potential near −70 mV (Fig. S6). This was unlikely a pH effect on Clomeleon fluorescence because the pHi effects (29) would alter Clomeleon fluorescence in the opposite direction from those observed (18). Thus, increases in [A]i are accompanied by reductions in [Cl−]i, so that the sum of [A]i + [Cl−]i is constant.
Prediction 3
If [A]o sets the local extracellular chloride concentration [Cl−]o, then cation-chloride cotransport should come to equilibrium at [Cl−]i that reflects the local [Cl−]o.. Thus, [A]o should vary inversely with [Cl−]i. The polysulfated proteoglycans of the extracellular matrix create extracellular negative charge densities of 50 – 350 mEq/liter and thus could comprise [A]o (30). We first verified that extracellular Na+ does not exert a significant Donnan effect (21) (Fig. S7). [Cl−]i was measured in organotypic Clomeleon hippocampal slices (DIV12-14). Then, slices were fixed and incubated with Alcian blue at pH 0.5 to specifically stain sulphated glycosaminoglycans (31). [A]o as assayed by Alcian blue staining was negatively correlated with [Cl−]i (R = −0.7, P < 0.001; Fig. 2D-G; S8). These data support the possibility that polysulfated proteoglycans of the extracellular matrix comprise [A]o and that together with [A]i these macromolecular anions constrain the local [Cl−] to set the local ECl.
Prediction 4
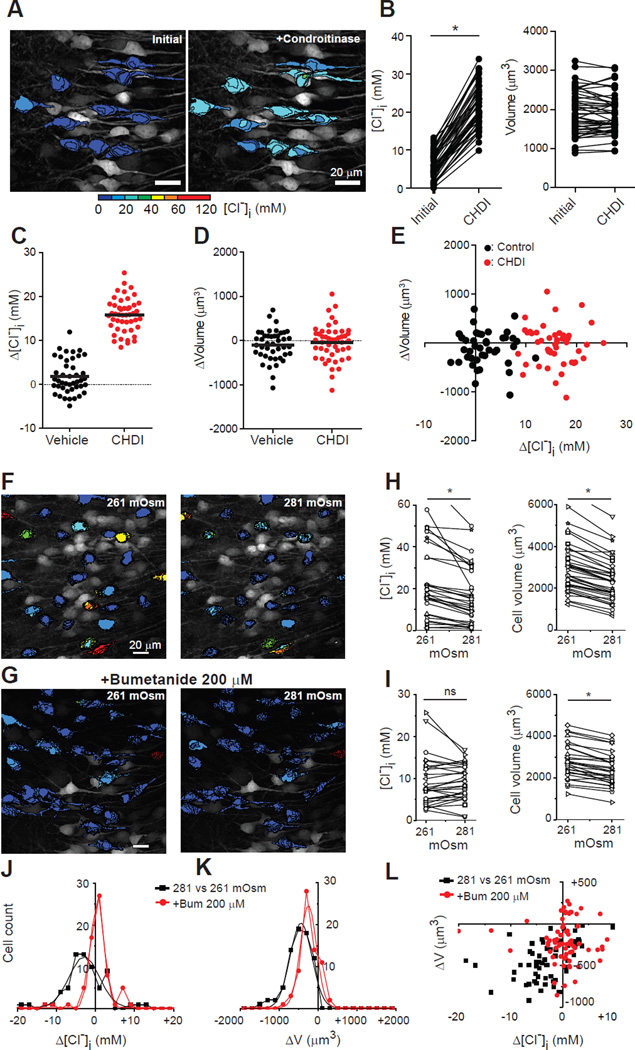
Decreasing [A]o will increase [Cl−]i. If the sulfated carbohydrate moieties of the proteoglycans in the extracellular matrix subserve this function, then their lysis would release SO4− and reduce [A]o. This would increase local [Cl−]o and shift the equilibrium condition for transmembrane cation-chloride transport toward higher [Cl−]i. Accordingly, when organotypic hippocampal slices (DIV12-14) were incubated in chondroitinase ABC (CDHI) for 1 hour, neuronal [Cl−]i increased from 5.9 ± 3.4 to 20.2 ± 5.7 mM (n = 47 neurons; 4 slices, P < 0.0001, Wilcoxon signed rank test; Fig. 3A-E). The [Cl−]i change was significantly higher (P < 0.0001) than that by incubation with vehicle solution (from 9.2 ± 2.7 to 10.9 ± 4.3 mM, n = 43; 4 slices, P = 0.02). Somatic volume was unchanged at ~80 minutes (Fig. 3B, D-E), likely reflecting successful compensatory volume regulation. Cell viability was not altered by incubation in CDHI (Fig. S9).
Fig. 3. [Cl−]i is changed by disruption of the extracellular matrix and by increased extracellular osmolarity.
A: Pseudo-colored, two-photon z-stack image before and after incubation with chondroitinase ABC (CHDI) in an organotypic hippocampal slice. B: Significant effect of CHDI on [Cl−]i, but not volume. C: [Cl−]i change by incubation with vehicle vs. CHDI solution (Vehicle: +0.8 ± 3.9 mM, n = 43; 4 slices; CHDI; +15.8 ± 3.8 mM, n = 47; 4 slices; P < 0.0001, unpaired t-test). D: As C but plotting somatic volume (vehicle; −52 ± 174 µm3, CHDI; −23 ± 203 µm3, P = 0.46 unpaired t-test. Change in vehicle: 1699 ± 480 to 1647 ± 464 µm3, P = 0.08. Change in CHDI: 1905 ± 564 to 1882 ± 516 µm3, P = 0.51, Wilcoxon signed rank test, same cells as C). E: Volume vs. [Cl−]i changes for each neuron in C-D. * P < 0.0001. F, G: Organotypic hippocampal slices expressing Clomeleon (two-photon) after increasing osmolarity with 20 mM Mannitol (8% increase), and in bumetanide (G). H-I: [Cl−]i and neuronal volume changes in mannitol (H) and plus bumetanide (I). J-K: [Cl−]i and neuronal somatic volume changes induced by increased extracellular osmolarity. L: Neuronal volume changes as a function of [Cl−] changes.
Prediction 5
In a system in which [A]i, [A]o, and [Cl−]i are in ionic and osmotic equilibrium, acutely altering the osmotic balance should alter [Cl−]i. Water is transported with cations and Cl− by CCCs (6, 7) and neurons lack known aquaporins (8). Thus, water movement contributes to the free energy of cotransport (6). An increase in extracellular osmolarity would favor CCC movement of water and Cl− out of the neuron, increasing the fractional cytoplasmic concentration of [A]i and decreasing [Cl−]i. This is opposite to what would be predicted by free transmembrane water permeability: loss of intracellular water would increase [Cl−]i (7). Two-photon multiplanar micrographs of individual pyramidal cells from organotypic hippocampal slices (DIV8-12) expressing Clomeleon were reconstructed in 3 dimensions to measure volume and [Cl−]i (Fig. 3F-I). In control conditions, the somatic volume and [Cl−]i were stable as a function of time (Fig. S4). Increasing extracellular osmolarity by 8% (from 261 [osmolarity of culture media] to 281 mOsm) via the addition of 20 mM mannitol resulted in a rapid 21% decrease in somatic volume from 2333 ± 1192 to 1842 ± 967 µm3 (mean ± SD, n=73 neurons; 4 slices; P < 0.001, paired t-test), and a 9% decrease in somatic [Cl−]i from 16.6 ± 14.6 to 15.1 ± 11.1 mM (P<0.001, Wilcoxon signed rank test; Fig. 3H,J,L). Using the same protocol but blocking NKCC1 and KCC2 (bumetanide 200 µM) resulted in 9% decrease in somatic volume from 2365 ± 1005 to 2147 ± 953 µm3 (n = 68 neurons; 3 slices; P < 0.0001, paired t-test; Fig. 3I,K), and no change in somatic [Cl−]i from 7.1±10 to 8.3±7.9 mM (P=0.074, Wilcoxon signed rank test; Fig. 3I-L). The unlinking of volume and [Cl−]i changes when CCCs were blocked is likely to represent volume regulation by other systems (7).
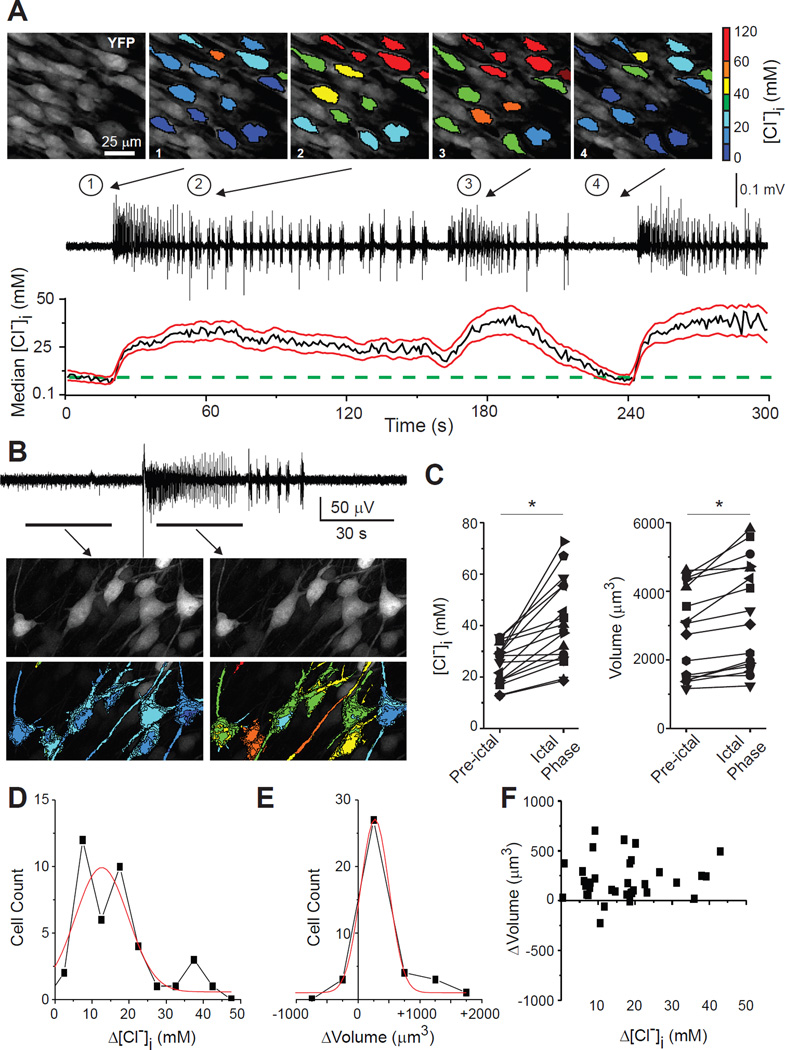
Finally, a consequence of the co-transport of water and salt by NKCC1 and KCC2 is that near-isotonic saline moves across the membrane, so that both neuronal volume and [Cl−]i should change in parallel during cotransport. Several conditions including hypoxic ischemic injury, trauma, and prolonged seizures have in common the development of cytotoxic edema and increased neuronal [Cl−]i (15, 32, 33). To test whether seizure-induced increases in neuronal volume are associated with acute increases in [Cl−]i, we performed extracellular field recording and two-photon microscopy to measure cytoplasmic volume and [Cl−]i in organotypic hippocampal slice cultures (DIV10-12) that spontaneously develop ictal-like epileptiform discharges (34). Prolonged seizure activity increased both neuronal volume and [Cl−]i (Fig. 4). Somatic volume increased by 10% from 2530 ± 1658 to 2775 ± 1741 µm3 (mean ± SD; n = 42 neurons; 4 slices; P = 0.006, paired t-test) and [Cl−]i increased from 25.4 ± 7.4 to 40.8 ± 13.8 mM (P < 0.001, Wilcoxon signed rank test). The increase in [Cl−]i during seizures is mediated by NKCC1 as it can be reduced by bumetanide (15).
Fig. 4. [Cl−]i and intracellular volume changes during ictal activity.
A: [Cl−]i transients during spontaneous seizures in the CA1 pyramidal cell layer, organotypic hippocampal slice (DIV12). Top, Pseudo-colored, two-photon images of CA1 neurons before and during seizures. Middle, Extracellular field potential recording. Bottom, [Cl−]i changes in a population of neurons (median, black; SD, red lines; green dotted line indicates initial [Cl−]i). B: Extracellular recording of spontaneous seizure in CA1 region of organotypic hippocampal slice (DIV12). Below, z-stack image of neurons before and during spontaneous seizure. C: [Cl−]i and somatic volume changes in individual neurons during spontaneous seizure activity (*, P < 0.05). D-F: [Cl−]i and somatic volume changes during recurrent seizures (n = 40 neurons; 4 slices).
These data indicate that the local [A]i and [A]o determine [Cl−]i,, [Cl−]o, and therefore EGABA, and CCCs serve to maintain [Cl−]i at this set point. Therefore: 1) The widely observed intercellular and intracellular variances in [Cl−]i and EGABA are best explained by local variation in [A]i and [A]o. The species of CCC and other cellular features are likely to correlate with [A]i and [Cl−]i (e.g. (14, 24)). 2) The developmental reduction in [Cl−]i would follow from the increase in neuronal [A]i during development (35) and experience (36), paralleled by increases in the proteoglycans of the extracellular matrix (37). 3) The subcellular variance in [Cl−]i arises from corresponding differences in the concentrations of relatively immobile cytoplasmic macromolecular anions (38). The variance in [A]i creates intracytoplasmic Gibbs-Donnan effects (39) such that local [Cl−]i is at equilibrium at different local [A]i. Thus, CCCs are not required to compensate for intra-cytoplasmic Cl− diffusion. 4) The influence of [A]o and [A]i on local [Cl−]i, and [Cl−]o open possibilities for developmental and experience-dependent plasticity of EGABA and Eglycine at individual synapses, so that the variance in extracellular sulfated proteoglycans comprises a potential locus of analog information storage and pathologically, a rich variety of antigens. 5) Pathological conditions that alter [A]o or [A]i will have secondary effects on both cell volume and [Cl−]i. This may explain the correlation between MRI evidence of cytotoxic edema after brain injury and anticonvulsant-resistant seizures, which can occur when increased [Cl−]i compromises GABAAR-mediated inhibition (15, 40). Thus, the magnitude and direction of GABAAR currents at individual synapses are among the wide variety of signaling functions subserved by intra- and extracellular macromolecular networks.
Supplementary Material
Acknowledgments
JG, VD and KE contributed equally. Work supported by NIH (NINDS) Grant NS 40109-06. JG supported by the American Epilepsy Society Post-doctoral fellowship and NIH R25. KE supported by The Japan Foundation for Pediatric Research. KTK supported by the Manton Center for Orphan Disease Research and NIH R25.
References and notes
- 1.Misgeld U, Deisz Ra, Dodt HU, Lux HD. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986;232:1413–1415. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlin NL, Dingledine R. GABAergic inhibition and the induction of spontaneous epileptiform activity by low chloride and high potassium in the hippocampal slice. Brain Res. 1988;445:12–18. doi: 10.1016/0006-8993(88)91068-2. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of chloride transporters and channels in the nervous system: From molecules to diseases. Academic Press; 2009. [Google Scholar]
- 4.DeFazio R, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne J. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am. J. Physiol. Physiol. 1997;273:C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 6.Zeuthen T. Water-transporting proteins. J. Membr. Biol. 2010;234:57–73. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann E, Lambert I, Pedersen S. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 8.Andrew RD, Labron MW, Boehnke SE, Carnduff L, Kirov SA. Physiological evidence that pyramidal neurons lack functional water channels. Cereb. cortex. 2007;17:787–802. doi: 10.1093/cercor/bhk032. [DOI] [PubMed] [Google Scholar]
- 9.Balakrishnan V, et al. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J. Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glykys J, et al. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63:657–672. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebihara S, Shirato K, Harata N, Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J. Physiol. 1995;484(Pt 1):77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada J, et al. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 2004;557:829–841. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyzio R, et al. Postnatal changes in somatic gamma-aminobutyric acid signalling in the rat hippocampus. Eur. J. Neurosci. 2008;27:2515–2528. doi: 10.1111/j.1460-9568.2008.06234.x. [DOI] [PubMed] [Google Scholar]
- 14.Martina M, Royer S, Paré D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J. Neurophysiol. 2001;86:2887–2895. doi: 10.1152/jn.2001.86.6.2887. [DOI] [PubMed] [Google Scholar]
- 15.Dzhala VI, et al. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J. Neurosci. 2010;30:11745–11761. doi: 10.1523/JNEUROSCI.1769-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabadics J, et al. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 17.Doyon N, et al. Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Comput. Biol. 2011;7:e1002149. doi: 10.1371/journal.pcbi.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuner T, Augustine GJ. A genetically encoded ratiometric indicator for chloride: capturing chloride transients in cultured hippocampal neurons. Neuron. 2000;27:447–459. doi: 10.1016/s0896-6273(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 19.Gianazza E, Righetti P. Size and charge distribution of macromolecules in living systems. J. Chromatogr. A. 1980;193:1–8. [Google Scholar]
- 20.Leaf A. On the mechanism of fluid exchange of tissues in vitro. Biochem. J. 1956;62:241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Leefmans F, Gamiño S, Reuss L. Cell volume changes upon sodium pump inhibition in Helix aspersa neurones. J. Physiol. 1992:603–619. doi: 10.1113/jphysiol.1992.sp019436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 23.Isaev D, et al. Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J. Neurosci. 2007;27:11587–11594. doi: 10.1523/JNEUROSCI.2033-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Delpire E, et al. Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minton A. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 27.Ellis RJ. Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr. Opin. Struct. Biol. 2001;11:114–119. doi: 10.1016/s0959-440x(00)00172-x. [DOI] [PubMed] [Google Scholar]
- 28.Poole R, Halestrap A. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 29.Ruusuvuori E, Kirilkin I, Pandya N, Kaila K. Spontaneous network events driven by depolarizing GABA action in neonatal hippocampal slices are not attributable to deficient mitochondrial energy metabolism. J. Neurosci. 2010;30:15638–15642. doi: 10.1523/JNEUROSCI.3355-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chahine NO, Chen FH, Hung CT, Ateshian Ga. Direct measurement of osmotic pressure of glycosaminoglycan solutions by membrane osmometry at room temperature. Biophys. J. 2005;89:1543–1550. doi: 10.1529/biophysj.104.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murakami T, Ohtsuka A, Piao X. Perineuronal sulfated proteoglycans in the human brain are identical to Golgi’s reticular coating. Arch. Histol. Cytol. 1996;59:233–237. doi: 10.1679/aohc.59.233. [DOI] [PubMed] [Google Scholar]
- 32.Pond BB, et al. The chloride transporter Na(+)-K(+)-Cl- cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J. Neurosci. 2006;26:1396–1406. doi: 10.1523/JNEUROSCI.1421-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Pol A, Obrietan K, Chen G. Excitatory actions of GABA after neuronal trauma. J. Neurosci. 1996;16:4283–4292. doi: 10.1523/JNEUROSCI.16-13-04283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berdichevsky Y, Dzhala V, Mail M, Staley KJ. Interictal spikes, seizures and ictal cell death are not necessary for post-traumatic epileptogenesis in vitro. Neurobiol. Dis. 2012;45:774–785. doi: 10.1016/j.nbd.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClatchy DB, Liao L, Park SK, Venable JD, Yates JR. Quantification of the synaptosomal proteome of the rat cerebellum during post-natal development. Genome Res. 2007;17:1378–1388. doi: 10.1101/gr.6375007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka J, Horiike Y, Matsuzaki M. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1688. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frischknecht R, et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 38.Song H, Sokolov M. Analysis of protein expression and compartmentalization in retinal neurons using serial tangential sectioning of the retina. J. Proteome Res. 2008;8:346–351. doi: 10.1021/pr800631d. [DOI] [PubMed] [Google Scholar]
- 39.Ricka J, Tanaka T. Swelling of ionic gels: quantitative performance of the Donnan theory. Macromolecules. 1984;17:2916–2921. [Google Scholar]
- 40.Glass HC, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J. Pediatr. 2011;159:731–735. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.