Abstract
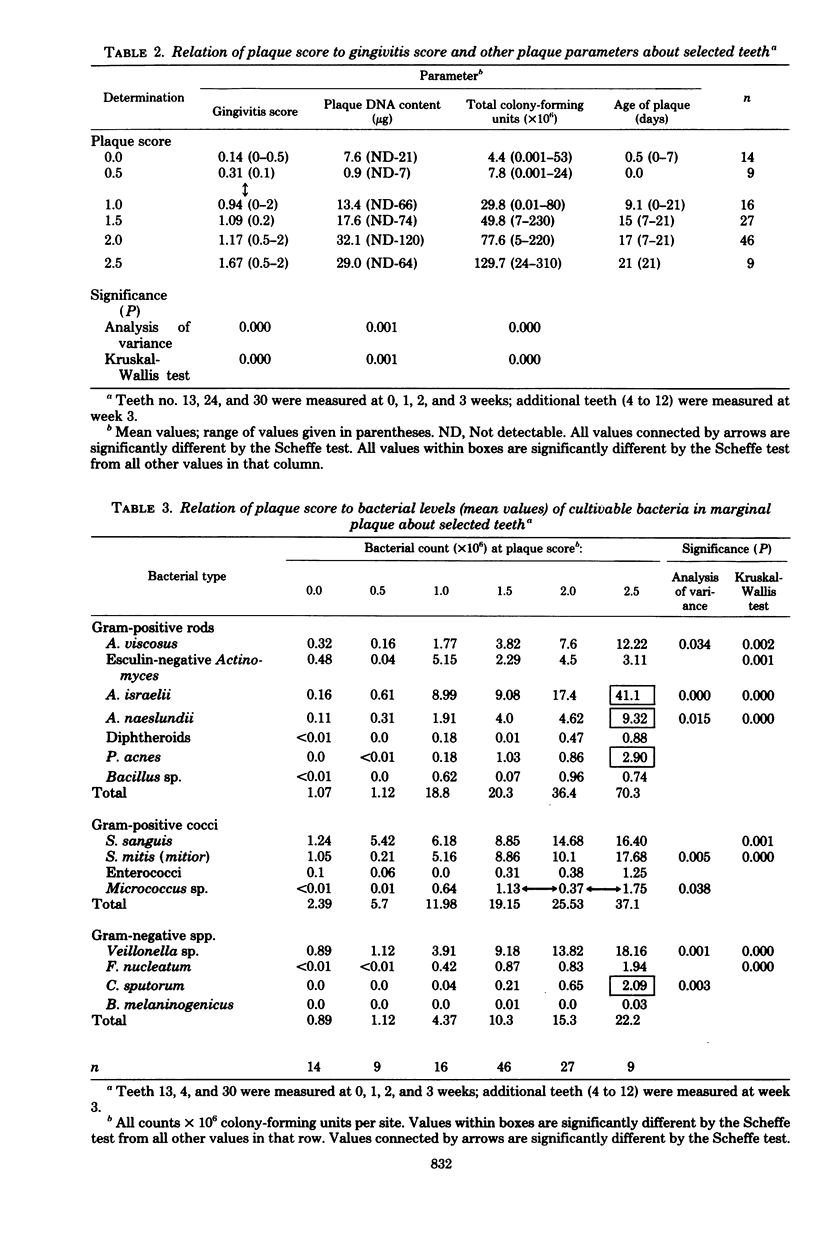
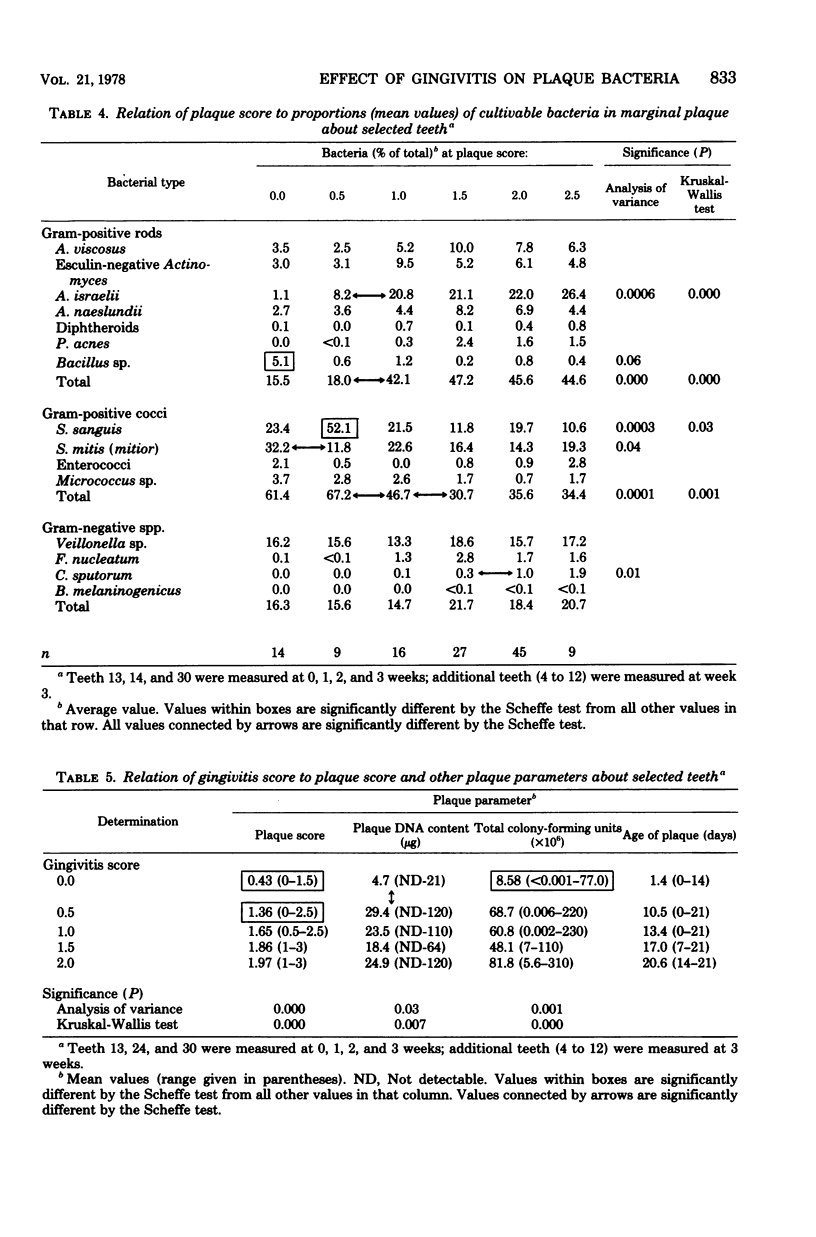
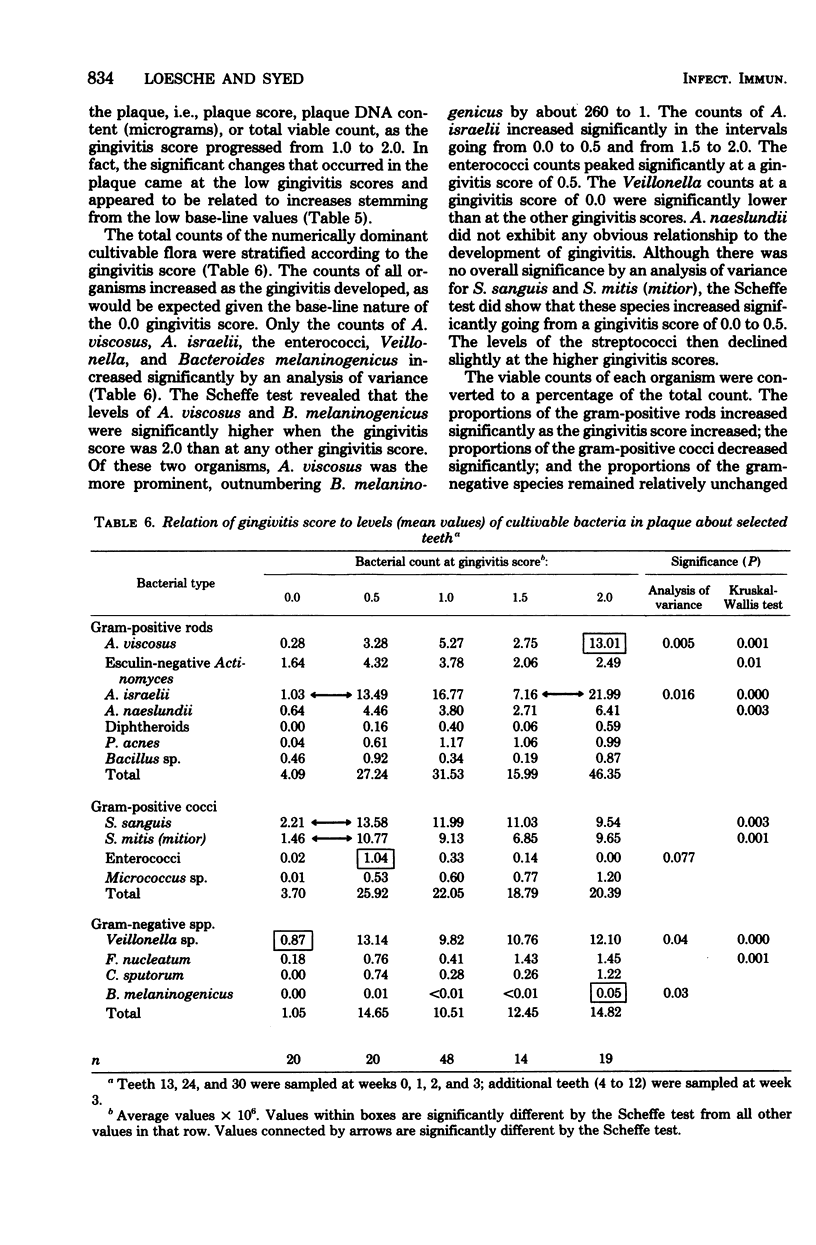
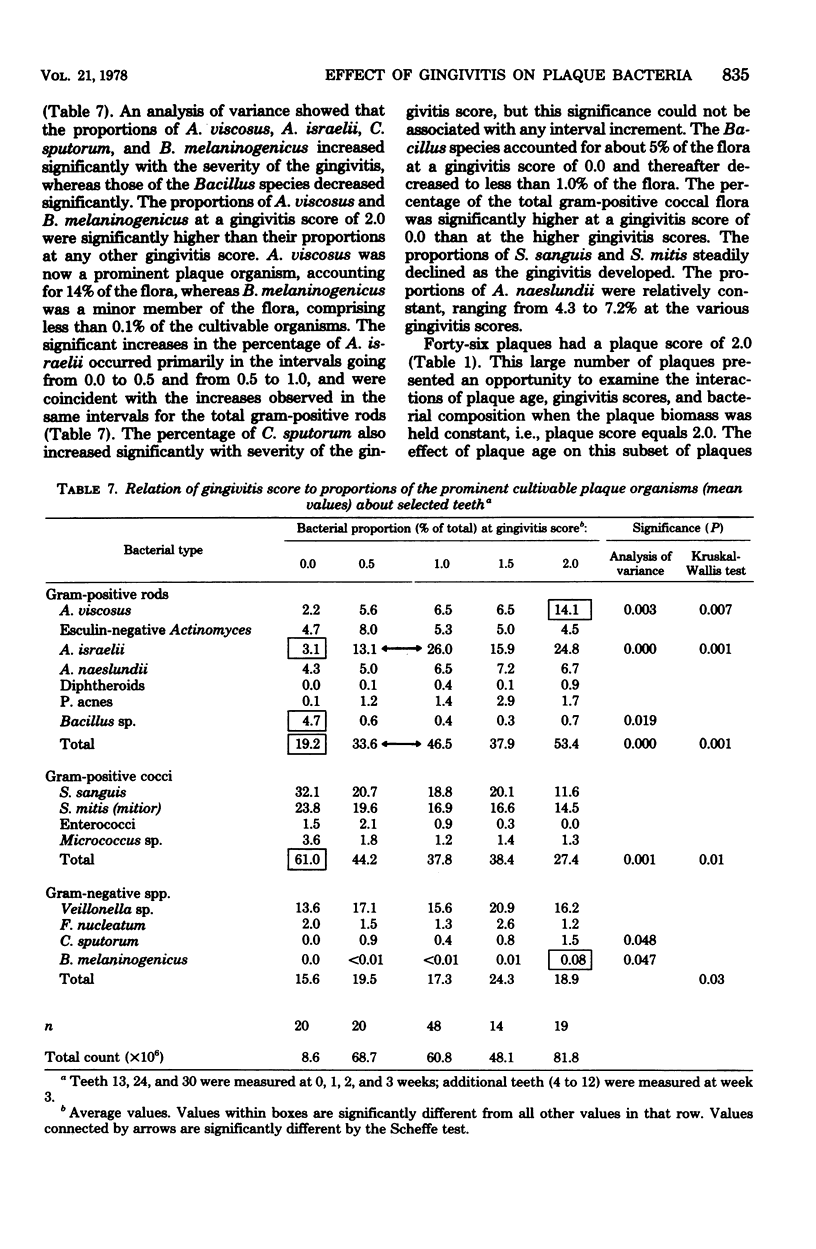
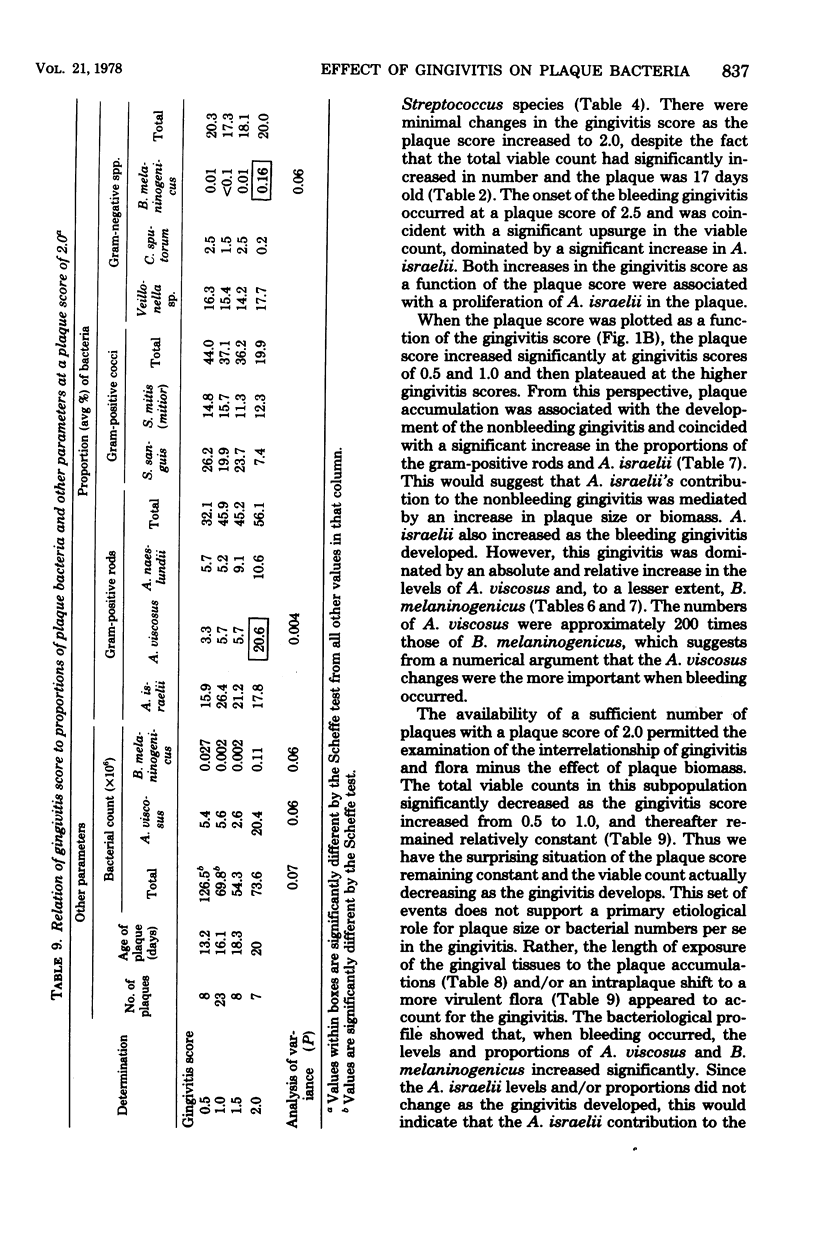
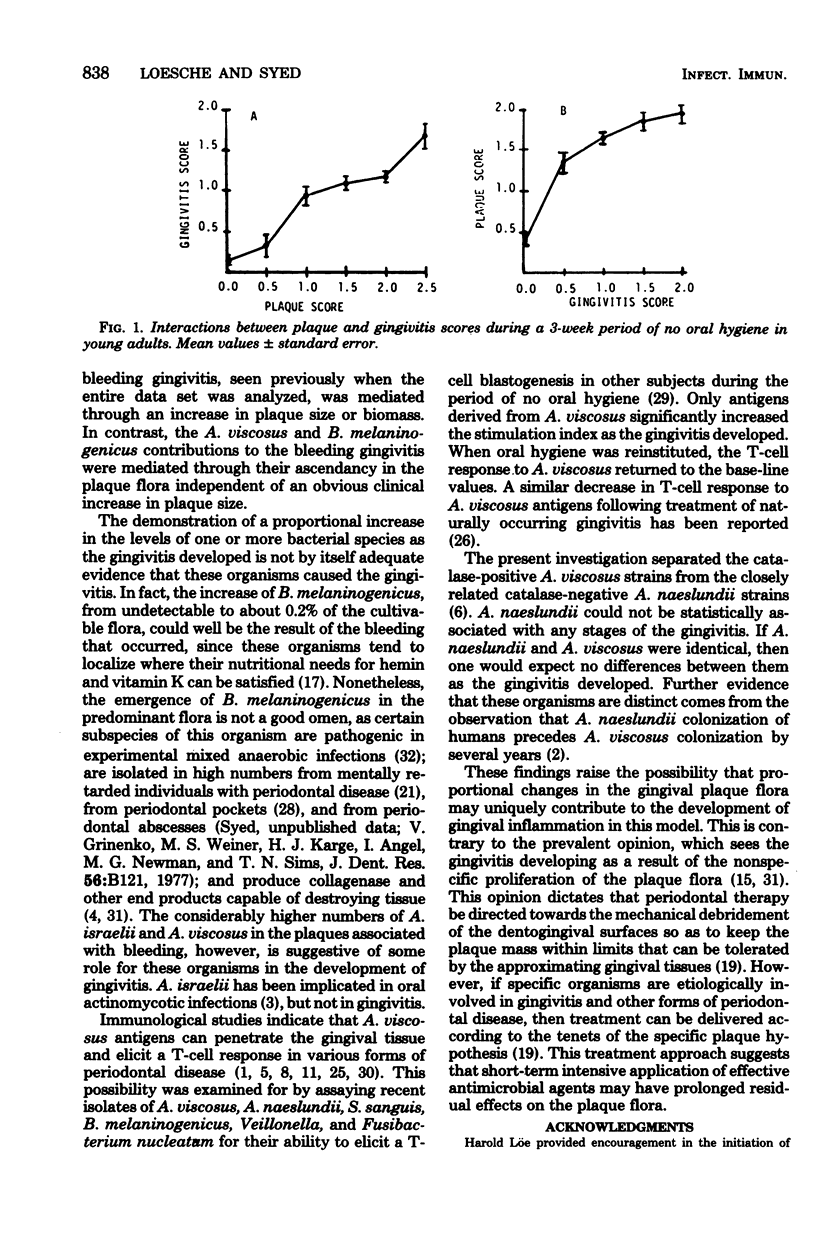
The plaque flora isolated from discrete dentogingival sites during a human gingivitis experiment was analyzed as a function of the plaque score and of the gingivitis score. When the gingivitis score was plotted as a function of the plaque score, a nonbleeding gingivitis was associated with a proportional increase in the Actinomyces sp. at the expense of the Streptococcus sp. In particular, the percentage of Actinomyces israelii increased significantly, while the percent Streptococcus sanguis decreased significantly. A. israelii also increased significantly when a bleeding gingivitis developed. When the plaque score was plotted as a function of the gingivitis score, A. israelii increased significantly as the nonbleeding gingivitis developed, but A. viscosus and Bacteroides melaninogenicus increased significantly when the bleeding gingivitis developed. The availability of a sufficient number of plaques with a plaque score of 2.0 permitted the examination of the interrelationship of gingivitis and flora minus the effect of plaque biomass. The bacteriological profile showed that when bleeding occurred, the levels and proportions of A. viscosus and B. melaninogenicus increased significantly. These findings raise the possibility that proportional changes in the gingival plaque flora may uniquely contribute to the development of gingival inflammation in this experimental model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. J., Chan S. P., Socransky S. S., Oppenheim J. J., Mergenhagen S. E. Importance of Actinomyces and certain gram-negative anaerobic organisms in the transformation of lymphocytes from patients with periodontal disease. Infect Immun. 1976 May;13(5):1363–1368. doi: 10.1128/iai.13.5.1363-1368.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Degradation of collagenous substrates by Bacteroides melaninogenicus. J Bacteriol. 1961 Apr;81:614–621. doi: 10.1128/jb.81.4.614-621.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Pedersen P., Agerbaek N., Theilade E. Experimental gingivitis in young and elderly individuals. J Clin Periodontol. 1975 Feb;2(1):14–24. doi: 10.1111/j.1600-051x.1975.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Numerical taxonomy and laboratory identification of Bacterionema matruchotii, Rothia dentocariosa, Actinomyces naeslundii, Actinomyces viscosus, and some related bacteria. J Gen Microbiol. 1973 May;76(1):43–63. doi: 10.1099/00221287-76-1-43. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch Oral Biol. 1970 Nov;15(11):1089–1096. doi: 10.1016/0003-9969(70)90121-4. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H., Bellack S. Periodontal lesions in hamsters and gnotobiotic rats infected with actinomyces of human origin. J Periodontal Res. 1972;7(1):21–28. doi: 10.1111/j.1600-0765.1972.tb00627.x. [DOI] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Lang N. P., Cumming B. R., Löe H. Toothbrushing frequency as it relates to plaque development and gingival health. J Periodontol. 1973 Jul;44(7):396–405. doi: 10.1902/jop.1973.44.7.396. [DOI] [PubMed] [Google Scholar]
- Lang N. P., Smith F. N. Lymphocyte blastogenesis to plaque antigens in human periodontal disease. I. Populations of varying severity of disease. J Periodontal Res. 1977 Jul;12(4):298–309. doi: 10.1111/j.1600-0765.1977.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Lehner T., Wilton J. M., Challacombe S. J., Ivanyi L. Sequential cell-mediated immune responses in experimental gingivitis in man. Clin Exp Immunol. 1974 Mar;16(3):481–492. [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Hockett R. N., Syed S. A. The predominant cultivable flora of tooth surface plaque removed from institutionalized subjects. Arch Oral Biol. 1972 Sep;17(9):1311–1325. doi: 10.1016/0003-9969(72)90164-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Importance of nutrition in gingival crevice microbial ecology. Periodontics. 1968 Dec;6(6):245–249. [PubMed] [Google Scholar]
- Loesche W. J., Nafe D. Reduction of supragingival plaque accumulations in institutionalized Down's syndrome patients by periodic treatment with topical kanamycin. Arch Oral Biol. 1973 Sep;18(9):1131–1143. doi: 10.1016/0003-9969(73)90087-3. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Murray R. J., Mellberg J. R. Effect of topical acidulated phosphate fluoride on percentage of Streptococcus mutans and Streptococcus sanguis in plaque. II. Pooled occulusal and pooled approximal samples. Caries Res. 1975;9(2):139–155. doi: 10.1159/000260153. [DOI] [PubMed] [Google Scholar]
- Loesche W., Green E. Comparison of various plaque parameters in individuals with poor oral hygiene. J Periodontal Res. 1972;7(2):173–179. doi: 10.1111/j.1600-0765.1972.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol. 1967 Nov-Dec;38(6 Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Mühlemann H. R., Son S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv Odontol Acta. 1971 Oct;15(2):107–113. [PubMed] [Google Scholar]
- Nisengard R. J. The role of immunology in periodontal disease. J Periodontol. 1977 Sep;48(9):505–516. doi: 10.1902/jop.1977.48.9.505. [DOI] [PubMed] [Google Scholar]
- Patters M. R., Sedransk N., Genco R. J. Lymphoproliferative response during resolution and recurrence of naturally occurring gingivitis. J Periodontol. 1977 Jul;48(7):373–380. doi: 10.1902/jop.1977.48.7.373. [DOI] [PubMed] [Google Scholar]
- Payne W. A., Page R. C., Ogilvie A. L., Hall W. B. Histopathologic features of the initial and early stages of experimental gingivitis in man. J Periodontal Res. 1975 May;10(2):51–64. doi: 10.1111/j.1600-0765.1975.tb00008.x. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J. REQUIRED ROLE OF BACTEROIDES MELANINOGENICUS IN MIXED ANAEROBIC INFECTIONS. J Infect Dis. 1965 Jun;115:247–253. doi: 10.1093/infdis/115.3.247. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Smith F. N., Lang N. P., Löe H. A. Cell mediated immune responses to plaque antigens during experimental gingivitis in man. J Periodontal Res. 1978 May;13(3):232–239. doi: 10.1111/j.1600-0765.1978.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Snyderman R. Immunological mechanisms of periodontal tissue destruction. J Am Dent Assoc. 1973 Oct;87(5):1020–1026. doi: 10.14219/jada.archive.1973.0002. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]


