Abstract
Objective: The purpose of this study was to evaluate the effects of the antimicrobial photodynamic therapy (a-PDT) with blue light and curcumin on oral disinfection during the 2 h after treatment. Background data: a-PDT is a technique that can potentially affect the viability of bacterial cells, with selective action targeting only areas with photosensitizer accumulation. Materials and methods: A randomized controlled trial was undertaken. Twenty-seven adults were randomly divided into three groups: (1) the PDT group, which was treated with the drug, curcumin, and blue light (n=9); (2) the light group, which was treated only with the blue light, and no drug (n=9) and; (3) the curcumin group, which was treated only with the drug, curcumin, and no light (n=9). The irradiation parameters were: blue light-emitting diode (LED) illumination (455±30 nm), 400 mW of average optical power, 5 min of application, illumination area of 0.6 cm2, 600 mW/cm2 of intensity, and 200 J/cm2 of fluence. A curcumin concentration of 30 mg/L was used. The saliva samples were collected for bacterial counts at baseline and after the experimental phases (immediately after treatment, and 1 and 2 h after treatment). Serial dilutions were performed, and the resulting samples were cultured on blood agar plates in microaerophilic conditions. The number of colony-forming units (CFU) was determined. Results: The PDT group showed a significant reduction of CFU immediately after treatment (post-treatment) with PDT (5.71±0.48, p=0.001), and 1 h (5.14±0.92, p=0.001) and 2 h (5.35±0.76, p=0.001) after treatment, compared with pretreatment (6.61±0.82). There were no significant changes for the light group. The curcumin group showed a significant increase of CFU 1 h after treatment (6.77±0.40, p=0.02) compared with pretreatment (5.57±0.91) falling to baseline values at 2 h after treatment (5.58±0.70). Conclusions: The PDT group showed significant difference in microbial reduction (p<0.05) compared with both the light and curcumin groups until 2 h post-treatment. The new blue LED device for PDT using curcumin may be used for reduction of salivary microorganisms, leading to overall disinfection of the mouth (e.g., mucosa, tongue, and saliva), but new protocols should be explored.
Introduction
The normal mouth has a large number of bacteria. It results in increased risk of infection when many types of surgical procedures are performed, mainly intraoral surgery. In these cases, prophylactic systemic antibiotics are used, but these drugs may be associated with unfavorable side effects. Oral antiseptics (for example, chlorhexidine) can also be used in these cases, but the reduction of intraoral bacterial counts is temporary.1 In this context, new procedures for oral disinfection should be investigated.
Blue light (405–470 nm) without the addition of exogenous photosensitizers (PS) has intrinsic antimicrobial effect and shows fewer deleterious effects to mammalian cells than ultraviolet irradiation.2 According Soukos et al.,3 the amount of endogenous porphyrin and/or other cell pigments produced in Prevotella intermedia, Porphyromonas gingivalis, Prevotella melaninogenica, and Prevotella nigrescens can explain a susceptibility to blue light resulting in oral disinfection.
Moreover, curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5,-dione] shows antimicrobial activity as well. Curcumin is the principal yellow pigment isolated from turmeric (Curcuma longa Linn).4 Several pharmacological properties of curcumin have been reported, such as antioxidant and anti-inflammatory,4,5 antibacterial,6 antifungal,7 and anticarcinogenic5 effects, mainly with high doses of curcumin alone.8 Because of the extended antimicrobial activity of curcumin and its safety assessed by clinical trials in humans,9 it was used as a structural sample to design the new antimicrobial agents with modified and increased antimicrobial activities through the synthesis of various derivatives related to curcumin.10 Moreover, curcumin-mediated antimicrobial photodynamic therapy (a-PDT) can be used at low doses in combination with light exposure, with considerable antibacterial effect. In this context, curcumin has a rather broad absorption peak (range 300–500 nm), with a maximum absorption band at wavelength 430 nm, and it can be used as PS in a-PDT.11
PDT uses a nontoxic drug called a PS, which is activated by exposure to light of a specific wavelength in the presence of oxygen. This results in the production of reactive oxygen species (ROS), which can potentially affect the viability of bacterial cells with selective action targeting only areas with PS accumulation.12,13 In this context, PDT has no side effects, and bacteria do not develop resistance to ROS.1
Several in vitro studies and clinical trials were performed to investigate bactericidal action and oral disinfection using blue light3,14,15 or curcumin,16,17 or by performing PDT with blue light and curcumin.18–22 In dentistry, a-PDT may reduce both dental plaque and the risk of developing caries, as well as contributing to treating gingivitis, periodontitis, peri-implantitis and endodontic diseases.23
Previous clinical trials from our group have found that PDT with blue light and curcumin significantly reduced salivary microorganisms pre- and post-treatment.1 However, oral disinfection as a function of time, to our knowledge, has not been investigated. Therefore, the aim of this study was to evaluate the effects of the PDT with blue light and curcumin on oral disinfection during 2 h after treatment. Our target was overall oral flora (mucosa, tongue, saliva), conducting analyses of salivary pathogens before and after antimicrobial photodynamic therapy. Our hypothesis was that blue light with or without curcumin, as well as only curcumin, could reduce colony-forming units (CFU).
Materials and Methods
The current research has been approved by the Ethics Committee of the Federal University of São Carlos (UFSCar) in São Carlos, Brazil (N. 258.461). The study was registered with the National Institutes of Health (NIH) ClinicalTrials (NCT02152475). All subjects signed written informed consent forms before their participation in the study.
A randomized controlled trial was undertaken. The inclusion criteria were healthy adults not using any antibiotic therapy who did not perform any oral hygiene, such as flossing, brushing, or use of antiseptic mouthwash, and who had fasted for 12 h prior the treatment and measurements. The exclusion criteria were having had oral cancer, smoking, pregnancy, or wearing partial or total dentures or orthodontic brackets. We performed simple randomization by a computer program. Twenty-seven healthy adult females and males between 20 and 35 years of age were randomly divided into three groups: (1) the PDT group, which was treated with the drug, curcumin, and blue light (n=9); (2) the light group, which was treated only with the blue light, and no drug (n=9); and (3) the curcumin group, which was treated only with the drug, curcumin, and no light (n=9).
Instrumentation to perform PDT
In order to perform PDT on the oral cavity, a device based on blue light-emitting diode (LED) (455±30 nm) with transparent acrylic diffuser tip and cylindrical shape (89 mm length and 6.73 mm diameter) was developed by researchers of the industry (MM Optics, São Carlos, SP, Brazil) and the Optics Group of the Physics Institute of São Carlos (IFSC), University of São Paulo (USP). An optical power meter model FieldMaster TO-II (Coherent Inc., Santa Clara, CA) linked to a photodetector was used to calibrate this device, and to reveal a 400 mW average optical power, a 0.6 cm2 illumination area, and a 600 mW/cm2 intensity. The light was applied for 5 min, which led to an energy density (radiation dose) delivered of ∼200 J/cm2. We considered a total energy per unit of area reaching the surface as the delivered dose, but this was not necessarily uniformly absorbed. The dose delivery was approximated, because different areas and several distances were irradiated. The new blue LED device in the oral cavity can be seen in Fig. 1. Although we applied the blue LED for 5 min as in the clinical trial of Araújo and collaborators,1 the device geometry used in our study was different; therefore, the parameters also were different.
FIG. 1.
New blue light-emitting diode (LED) device for photodynamic therapy (PDT) in the oral cavity.
Curcumin
A stock solution (1.5 g/L) of curcumin (PDT Pharma, Cravinhos, SP, Brazil) was prepared in dimethylsulfoxide (DMSO) (0.1%) and then diluted in autoclaved distilled water (980 mL) to obtain the concentration used (30 mg/L). The literature explores different concentrations.18–20,22 In the clinical trial of Araújo and collaborators,1 the curcumin salt used had 1 g of salt containing 0.654 g of the cucumin plus curcuminoid, but in our study, natural curcumin (curcumin 53.4% and curcuminoid 46.16%) was used.
Treatment for oral disinfection
The volunteers in the PDT group used mouthwash with 20 mL of curcumin solution for 5 min, after which the solution was expelled and a blue light was introduced to activate the curcumin for 5 min. In the same way, the oral cavity of the light group was illuminated with blue light for 5 min and the curcumin group used mouthwash with 20 mL of curcumin solution for 5 min.1 We did not use similar parameters of the blue illumination and curcumin concentration to those applied in in vitro studies, because in vivo studies show complexity regarding variety of biological tissues in the oral cavity and in immunological response.
Microbiological analyses
Two saliva samples from each volunteer were collected at each time point (pretreatment, post-treatment, post 1 h, and post 2 h) and stored in sterile containers. The saliva samples underwent serial dilutions and 100 μL aliquots were plated on Brain Heart Infusion Agar (BHIA) with 10% Sheep Blood (Difco Laboratories, Detroit, MI) plates (in duplicate) and then incubated under microaerophilic conditions for 48 h at ∼36°C. After incubation, the total number of CFUs was determined.1
Statistical analysis
The data were expressed as means and standard deviations. In order to assess the effect of the treatments, CFU/mL values were transformed to logarithm (log10). The Shapiro–Wilk test was used to analyze data normality and the homogeneity of variances using Levene's test. Two way repeated measures ANOVA with post-hoc Tukey tests were used to compare changes in CFUs as a function of time. The independent factors were group (with three levels: PDT, light, and curcumin groups) and time (with four levels: pretreatment, post-treatment, post 1 h, and post 2 h), which was also considered a repeated measurement (intragroup differences). The survival fraction normalized and the delta CFU between the situations before and after the treatments (post-treatment, post 1 h, and post 2 h) was performed for intergroup comparisons using a one-way ANOVA with post-hoc Tukey tests. The Statistica for Windows Release 7 software (Statsoft Inc., Tulsa, OK) was used for the statistical analysis, and the significance level was set at 5% (p<0.05).
Results
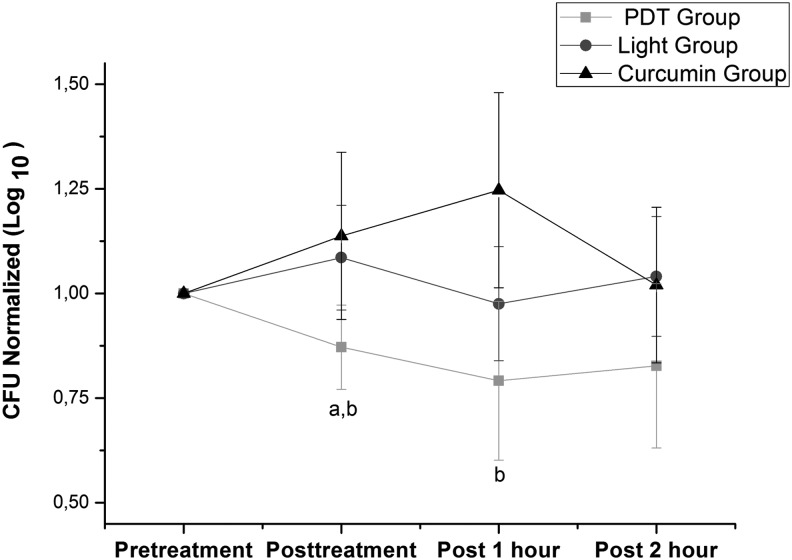
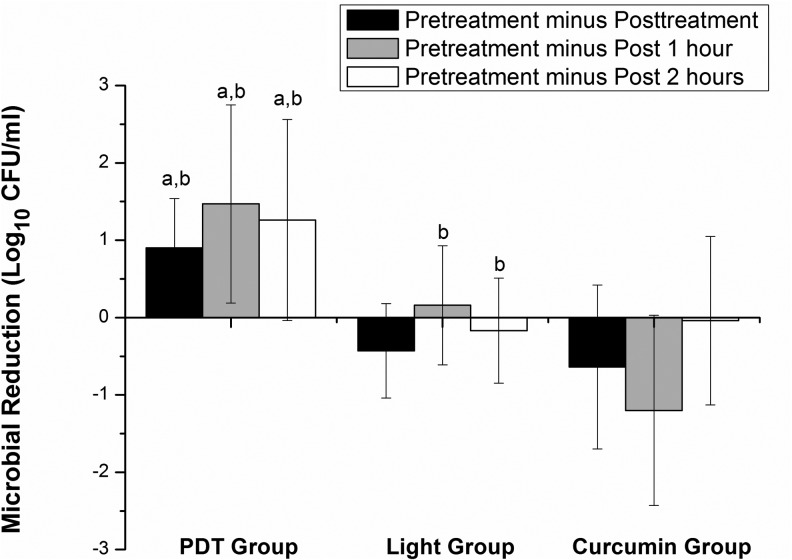
The PDT group showed a significant reduction in CFUs (1 log reduction) at post-treatment, post 1 h, and post 2 h (p<0.01) compared with instance pretreatment. There were no significant changes for the light group. The curcumin group showed a significant increase in CFUs at post 1 h (p<0.05) compared with pretreatment, falling to baseline values at post 2 h. These intragroup differences can be seen in the Table 1. The PDT group showed a significant difference (p<0.05) in both normalized CFUs (Fig. 2) and microbial reduction (Fig. 3) compared with both the light and curcumin groups.
Table 1.
Effects of the Blue LED Illumination With or Without Curcumin
| Pretreatment | Post-treatment | Post 1 h | Post 2 h | |
|---|---|---|---|---|
| PDT group | 6.61±0.82 | 5.71±0.48a | 5.14±0.92a | 5.35±0.76a |
| Light group | 5.67±0.82 | 6.10±0.62 | 5.51±0.93 | 5.84±0.65 |
| Curcumin group | 5.57±0.91 | 6.21±0.58 | 6.77±0.40a | 5.58±0.70b |
Data represent the log10 CFU/mL.
Significant intragroup difference compared with pretreatment (two way ANOVA with post-hoc Tukey, p<0.01).
Significant intragroup difference compared with period immediately before (two way ANOVA with post-hoc Tukey, p<0.05).
LED, light-emitting diode; PDT, photodynamic therapy; CFU, colony-forming units.
FIG. 2.
Normalized colony-forming units (CFU). aSignificant intergroup difference compared with light group (one way ANOVA with post-hoc Tukey, p <0.05). bSignificant intergroup difference compared with curcumin group (one way ANOVA with post-hoc Tukey, p <0.01).
FIG. 3.
Viable counts of colony-forming units (CFU) between pretreatment and after treatment. Significant intergroup difference (one way ANOVA with post-hoc Tukey, p<0.05). The photodynamic therapy (PDT) group showed significant microbial reduction compared with both the lighta and curcuminb groups at pretreatment minus post-treatment (p=0.02 and p=0.03), pretreatment minus post 1 h (p=0.01 and p=0.04), and pretreatment minus post 2 h (p=0.04 and p=0.03). The light group showed significant difference compared with the curcuminb group (p=0.01).
Discussion
The main finding of this study was that the PDT group showed reduction in CFUs immediately post-treatment. Surprisingly, this antimicrobial effect was observed for 1 h after PDT (∼1 log reduction). These findings corroborate study of Araújo et al.,1 which investigated the immediate effects of PDT with blue light and curcumin in a clinical trial. Other studies also showed the positive effects of a-PDT. However, these studies used first and second generation photosensitizers such as porphyrin derivative,24 phthalocyanines,13 chlorine,25 toluidine blue,26 and methylene blue,27 which may target both gram-negative and gram-positive bacteria.
Regarding a-PDT with curcumin and blue light, several in vitro studies were performed. Dovigo et al.18,19 showed that low curcumin concentrations were effective for inactivating Candida albicans when associated with blue LED (450 nm) excitation. In similar studies, Araújo et al.22,28 found reduction of Streptococcus mutans and Lactobacillus acidophilus on planktonic cultures,28 and this reduction was more effective in biofilm compared with carious dentine conditions.22 In a recent study, Pileggi et al.20 showed that curcumin associated with a dental quartz-tungsten-halogen light source, emitting blue light (380–500 nm) inactivated Enterococcus faecalis on planktonic cultures or in biofilm cultures. In another recent study, Panhóca et al.29 performed PDT with blue LED and curcumin associated with surfactant (sodium dodecyl sulfate 0.1%), and showed inactivation of S. mutans in biofilm, optimizing a-PDT. In the same line, Paschoal et al.21 showed that a low concentration of curcumin associated with white light (400–700 nm with a central wavelength of 550 nm) illumination lead to inactivation of S. mutans.
These results with regard to a-PDT are limited to an in vitro model; however, they may support our clinical trial results. Moreover, these in vitro model results also showed that blue light irradiation alone or curcumin alone did not reduce CFU.
Unlike other in vitro studies, the work of Lipovsky et al.30 showed bacterial reduction (Staphylococcus aureus and Escherichia coli) with blue light (415 and 455 nm) alone, mainly at higher fluences (120 J/cm2); however, at low fluences, blue light enhanced bacterial proliferation.31 In addition, Feuerstein et al.14 showed bacterial reduction (P. gingivalis and Fusobacterium nucleaturn) with blue light (450 nm) at fluences of 62, 78, and 94 J/cm2 under aerobic condition; however, this phototoxic effect was not observed when the bacteria were exposed to light under anaerobic conditions. Considering blue LED applications, several clinical trials for treatment of acne showed positive effects of this wavelength.31,32 It is also evidenced by decrease numbers of Propionibacterium acnes in vitro.32 Therefore, the antibacterial effect of blue light is dose dependent as well as dependent upon the response of an organism to O2 in its environment.
Regarding use of curcumin alone, our study showed that CFU increased significantly, suggesting that curcumin probably caused disaggregation of dental plaque clumps on tooth enamel leading to saliva. Curcumin has been tested as a compound to inhibit fibril formation. Rabbie et al.33 observed disaggregation of preformed fibrils upon addition of curcumin. Overall, this compound appears to be able to interact with native, intermediate, and fibrillar forms.34,35 There is a fair amount of support from oral research to suggest that bacteria fibrils are made of protein, and some evidence that suggests that some are even made of glycoprotein. They are difficult to remove, and some strains of oral streptococci have tufts of fibrils (that were grouped together into a new species and given the name “Streptococcus cristae”).36 There is evidence that fibril tufts and coaggregation may also be involved in adhesion, this time to rod-shaped bacteria, to make the structures commonly found in mature dental plaque called “corncob-configuration.”37
Considering the action on adhesion, our result in which curcumin alone increased CFUs significantly, suggests that this mouth rinse disrupts coaggregation bacteria attachment to a tooth surface, leading bacteria to saliva. However, several studies have shown that curcumin and other oral disinfectants reduced CFU. Curcumin mouth rinse may be dependent upon several factors, including duration of fasting28 and time of mouth rinse.38 Although there is a growing number of publications about the effect of curcumin on bacterial reduction,16,18,28,39,41 few publications28,39 such as this study observed the magnitude of curcumin as a mouth rinse.
In an in vitro model, Hegde and Kesaria17 showed that curcumin reduced only C. albicans, and that sodium hypochlorite and neem (Azadirachta indica) were more effective in microbial inactivation, because it reduced both E. faecalis and C. albicans. In a clinical trial, Bhat et al.16 showed that the antimicrobial efficacy of neem (3%) was highest, followed by cetylpyridinium chloride (0.5%), curcumin (5%), and chlorhexidine gluconate (0.2%) when the CFU (S. mutans) reduction was measured. Chlorhexidine is widely used in dentistry for decontamination. In the study by Hayek et al.,41 Prevotella sp., Fusobacterium sp., and Streptococcus beta-haemolyticus were significantly reduced in ligature-induced peri-implantitis in dogs; however, no significant differences were observed between chlorhexidine and PDT with paste-based azulene and GaAlAs laser (λ=660 nm).
The action of curcumin alone as mouth rinse is quite encouraging for a further development of the technique, as prewash plaque remover and the reduction of 1 log for 2 h after PDT is an excellent result, considering the advantages of curcumin being a natural substance and harmless to the oral tissues. Overall, decontamination using a simple procedure is desirable for general use in dentistry. In this context, the reduction of bacterial counts and its maintenance during 2 h are important for several intraoral surgical procedures during a single session. Moreover, bacterial reduction is initiated on superficial surfaces of the oral environment, and after multiples sessions of PDT, deeper layers can be achieved. PDT is cumulatively bactericidal.42 However, some limitations of our study were the small sample size per group, and not having used different procedures for optimizing a-PDT. Future studies should explore these aspects.
Conclusions
In conclusion, in this study, the results indicate that curcumin has a potential to disaggregate oral plaque, and the new blue LED device for PDT-curcumin may be used for reduction of salivary microorganisms lasting 2 h, leading to overall disinfection of the mouth for several intraoral surgical procedures during a single session of dentistry. However, new protocols should be explored to optimize a-PDT.
Acknowledgments
The authors thank the National Council for Scientific and Technological Development (CNPq) - grant no. 573587/2008 and the São Paulo Research Foundation (FAPESP) - grant nos. 2013/07276-1 and 2013/14001-9 for financial support. The authors also acknowledge scientific contributions and helpful advice from Hérica Ricci and Vitor Hugo Panhóca.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Araújo N.C., Fontana C.R., Gerbi M.E., and Bagnato V.S. (2012). Overall-mouth disinfection by photodynamic therapy using curcumin. Photomed. Laser Surg. 30, 96–101 [DOI] [PubMed] [Google Scholar]
- 2.Dai T., Gupta A., Murray C.K., Vrahas M.S., Tegos G.P., and Hamblin M.R. (2012). Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. 15, 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soukos N.S., Som S., Abernethy A.D., Ruggiero K., Dunham J., Lee C, Doukas A.G., and Goodson J.M. (2005). Phototargeting oral black-pigmented bacteria. Antimicrob. Agents Chemother. 49, 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somparn P., Phisalaphong C., Nakornchai S., Unchern S., and Morales N.P. (2007). Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 30, 74–78 [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal B.B., Kumar A., and Bharti A.C. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23, 363–398 [PubMed] [Google Scholar]
- 6.Tajbakhsh S., Mohammadi K., Deilami I., Keivan Z., Fouladvand M., Ramedani E., and Asayesh G. (2008). Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 7, 3832–3835 [Google Scholar]
- 7.Martins C.V., da Silva D.L., Neres A.T., Magalhães T.F., Watanabe G.A., Modolo L.V., Sabino A.A., de Fátima A., and de Resende M.A. (2009). Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 63, 337–339 [DOI] [PubMed] [Google Scholar]
- 8.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., and Zandi K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014:186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatcher H., Planalp R., Cho J., Torti F.M., and Torti S.V. (2008). Curcumin: from ancient medicine to current clinical trials. Cell Mol. Life Sci. 65, 1631–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand P., Kunnumakkara A.B., Newman R.A., and Aggarwal B.B. (2007). Bioavailability of curcumin: problems and promises. Mol Pharm. 4, 807–818 [DOI] [PubMed] [Google Scholar]
- 11.Haukvik T., Bruzell E., Kristensen S., and Tønnesen H.H. (2010). Photokilling of bacteria by curcumin in selected polyethylene glycol 400 (PEG 400) preparations. Studies on curcumin and curcuminoids, XLI. Pharmazie 65, 600–606 [PubMed] [Google Scholar]
- 12.Dai T., Huang Y.Y., and Hamblin M.R. (2009). Photodynamic therapy for localized infections state of the art. Photodiagnosis Photodyn Ther. 6, 170–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longo J.P., Leal S.C., Simioni A.R., de Fátima M.M.A.S., Tedesco A.C., and Azevedo R.B. (2012). Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: an in vitro and clinical study. Lasers Med. Sci. 27, 575–584 [DOI] [PubMed] [Google Scholar]
- 14.Feuerstein O., Ginsburg I., Dayan E., Veler D., and Weiss E.I. (2005). Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. Photochem. Photobiol. 81, 1186–1189 [DOI] [PubMed] [Google Scholar]
- 15.Bumah V.V., Masson–Meyers D.S., Cashin S.E., and Enwemeka C.S. (2013). Wavelength and bacterial density influence the bactericidal effect of blue light on methicillin-resistant Staphylococcus aureus (MRSA). Photomed. Laser Surg. 31, 547–553 [DOI] [PubMed] [Google Scholar]
- 16.Bhat P.K., Badiyani B.K., Sarkar S., Chengappa S., and Bhaskar N.N. (2012). Effectiveness of antimicrobial solutions on Streptococcus mutans in used toothbrushes. World J. Dent. 3, 6–10 [Google Scholar]
- 17.Hegde V., and Kesaria D.P. (2013). Comparative evaluation of antimicrobial activity of neem, propolis, turmeric, liquorice and sodium hypochlorite as root canal irrigants against E. Faecalis and C. Albicans – An in vitro study. Endodontology 25, 38–45 [Google Scholar]
- 18.Dovigo L.N., Pavarina A.C., Carmello J.C., Machado A.L., Brunetti I.L., and Bagnato V.S. (2011). Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg. Med. 43, 927–934 [DOI] [PubMed] [Google Scholar]
- 19.Dovigo L.N., Pavarina A.C., Ribeiro A.P., Brunetti I.L., Costa C.A., Jacomassi D.P., Bagnato V.S., and Kurachi C. (2011). Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 87, 895–903 [DOI] [PubMed] [Google Scholar]
- 20.Pileggi G., Wataha J.C., Girard M., Grad I., Schrenzel J., Lange N., and Bouillaguet S. (2013). Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagnosis Photodyn. Ther. 10, 134–140 [DOI] [PubMed] [Google Scholar]
- 21.Paschoal M.A., Santos–Pinto. L., Lin M., and Duarte S. (2014). Streptococcus mutans photoinactivation by combination of short exposure of a broad-spectrum visible light and low concentrations of photosensitizers. Photomed. Laser Surg. 32, 175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araújo N.C., Fontana C.R., Bagnato V.S., and Gerbi M.E. (2014). Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med. Sci. 29, 629–635 [DOI] [PubMed] [Google Scholar]
- 23.Gursoy H., Ozcakir–Tomruk C., Tanalp J., and Yilmaz S. (2013). Photodynamic therapy in dentistry: a literature review. Clin. Oral Investig. 17, 1113–1125 [DOI] [PubMed] [Google Scholar]
- 24.Fontana C.R., Lerman M.A., Patel N., Grecco C., Costa C.A., Amiji M.M., Bagnato V.S., and Soukos N.S. (2013). Safety assessment of oral photodynamic therapy in rats. Lasers Med. Sci. 28, 479–486 [DOI] [PubMed] [Google Scholar]
- 25.Fekrazad R., Bargrizan M., Sajadi S., and Sajadi S. (2011). Evaluation of the effect of photoactivated disinfection with Radachlorin® against Streptococcus mutans (an in vitro study). Photodiagnosis Photodyn. Ther. 8, 249–253 [DOI] [PubMed] [Google Scholar]
- 26.Kömerik N., Nakanishi H., MacRobert A.J., Henderson B., Speight P., and Wilson M. (2003). In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob. Agents Chemother. 47, 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soukos N.S., Chen P.S., Morris J.T., Ruggiero K., Abernethy A.D., Som S., Foschi F., Doucette S., Bammann L.L., Fontana C.R., Doukas A.G., and Stashenko P.P. (2006). Photodynamic therapy for endodontic disinfection. J. Endod. 32, 979–984 [DOI] [PubMed] [Google Scholar]
- 28.Araújo N.C., Fontana C.R., Bagnato V.S., and Gerbi M.E. (2012). Photodynamic effects of curcumin against cariogenic pathogens. Photomed. Laser Surg. 30, 393–399 [DOI] [PubMed] [Google Scholar]
- 29.Panhóca V.H., Geralde M.C., Corrêa T.Q., Carvalho M.T., Souza C.W.O.S., and Bagnato V.S. (2014). Enhancement of the photodynamic therapy effects on Streptococcus Mutans biofilm. J. Phys. Sci. Applic. 4, 107–114 [Google Scholar]
- 30.Lipovsky A., Nitzan Y., Gedanken A., and Lubart R. (2010). Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers Surg. Med. 42, 467–472 [DOI] [PubMed] [Google Scholar]
- 31.Wheeland R.G., and Koreck A. (2012). Safety and effectiveness of a new blue light device for the self-treatment of mild-to-moderate acne. J. Clin. Aesthet. Dermatol. 5, 25–31 [PMC free article] [PubMed] [Google Scholar]
- 32.Kawada A., Aragane Y., Kameyama H., Sangen Y., and Tezuka T. (2002). Acne phototherapy with a high–intensity, enhanced, narrow-band, blue light source: an open study and in vitro investigation. J. Dermatol. Sci. 30, 129–135 [DOI] [PubMed] [Google Scholar]
- 33.Rabiee A., Ebrahim–Habibi A., Ghasemi A., and Nemat–Gorgani M. (2013). How curcumin affords effective protection against amyloid fibrillation in insulin. Food Funct. 4, 1474–1480 [DOI] [PubMed] [Google Scholar]
- 34.Palmal S., Maity A.R., Singh B.K., Basu S., Jana N.R., and Jana N.R. (2014). Inhibition of amyloid fibril growth and dissolution of amyloid fibrils by curcumin-gold nanoparticles. Chemistry 20, 6184–6191 [DOI] [PubMed] [Google Scholar]
- 35.Marchiani A., Rozzo C., Fadda A., Delogu G., and Ruzza P. (2014). Curcumin and curcumin-like molecules: from spice to drugs. Curr. Med. Chem. 21, 204–222 [DOI] [PubMed] [Google Scholar]
- 36.Handley P.S., Correia F.F., Russell K., Rosan B., and DiRienzo J.M. (2005). Association of a novel high molecular weight, serine-rich protein (SrpA) with fibril-mediated adhesion of the oral biofilm bacterium Streptococcus cristatus. Oral Microbiol. Immunol. 20, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lévesque C., Lamothe J., and Frenette M. (2003). Coaggregation of Streptococcus salivarius with periodontopathogens: evidence for involvement of fimbriae in the interaction with Prevotella intermedia. Oral Microbiol. Immunol. 18, 333–337 [DOI] [PubMed] [Google Scholar]
- 38.Muglikar S., Patil K.C., Shivswami S., and Hegde R. (2013). Efficacy of curcumin in the treatment of chronic gingivitis: a pilot study. Oral Health Prev. Dent. 11, 81–86 [DOI] [PubMed] [Google Scholar]
- 39.Bhawana , Basniwal R.K., Buttar H.S., Jain V.K., and Jain N. (2011). Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. 59, 2056–2061 [DOI] [PubMed] [Google Scholar]
- 40.Vimala K., Varaprasad K., Sadiku R., Ramam K., and Kanny K. (2013). Development of novel protein-Ag nanocomposite for drug delivery and inactivation of bacterial applications. Int. J. Biol. Macromol. 63, 75–82 [DOI] [PubMed] [Google Scholar]
- 41.Hayek R.R., Araújo N.S., Gioso M.A., Ferreira J., Baptista–Sobrinho C.A., Yamada A.M., and Ribeiro M.S. (2005). Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J. Periodontol. 76, 1275–1281 [DOI] [PubMed] [Google Scholar]
- 42.Müller Campanile V.S., Giannopoulou C., Campanile G., Cancela J.A., and Mombelli A. (2013). Single or repeated antimicrobial photodynamic therapy as adjunct to ultrasonic debridement in residual periodontal pockets: clinical, microbiological, and local biological effects. Lasers Med. Sci. 2013May10 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]