Abstract
Purpose: Treatment with topical eye drops for long-standing ocular diseases like allergy can induce detrimental side effects. The purpose of this study was to investigate in vitro cytotoxicity of commercially preserved and unpreserved anti-allergic eye drops on the viability and barrier function of monolayer and stratified human corneal-limbal epithelial cells.
Methods: Cells were treated with unpreserved ketotifen solution, benzalkonium chloride (BAC)-containing anti-allergic drugs (ketotifen, olopatadine, levocabastine) as well as BAC alone. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was used to determine cell viability. Effects of compounds on barrier function were analyzed measuring transepithelial electrical resistance (TEER) to determine paracellular permeability and rose bengal assays to evaluate transcellular barrier formation.
Results: The BAC-preserved anti-allergic formulations and BAC alone significantly reduced cell viability, monolayer cultures being more sensitive to damage by these solutions. Unpreserved ketotifen induced the least diminution in cell viability. The extent of decrease of cell viability was clearly dependent of BAC presence, but it was also affected by the different types of drugs when the concentration of BAC was low and the short time of exposure. Treatment with BAC-containing anti-allergic drugs and BAC alone resulted in increased paracellular permeability and loss of transcellular barrier function as indicated by TEER measurement and rose bengal assays.
Conclusions: The presence of the preservative BAC in anti-allergic eye drop formulations contributes importantly to the cytotoxic effects induced by these compounds. Stratified cell cultures seem to be a more relevant model for toxicity evaluation induced on the ocular surface epithelia than monolayer cultures.
Introduction
The incidence of allergic conjunctival diseases in industrial countries is continuously growing. Various forms of allergic conjunctival diseases have been identified, including seasonal allergic conjunctivitis, perennial allergic conjunctivitis, atopic keratoconjunctivitis, and giant papillary conjunctivitis.1 Topical medications using antihistamine agents like levocabastine or multiple action agents with mast cell stabilizing and antihistaminic properties such as olopatadine and ketotifen are a major form of treatment,2 which can be continued for several months or even a year. This long-term use of eye drops can induce adverse effects on the ocular surface. These detrimental effects can be caused by the anti-allergic active component and, also, preservatives used to prevent multidose eye drop microbial contamination can contribute to ocular surface toxic effects and deleterious reactions when used over long-term periods. Indeed, it has been shown in in vitro and in vivo studies that benzalkonium chloride (BAC), the mostly used preservative, can induce toxic and inflammatory effects on the ocular surface causing ocular discomfort and dry eyes.3–5
To predict the toxicity of topical ophthalmic formulations, the conventional method used is the Draize rabbit eye test.6 However, this method has several disadvantages: a significant number of animals are necessary for testing purposes, rabbits have less effective tearing mechanisms and a nictitating membrane, and there is a considerable inter-laboratory variability in the results.7,8 Consequently, there is a noteworthy demand for the development and validation of new tests to replace this method. One useful alternative to animal models relies on cell cultures. Cell culture models offer the advantage of a defined system, in which parameters and conditions can be easily modified. The results are often more reproducible as compared with in vitro studies with excised animal tissue. Moreover, the use of human cell lines precludes the species related applicability problems that might arise when using animal tissue for in vitro experiments.9 Thus, several corneal, epithelial, and conjunctival cell lines have been used for ocular toxicology.10–12 A limitation of these cell models is that the vast majority of them form a monolayer, a culture state that does not mimic the cellular architecture of the ocular surface where corneal and conjunctival epithelia are stratified. In an attempt to resemble ocular surface epithelium, air-lifting 3-dimensional (3D) cultures of corneal and conjunctival epithelial cells have been established in the last years.13–15
In this study, we investigated the effect on cell viability of several common anti-allergic drugs, some of them containing BAC as preservative, as well as the effect of the preservative alone, analyzing differences in response of monolayer and stratified cell cultures. For these experiments, a human corneal-limbal epithelial cell line that can stratify in culture medium rather than at an air interface16 was used. Moreover, we performed assays focused on functional characteristic of the corneal epithelium after exposure to anti-allergic drugs, namely the barrier function. Maintenance of an effective epithelial barrier on ocular surface requires both transcellular and paracellular exclusion of macromolecules and pathogens. The paracellular barrier is provided by the tight junctions that seal the intercellular space and connect individual epithelial cell membranes.17,18 In addition to this paracellular barrier, recently, a mechanism for transcellular barrier formation at the ocular surface has been proposed and involves interaction of cell surface-associated mucins and their O-glycans with the carbohydrate-binding protein galectin-3.19,20 The effect of anti-allergic drugs on the integrity of both barriers was evaluated by transepithelial electrical resistance (TEER) measurement and rose bengal assays.
Methods
Cell culture and treatments
Telomerase-immortalized human corneal-limbal epithelial (HCLE) cells were previously established16 and kindly provided by Dr. Ilene Gipson. Cells were routinely grown in a keratinocyte serum-free medium (Invitrogen, Carlsbad, CA) supplemented with 25 μg/mL bovine pituitary extract, 0.2 ng/mL epidermal growth factor, 0.4 mM CaCl2, and antibiotics, and maintained at 37°C in 5% CO2. To promote stratification and differentiation, after reaching the confluence, the culture medium was replaced and cells were grown in Dulbecco's modified Eagle's medium (DMEM)/F12 medium supplemented with 10% calf serum and 10 ng/mL epidermal growth factor for 7 days as previously reported.16
Four anti-allergic products, used routinely in allergic conjunctivitis treatment were tested: 0.025% preservative-free ketotifen fumarate (Zaditen, Thea), 0.025% ketotifen fumarate (Bentifen, Thea, 0.01% BAC), 0.1% olopatadine (Opatanol, Alcon, 0.01% BAC), 0.05% levocabastine (Bilina, Esteve, 0.015% BAC). Each formulation was diluted: 1:2 (50%), 1:4 (25%), and 1:10 (10%) in DMEM/F12 medium and the times of exposure were 20 min, 1, 3, and 24 h. Furthermore, different concentrations of BAC (0.005%, 0.0025%, and 0.001%) were analyzed, which correspond to the concentration of BAC contained within the formulations (olopatadine and ketotifen fumarate solutions) after the different dilutions (50%, 25%, and 10%).
MTT assay
Monolayer or stratified cultures were exposed to solutions at different times. After exposure, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) test was used to assess cellular viability as described previously.21,22 Briefly, fresh MTT solution (0.5 mg/mL) was added and cells were incubated for 2 h at 37°C. The cells were then lysed and purple formazan dissolved using dimethyl sulfoxide. Absorbance was measured on the Gen5 plate reader (BioTek, Winooski, VT) at 570 and corrected for background by subtracting the absorbance at 690 nm from that at 570 nm. The mean absorbance values corresponding to the nontreated cells were taken as 100% and results were expressed as a percentage of cell viability compared with the control (nontreated) cells. Experiments were conducted in triplicate.
Transepithelial electrical resistance
TEER was determined on stratified cells grown in Transwell® cell culture inserts with an Evom2 Epithelial Voltohmmeter (World Precision Instruments, Sarasota, FL). Before each measurement, the Evom2 was zeroed according to the manufacturer's instructions. One Transwell insert was left empty as a control to determine the intrinsic resistance of the filter, which was subtracted from all readings. Experiments were performed in triplicate and the TEER (ohm-square centimeters) was calculated by multiplying the measured electrical resistance by the area of the filter (1.12 cm2). Results were expressed as mean±standard deviation.
Rose bengal assays
After incubation with the anti-allergic products, stratified epithelial cell cultures grown on culture chamber slides (Lab-Tek, Naperville, IL) were rinsed with phosphate-buffered saline (PBS), and incubated for 5 min with 0.1% rose bengal (Sigma-Aldrich, St. Louis, MO) in PBS, Ca2+/Mg2+-free, pH 7.4. Rose bengal was then aspirated and the cell layer was examined to assess the extent of dye penetration using a Zeiss Axiovert 200M confocal microscope equipped with an LSM 5 Pascal confocal module (Carl Zeiss Meditec GmbH, Jena, Germany). Pictures were taken at 10×with an AxioCam camera (Carl Zeiss Meditec GmbH). Images were processed further for dye penetration quantification using the ImageJ software (NIH, Bethesda, MD). Uptake is represented as the integrated density of stained areas, and is normalized to control conditions.
Statistical analysis
Statistical comparisons of treated and nontreated control cells were performed using the 1-way ANOVA analysis followed by the Dunnet test. All the statistical analyses were performed using the InStat3 software (GraphPad Software, La Jolla, CA). Differences were considered significant when P values<0.05.
Results
Cell viability assays
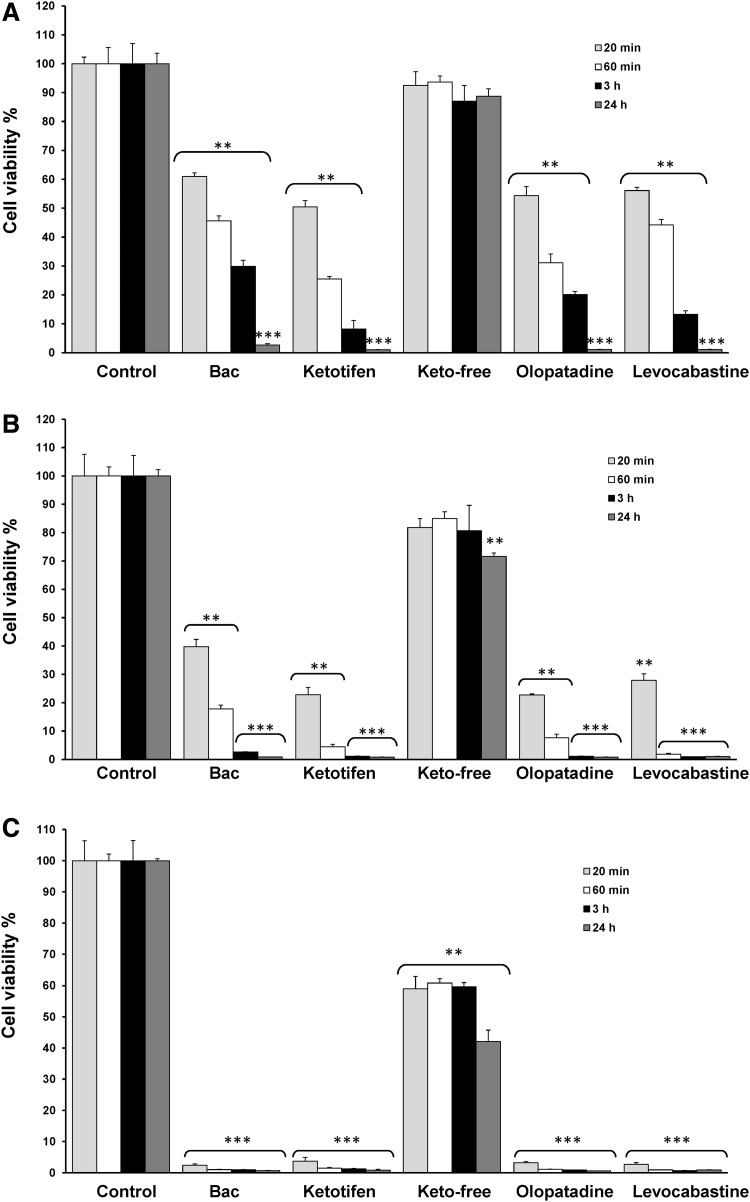
The viability of monolayer cultures after exposure to the anti-allergic formulations at different dilutions is shown in Figure 1. Cell viability significantly decreased to 61.00%, 50.47%, 54.34%, and 56.13% of the control value (P<0.01) for the 0.001% BAC solution and BAC-containing preparations of ketotifen, olopatadine, and levocabastine (all 1:10 diluted), respectively, at 20 min (Fig. 1A). In contrast, treatment with the preservative-free ketotifen did not show this cytotoxic effect and no significant changes in cell viability were detected. Cell viability decreased in a time-dependent manner and values were continuously reduced at a longer time exposure of 1 and 3 h with cell viability markedly decreasing to <5% at 24 h for 0.001% BAC solution and BAC-containing preparations (P<0.001). Only preservative-free ketotifen treatment maintained cell viability values around 90% even at longer exposure times.
FIG. 1.
Effects of anti-allergic formulations on cell viability. Monolayer human corneal-limbal epithelial (HCLE) cells were exposed to solutions diluted at different concentrations: (A) 10% of anti-allergic formulations (diluted 1:10) and 0.001% benzalkonium chloride (BAC), (B) 25% of anti-allergic formulations (diluted 1:4) and 0.0025% BAC, (C) 50% of anti-allergic formulations (diluted 1:2) and 0.005% BAC. The times of exposure were: 20 min (gray bars), 60 min (white bars), 3 h (black bars), and 24 h (dark gray bars). Cell viability was determined with 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay and data were normalized to the control value (100%) and expressed as the mean±standard deviation (n=3). **P<0.01 versus control, ***P<0.001 versus control.
Cytotoxic effects were also concentration-dependent, thus, when BAC-preserved anti-allergic formulations were used at 25% (1:4 diluted) or in the presence of BAC alone (0.0025%), cell viability was reduced to values lower than 40% and the decrease was particularly dramatic with agents used at 50% (1:2 diluted) that diminished cell viability to<5% (Fig. 1B, C). At this highest concentration, preservative-free ketotifen displayed a significantly more deleterious effect and cell viability, decreasing about 60% of the control value at 20 min, 1, and 3 h and 43% at 24 h (P<0.01) (Fig. 1C).
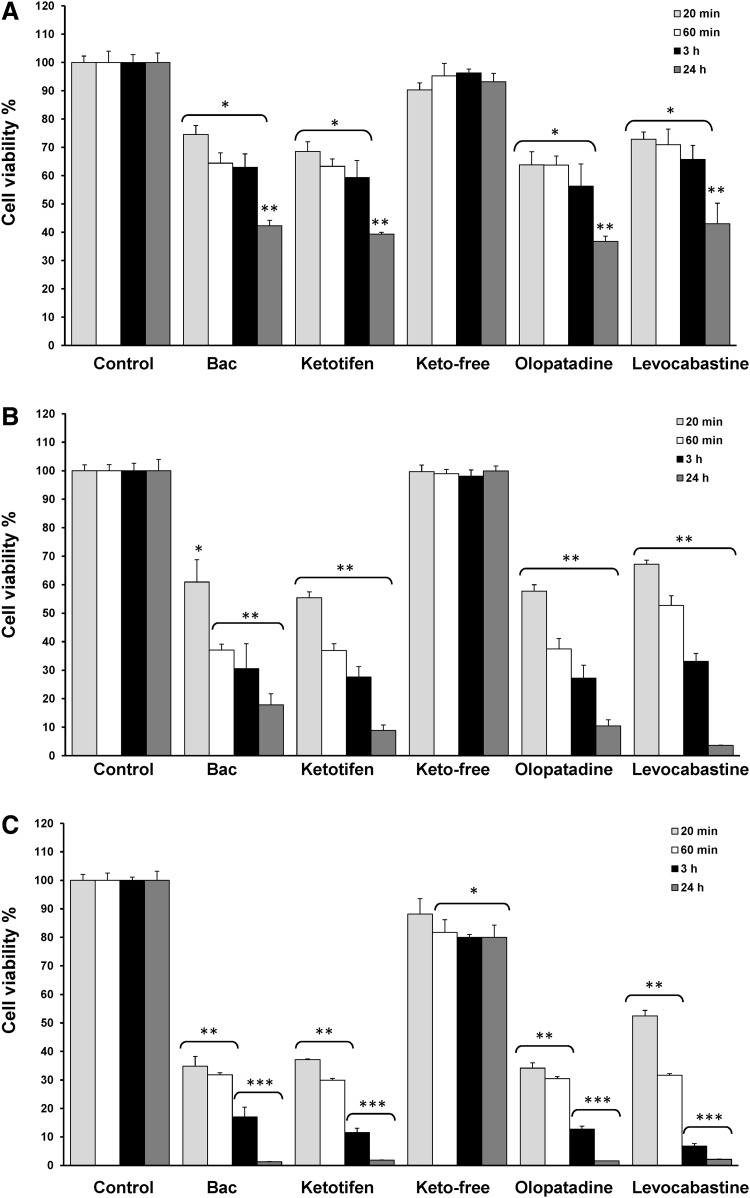
Stratified cell cultures were clearly less sensitive to the effect on cell viability of anti-allergic formulations than monolayer cells (Fig. 2). Thus, after 24 h of cell treatment with 0.001% BAC solution and anti-allergic preserved eye drops (all 1:10 diluted), cell viability levels were around 40% (42.26% for BAC, 39.35% for ketotifen, 36.74% for olopatadine, and 43.01% for levocabastine) (Fig. 2A), whereas values lower than 5% were detected for monolayer cultures (Fig. 1A).
FIG. 2.
Effects of anti-allergic formulations on cell viability. Stratified HCLE cells were exposed to solutions diluted at different concentrations: (A) 10% of anti-allergic formulations (diluted 1:10) and 0.001% BAC, (B) 25% of anti-allergic formulations (diluted 1:4) and 0.0025% BAC, (C) 50% of anti-allergic formulations (diluted 1:2) and 0.005% BAC. The times of exposure were: 20 min (gray bars), 60 min (white bars), 3 h (black bars), and 24 h (dark gray bars). Cell viability was determined with MTT assay and data were normalized to the control value (100%) and expressed as the mean±standard deviation (n=3). *P<0.05 versus control, **P<0.01 versus control, ***P<0.001 versus control.
As expected, the damage tended to be greater when longer time exposures or highest concentrations of preservative-containing anti-allergic solutions were used (Fig. 2B, C). In this sense, assays of cell viability after 20 min of exposure to 0.0025% BAC solution and 25% diluted preservative-containing drugs (1:4 diluted) yielded 60.96% for 0.0025% BAC, 55.39% for ketotifen, 57.71% for olopatadine, and 67.16% for levocabastine, and after exposure to 0.005% BAC solution and 50% diluted preservative-containing drugs (1:2 diluted) cell viability levels were reduced to 34.83% for BAC, 37.11% for ketotifen, 34.18% for olopatadine, and 52.49% for levocabastine (P<0.001 vs. control). Curiously, 25% diluted levocabastine showed relatively less cytotoxicity at 20 min and 1 h of treatment as compared with the other preservative-containing preparations and BAC solution, whereas at longer time exposure (3 and 24 h), its behavior was similar to that found with the other drugs.
Preservative-free ketotifen diluted at 10% and 25% did not induce significant changes in cell viability, only at 50% was possible to detect a slight, although significant, reduction of cell viability (81.69% of the control value at 1 h and around 80% at 3 and 24 h) (P<0.05 vs. control).
Analysis of TEER
Previous toxicological studies have indicated that the multilayer models exhibit a barrier function similar to the intact cornea that monolayer models cannot replicate,23 consequently barrier function studies were performed using stratified cell cultures.
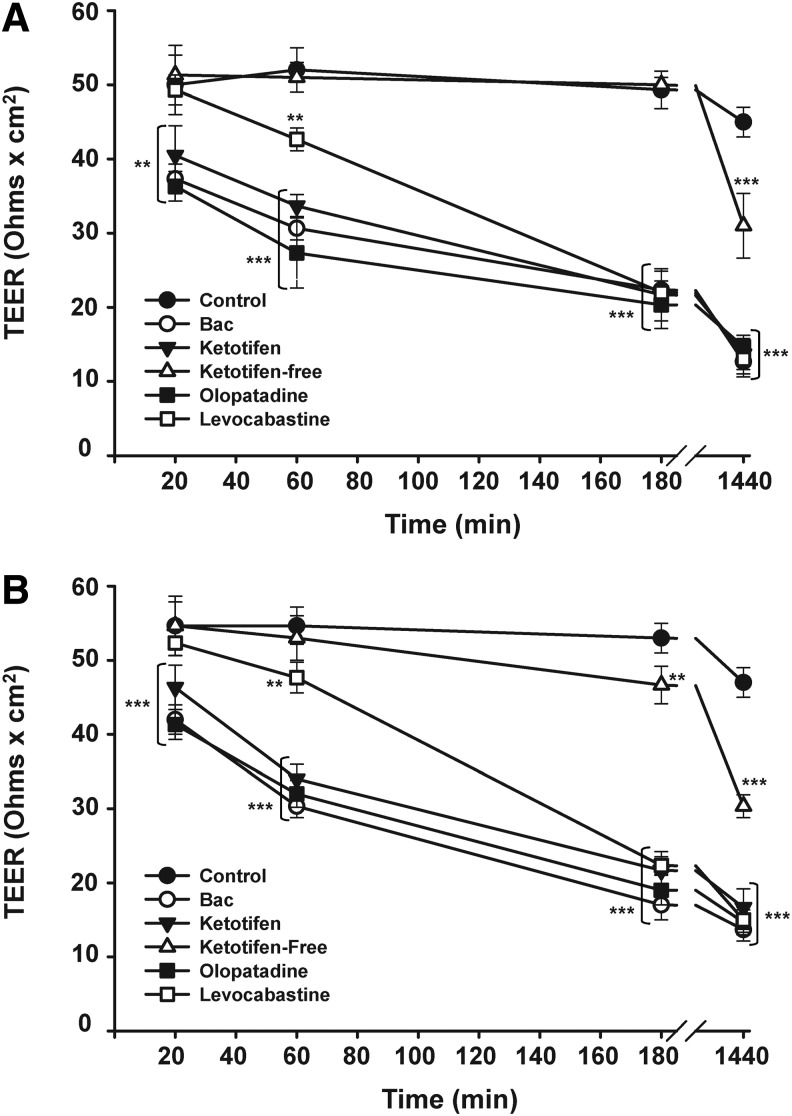
The effect of exposure to the anti-allergic products diluted 1:4 (25%) and 1:10 (10%) on paracellular barrier function provided by tight junctions of stratified layers of HCLE cells was assessed by TEER measurement (Fig. 3). A significant decrease in corneal TEER values was detected after 20 min of exposure to ketotifen, olopatadine, and 0.001% BAC (P<0.01) (Fig. 3A) with TEER values becoming significantly lower at longer time exposures. For instance, after 3 h of exposure, around 50% of reduction on corneal TEER was measured for all BAC-containing anti-allergic products and 0.001% BAC (P<0.001). Treatment with these compounds for 24 h reduced TEER values by 70% as compared with control levels (P<0.001). Levocabastine showed a different behavior at a short time exposure. Thus, levocabastine did not cause changes in corneal TEER after incubation for 20 min and, at 1 h, levocabastine caused a more moderated, although significant, reduction of corneal TEER (18% of reduction) (P<0.01). When the time exposure was increased (3 and 24 h), this compound induced a decrease in corneal TEER comparable to that triggered by the other BAC-containing formulations.
FIG. 3.
Changes in corneal transepithelial electrical resistance (TEER) induced by anti-allergic formulations. Stratified HCLE cells were exposed to commercial eye drops diluted at different concentrations: (A) 10% of anti-allergic formulations (diluted 1:10) and 0.001% BAC, (B) 25% of anti-allergic formulations (diluted 1:4) and 0.0025% BAC. TEER values were determined at 20, 60 min, 3, and 24 h. Each value represents the mean±standard deviation of 3 experiments, **P<0.01 versus control, ***P<0.001 versus control.
In contrast with the results for BAC-containing drugs, corneal TEER was not altered after applying the preservative-free ketotifen within 3 h of the exposure, although it reduced corneal TEER by 30% at 24 h.
Similar results were found when a higher concentration of drugs (25%; diluted 1:4) was used (Fig. 3B). Again, levocabastine induced a slightly more decrease in corneal TEER values at short time incubations as compared with the other preservative-containing formulations. Preservative-free ketotifen significantly modified corneal TEER at 3 h (12% of reduction) and 24 h (35% of reduction); nevertheless this compound clearly exhibited the lowest modification of corneal TEER as compared with the other anti-allergic products containing BAC.
Rose bengal assays
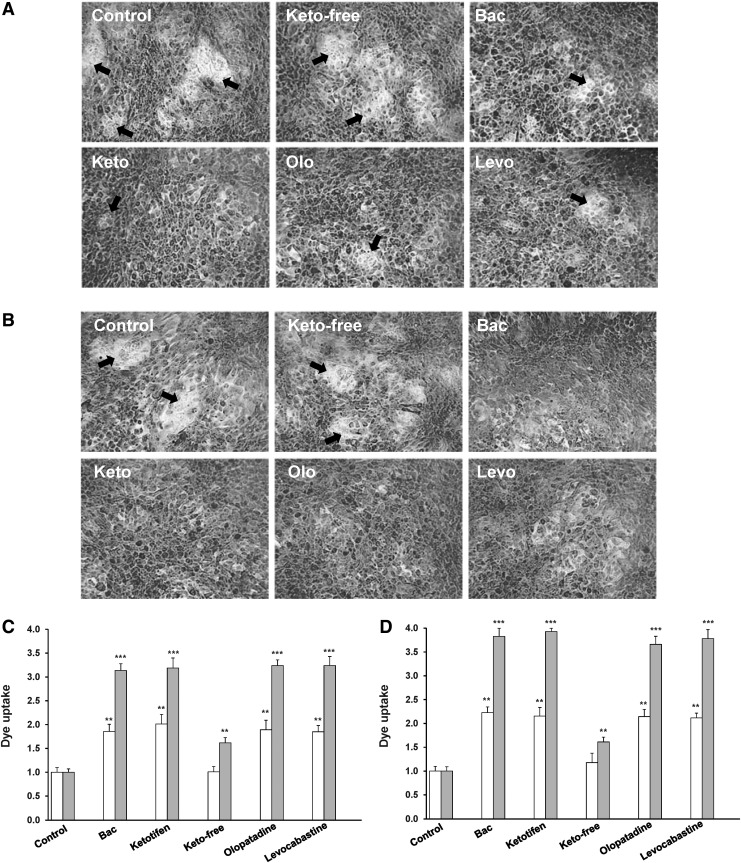
Rose bengal diagnostic dye was used to determine the effect of anti-allergic compounds on transcellular epithelial barrier function.19 In this assay, the appearance of islands that exclude rose bengal and protect from dye penetration into epithelial cells is indicative of the presence of a fully functional mucosal barrier, whereas penetration and positive staining of the epithelia by rose bengal indicates a compromised mucosal barrier. Representative images of rose bengal staining of stratified human corneal epithelial cells after the incubation with anti-allergic drugs, in this case diluted at 10% (1:10) as well as 0.001% BAC for 1 or 3 h are shown in Figure 4A and B, respectively. After 1 h of incubation, the presence of cell regions that excluded rose bengal (islands) was detected in control cells and cells treated with unpreserved ketotifen. In cells treated with 10% preservative-containing drugs and 0.001% BAC, it was also possible to observe small cell regions that were not stained by rose bengal, although these islands were much smaller and less frequent in the culture (Fig. 4A). Indeed, a statistically significant increase in dye uptake compared with control cells was measured (Fig. 4C). When the exposure time was extended to 3 h, unstained islands were only observed in control cells and ketotifen free treated cells (Fig. 4B) and rose bengal uptake significantly increased around 3-fold in cultures incubated with the preservative-containing anti-allergic compounds and BAC as compared with the control and it was higher than the dye uptake detected after 1 h of incubation (Fig. 4C).
FIG. 4.
Rose bengal penetration in control stratified human corneal epithelial cells and cells treated with the anti-allergic formulations. HCLE cells were exposed to anti-allergic solutions and rose bengal penetration was examined by confocal microscopy. Images were obtained using a 10×objective lens and processed for rose bengal penetration quantification using the ImageJ software. Representative images for 10% anti-allergic drugs are shown in the upper panel: (A) after 1 h of exposure and (B) at 3 h of exposure. Arrows indicate cell regions that exclude rose bengal. Bars represent rose bengal uptake (integrated density of stained areas normalized to control conditions) at 1 h (white bars) and 3 h (gray bars) when HCLE cells were exposed to solutions diluted 1:10 (10% of anti-allergic formulations) and 0.001% BAC (C) or diluted 1:4 (25% of anti-allergic formulations) and 0.0025% BAC (D). Experiments were performed in triplicate and represent the mean±standard deviation, **P<0.01 versus control, ***P<0.001 versus control.
Similarly, when cells were exposed to a higher concentration of the drug (anti-allergic drugs diluted at 25%, dil 1:4), a statistically significant increase in dye uptake (2-fold increase at 1 h and around 4-fold increase after 3 h of exposure) was detected for anti-allergic compound containing BAC as well as for the preservative alone at 0.0025% (Fig. 4D).
Discussion
Corneal epithelial cell viability decreased in a concentration- and time-dependent manner with the exposure of topical ocular anti-allergic agents. Our results reveal an increased sensitivity of monolayer cultures to anti-allergic drugs, particularly those containing BAC. Cell viability was markedly reduced to less than 5% at 24 h for BAC-containing drugs used at 10% (1:2 diluted). Under similar experimental conditions, previous studies suggested that olopatadine induced a lower toxicity than ketotifen on monolayer rabbit corneal epithelial cells Statens Seruminstitut Rabbit Cornea.24 On the contrary, our results of cell viability showed a very similar behavior between both compounds. Variations in the method used to determine changes in cell viability (MTT assay vs. lactate dehydrogenase release) as well as the distinct cell model tested may explain the different results found.
Even at the lowest drug concentrations, the decrease in cell viability detected in monolayer cultures was evidently higher than that detected in stratified cell cultures. This lower toxicity of ocular medication and preservatives on stratified cells is consistent with previous studies. Using the same cell line, Lim et al. reported that monolayer cultures were much less resistant to the adverse effects caused by multipurpose contact lens solutions as compared with stratified human corneal-limbal epithelial cells.25 Similar results were found in a study evaluating the preservative-induced toxicity on monolayer and stratified air-lifted cultures, Chang conjunctival cells.15 These findings suggest that multilayer cultures are more realistic models that should be preferentially chosen for in vitro ocular surface toxicology.
In contrast to any preserved anti-allergic drug (ketotifen, olopatadine, levocabastine), unpreserved ketotifen hardly induced cell toxicity, particularly when stratified cultures were examined. This result indicates the basic contribution of the benzalkonium chloride in the cytotoxic effects induced by anti-allergic drugs. In fact, treatment with the preservative alone caused a cell viability reduction similar to that disclosed by BAC-containing anti-allergic preparations especially at higher concentrations and longer exposures. These results are consistent with previous studies where cell viability was more affected in anti-allergic drugs containing BAC than those without the preservative.24,26–28 Similarly, BAC-containing drugs for the treatment of other chronic ocular pathologies like glaucoma induced a higher cytotoxicity than those preservative free.14,29–31 Indeed, the cytotoxic effects induced by BAC on several monolayer conjunctival or corneal epithelial cell models as well as on 3D model of human corneal epithelium have been previously established.32–34
Nevertheless, on the basis of our results, the cytotoxic effects induced by the anti-allergic drugs were not exclusively due to the preservative. When monolayer or stratified cultures were exposed for 20 min to ketotifen and olopatadine at the rate of dilution of 10% or 25%, both showed greater toxicity than the respective BAC concentrations (0.001% and 0.0025%) alone. Moreover, differences can be observed between anti-allergic drugs when low concentrations of BAC are present in the formulations and shorter times of exposures. Thus, levocabastine at lower concentration (10% and 25%) and shorter exposures (20 and 60 min) induced a less pronounced decrease in the viability of stratified human corneal epithelial cells as compared with BAC-containing ketotifen or olopatadine. Levocabastine is a selective histamine-1 receptor antagonist, whereas ketotifen and olopatadine have not only antihistaminic properties but also they stabilize mast cells. This different mechanism of action could be related to the different behavior on cell viability exhibited by levocabastine at lower concentrations. Moreover, as commercial eye drops were used in this study, we cannot rule out a possible protective effect of other additives present in the commercial formulation. Although this experimental approach limits the identification of the individual effects of each one of these components, the use of products currently prescribed for allergic conjunctivitis treatment, reinforces the clinical significance of the study.
Ocular and corneal health is maintained by the selective permeability and barrier function provided by the corneal epithelium. Transepithelial electric resistance was used to evaluate the expression of functional tight junctions sealing the paracellular pathway after treatment with anti-allergic drugs. During the initial times of exposures (20 and 60 min), TEER decreased significantly after treatment with the BAC-containing olopatadine, ketotifen, and the BAC alone. Similar to the results of cell viability of stratified cultures, TEER decrease triggered by levocabastine at shorter exposures was lower than that caused by the other anti-allergic preserved eye drops or by the preservative alone. Previous reports have suggested that the decrease in TEER is clearly dependent on the presence of BAC, however, this parameter may also be affected by the type of drug when the preservative concentration is low.35 In these experiments, the drug Alegysal® with a percentage of BAC equal to the other anti-allergic drugs scarcely decreased the corneal TEER.
When the time of exposure increased, all preserved anti-allergic drugs, including levocabastine, markedly reduced corneal TEER. Consistent with these results, it has been described as the disturbance of occludin, a tight-junction protein distribution in a human 3D-reconstituted corneal epithelial model after treatment with BAC-containing anti-allergic dugs and 0.01% BAC for 24 h.28 In vitro and in vivo studies have demonstrated that BAC can alter the expression and distribution of tight junction proteins in the corneal epithelium.4,34 Confirming this notion, exposure to BAC induces a continuous decline in TEER of rabbit corneas.36,37
In addition to analyzing the effect of anti-allergic drugs on the paracellular barrier formed by tight junctions, to our knowledge, this is the first study to compare the action of these drugs on the recently described apical epithelial barrier provided by cell surface-associated mucins.
A marked increase in rose bengal penetration, indicative of mucin barrier disruption, was detected after treatment with all BAC-containing compounds, whereas islands that exclude the dye remained in cells exposed to ketotifen free. These results suggest that the presence of BAC can compromise mucosal barrier. Supporting this notion, exposure of immortalized human corneal-limbal epithelial cells to 0.0025% BAC (the same concentration used to in our assays when the drug is diluted 1:4) resulted in a significant decrease of MUC1 and MUC16 protein levels.38 Likewise, patients treated with Ocuflox® eye drops containing 0.0025% BAC for 1 week showed a lower protein level of mucins compared with control subjects.38
Considering our experimental data, it is evident that BAC-containing anti-allergic drugs, apart from reducing cell viability, can also alter both paracellular and transcellular barrier function of the corneal epithelium, which could increase susceptibility to possible processes of ocular surface damage. Definitely, unpreserved ketotifen was the least toxic formulation that strongly supports the use of this benzalkonium-free solution in the treatment of patients suffering from allergic conjunctivitis. Thus, preservative-free anti-allergic medications should be the first choice to lower adverse effects on the ocular surface, improving the tolerability and quality of life of patients with ocular allergic diseases.
In addition, we have shown that monolayer cultures could overpredict the toxicity of ocular medications and preservatives and stratified cultures are more physiologically relevant as an in vitro test system. In this sense, the use of the HCLE cell line able to stratify in culture medium rather than at air interface could be particularly valuable as it resembles the corneal epithelium in vivo that is not at an air interface, but it is covered by the tear film. Thus, the use of HCLE cell model could represent an advantageous alternative with respect to the existing air-lifted models to study the bioactivity of eye drops in the cornea.
Acknowledgments
The authors thank Dr. Ilene Gipson, Schepens Eye Research Institute, Massachusetts Eye and Ear, Harvard Medical School, Boston, MA, for providing the HCLE cells. This work was supported by the Ministry of Economy (Project SAF 2010/16024 and SAF2013/44416R) and the Institute Carlos III (RETICS RD12/0034/0003).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kari O., and Saari K.M.Diagnostics and new developments in the treatment of ocular allergies. Curr. Allergy Asthma Rep. 12:232–239, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Mishra G.P., Tamboli V., Jwala J., and Mitra A.K.Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat. Inflamm. Allergy Drug Discov. 5:26–36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudouin C., Labbe A., Liang H., Pauly A., and Brignole-Baudouin F.Preservatives in eyedrops: the good, the bad and the ugly. Prog. Retin. Eye Res. 29:312–334, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Chen W., Li Z., Hu J., Zhang Z., Chen L., Chen Y., and Liu Z.Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. 6:e26103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Z., He H., Zhou T., Liu X., Wang Y., He H., Wu H., and Liu Z.A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest. Ophthalmol. Vis. Sci. 54:6314–6325, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Draize J.H., Woodard G., and Calvery H.O.Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 82:377–390, 1944 [Google Scholar]
- 7.Curren R.D., and Harbell J.W.Ocular safety: a silent (in vitro) success story. Altern. Lab. Anim. 30Suppl 2:69–74, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Wilhelmus K.R.The Draize eye test. Surv. Ophthalmol. 45:493–515, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hornof M., Toropainen E., and Urtti A.Cell culture models of the ocular barriers. Eur. J. Pharm. Biopharm. 60:207–225, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ammar D.A., Noecker R.J., and Kahook M.Y.Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv. Ther. 27:837–845, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Ayaki M., Yaguchi S., Iwasawa A., and Koide R.Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin. Exp. Ophthalmol. 36:553–559, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Epstein S.P., Chen D., and Asbell P.A.Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 25:415–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doucet O., Lanvin M., Thillou C., Linossier C., Pupat C., Merlin B., and Zastrow L.Reconstituted human corneal epithelium: a new alternative to the Draize eye test for the assessment of the eye irritation potential of chemicals and cosmetic products. Toxicol. In Vitro. 20:499–512, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Liang H., Pauly A., Riancho L., Baudouin C., and Brignole-Baudouin F.Toxicological evaluation of preservative-containing and preservative-free topical prostaglandin analogues on a three-dimensional-reconstituted corneal epithelium system. Br. J. Ophthalmol. 95:869–875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanochko G.M., Khoh-Reiter S., Evans M.G., and Jessen B.A.Comparison of preservative-induced toxicity on monolayer and stratified Chang conjunctival cells. Toxicol. In Vitro. 24:1324–1331, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Gipson I.K., Spurr-Michaud S., Argueso P., Tisdale A., Ng T.F., and Russo C.L.Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 44:2496–2506, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Anderson J.M., Balda M.S., and Fanning A.S.The structure and regulation of tight junctions. Curr. Opin. Cell Biol. 5:772–778, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Ban Y., Dota A., Cooper L.J., Fullwood N.J., Nakamura T., Tsuzuki M., Mochida C., and Kinoshita S.Tight junction-related protein expression and distribution in human corneal epithelium. Exp. Eye Res. 76:663–669, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Argueso P., Guzman-Aranguez A., Mantelli F., Cao Z., Ricciuto J., and Panjwani N.Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J. Biol. Chem. 284:23037–23045, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman-Aranguez A., Woodward A.M., Pintor J., and Argueso P.Targeted disruption of core 1 beta1,3-galactosyltransferase (C1galt1) induces apical endocytic trafficking in human corneal keratinocytes. PLoS One. 7:e36628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denizot F., and Lang R.Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 89:271–277, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Mosmann T.Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65:55–63, 1983 [DOI] [PubMed] [Google Scholar]
- 23.Ward S.L., Walker T.L., and Dimitrijevich S.D.Evaluation of chemically induced toxicity using an in vitro model of human corneal epithelium. Toxicol. In Vitro. 11:121–139, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Lee J.S., Lee J.E., Kim N., and Oum B.S.Comparison of the conjunctival toxicity of topical ocular antiallergic agents. J. Ocul. Pharmacol. Ther. 24:557–562, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Lim M.J., Hurst R.K., Konynenbelt B.J., and Ubels J.L.Cytotoxicity testing of multipurpose contact lens solutions using monolayer and stratified cultures of human corneal epithelial cells. Eye Contact Lens. 35:287–296, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Ayaki M., Iwasawa A., Yaguchi S., and Koide R.Preserved and unpreserved 12 anti-allergic ophthalmic solutions and ocular surface toxicity: in vitro assessment in four cultured corneal and conjunctival epithelial cell lines. Biocontrol Sci. 15:143–148, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Ayaki M., Iwasawa A., Yaguchi S., and Koide R.In vitro assessment of the cytotoxicity of anti-allergic eye drops using 5 cultured corneal and conjunctival cell lines. J. Oleo Sci. 60:139–144, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Pauly A., Brasnu E., Riancho L., Brignole-Baudouin F., and Baudouin C.Multiple endpoint analysis of BAC-preserved and unpreserved antiallergic eye drops on a 3D-reconstituted corneal epithelial model. Mol. Vis. 17:745–755, 2011 [PMC free article] [PubMed] [Google Scholar]
- 29.Brasnu E., Brignole-Baudouin F., Riancho L., Guenoun J.M., Warnet J.M., and Baudouin C.In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr. Eye Res. 33:303–312, 2008 [DOI] [PubMed] [Google Scholar]
- 30.De Saint Jean M., Debbasch C., Brignole F., Rat P., Warnet J.M., and Baudouin C.Toxicity of preserved and unpreserved antiglaucoma topical drugs in an in vitro model of conjunctival cells. Curr. Eye Res. 20:85–94, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Pellinen P., Huhtala A., Tolonen A., Lokkila J., Maenpaa J., and Uusitalo H.The cytotoxic effects of preserved and preservative-free prostaglandin analogs on human corneal and conjunctival epithelium in vitro and the distribution of benzalkonium chloride homologs in ocular surface tissues in vivo. Curr. Eye Res. 37:145–154, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Debbasch C., Brignole F., Pisella P.J., Warnet J.M., Rat P., and Baudouin C.Quaternary ammoniums and other preservatives' contribution in oxidative stress and apoptosis on Chang conjunctival cells. Invest. Ophthalmol. Vis. Sci. 42:642–652, 2001 [PubMed] [Google Scholar]
- 33.Epstein S.P., Ahdoot M., Marcus E., and Asbell P.A.Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 25:113–119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pauly A., Meloni M., Brignole-Baudouin F., Warnet J.M., and Baudouin C.Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: early detection of toxic damage. Invest. Ophthal. Vis. Sci. 50:1644–1652, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Nakashima M., Nakamura T., Teshima M., To H., Uematsu M., Kitaoka T., Taniyama K., Nishida K., Nakamura J., and Sasaki H.Breakdown evaluation of corneal epithelial barrier caused by antiallergic eyedrops using an electrophysiologic method. J. Ocul. Pharmacol. Ther. 24:43–51, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kusano M., Uematsu M., Kumagami T., Sasaki H., and Kitaoka T.Evaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivo. Cornea. 29:80–85, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T., Yamada M., Teshima M., Nakashima M., To H., Ichikawa N., and Sasaki H.Electrophysiological characterization of tight junctional pathway of rabbit cornea treated with ophthalmic ingredients. Biol. Pharm. Bull. 30:2360–2364, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Chung S.H., Lee S.K., Cristol S.M., Lee E.S., Lee D.W., Seo K.Y., and Kim E.K.Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol. Vis. 12:415–421, 2006 [PubMed] [Google Scholar]