Abstract
Most of biological oxygen reduction is catalyzed by the heme-copper oxygen reductases. These enzymes are redox-driven proton pumps that take part in generating the proton gradient in both prokaryotes and mitochondria that drives synthesis of ATP. The enzymes have been divided into three evolutionarily related groups: the A-, B- and C-families. Recent comparative studies suggest that all oxygen reductases perform the same chemistry for oxygen reduction and comprise the same essential elements of the proton pumping mechanism, such as the proton loading and kinetic gating sites, which, however, appear to be different in different families. All species of the A-family, however, demonstrate remarkable similarity of the central processing unit of the enzyme, as revealed by their recent crystal structures. Here we demonstrate that cytochrome c oxidases (CcO) of such diverse organisms as a mammal (bovine heart mitochondrial CcO), photosynthetic bacteria (Rhodobacter sphaeroides CcO), and soil bacteria (Paracoccus denitrificans CcO) are not only structurally similar, but almost identical in microscopic electrostatics and thermodynamics properties of their key amino-acids. By using pKa calculations of some of the key residues of the catalytic site, D- and K- proton input, and putative proton output channels of these three different enzymes, we demonstrate that the microscopic properties of key residues are almost identical, which strongly suggests the same mechanism in these species. The quantitative precision with which the microscopic physical properties of these enzymes have remained constant despite different evolutionary routs undertaken is striking.
Keywords: heme-copper oxygen reductases, cytochrome c oxidase, electron transfer, proton transfer, pKa calculations, redox potentials
1. Introduction
The solution of the structure of Cytochrome c Oxidase (CcO) from several organisms, and the followed rapid advances in understanding the molecular mechanism of its proton pumping have prompted a discussion of intriguing evolutionary issues of the super-family of heme-Cu oxidases to which Cytochrome oxidase belongs. These enzymes are redox-driven proton pumps that generate the proton gradient in both prokaryotes and mitochondria that drives synthesis of ATP. In light of non-trivial mechanistic principles that the enzyme appear to be using, and overall complexity of the mechanism (four electrons and eight protons are involved in one turnover) by which oxygen reduction is coupled to proton translocation (see refs. 1-4), the evolutionary aspects of the proton pumping mechanism become particularly interesting.
The enzymes have been divided into three evolutionarily related groups: the A-, B- and C-families5. Recent comparative studies 6 suggest that all oxygen reductases perform the same chemistry for oxygen reduction and comprise the essential elements of the proton pumping mechanism, such as the proton loading and kinetic gating sites, which, however, appear to be different in different families. For example, the A-type enzymes require two proton input pathways (D- and K-channels) to transfer protons used for oxygen reduction chemistry and for proton pumping, with the D-channel transporting all pumped protons. But as recently has been discovered 6, the the B-family, does not contain a D-channel. Rather it utilizes only one proton input channel, analogous to that of the A-family K-channel, and it delivers protons to the active site for both chemistry and pumping.
All species of A-family, however, demonstrate remarkable similarity of the central processing unit of the enzyme, as revealed by recent crystal structures. Here we demonstrate that the A-family cytochrome c oxidases of such diverse organisms as a mammal (bovine heart mitochondrial CcO), photosynthetic bacteria (Rhodobacter sphaeroides CcO), and soil bacteria (Paracoccus denitrificans CcO) are not only structurally similar, but almost identical in microscopic electrostatics and thermodynamics properties of their key amino-acids. We have performed pKa calculations of some of the key residues of the catalytic site, D- and K- proton input, and putative proton output channels of these three different enzymes, and demonstrate that the microscopic properties of key residues are almost identical, which strongly suggest similar mechanism in these species. Many similar calculations have been reported in the past, including those by this group; however, a side-by-side comparison, using the same methods, has never been performed before. The quantitative precision with which the microscopic physical properties of these enzymes have remained constant in evolution despite differences in sequences and number of subunits is striking.
2. Proposed proton pumping mechanism
Computational studies7-9 point to a mechanism of pumping shown in Fig.1. Recent experiments10,11 support the mechanism in its general form; however, some details remain experimentally untested and may in fact be different (see e.g. discussion in refs10,11). The calculations reported previously were done on the structure of mitochondrial CcO from bovine heart (pdb code, 2OCC12). Here we extend these calculations to new structures. According to the computational model, during the pumping cycle, the stable state of the catalytic center, before an additional electron is supplied to the system, is that in which one of the metal centers is formally oxidized, e.g., Fe3+–H2O, or Cu2+ –H2O. This state is established in a previous step of the cycle, when a chemical proton is accepted by one of the hydroxyl ligands of the BNC. In this state, His291 (i.e., Nδ1 position) is deprotonated and Glu242 is protonated.
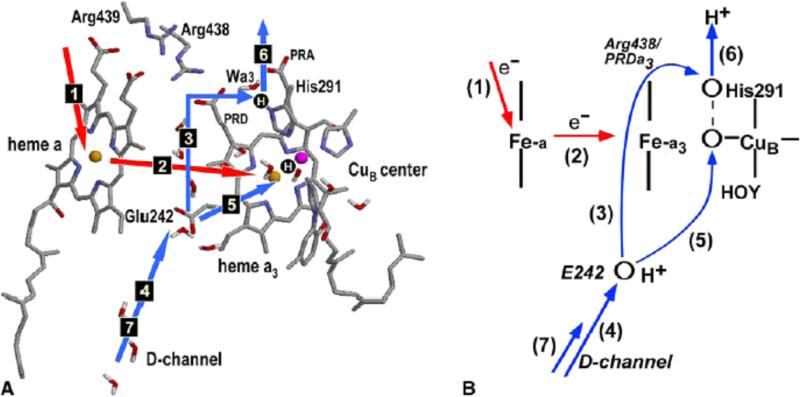
Fig.1.
(A) The key structural elements of the proposed pumping mechanism of CcO and the sequence of transitions during one pumping cycle. Two protonation sites, PLS and one in BNC, are shown as H-circles. PT and ET steps are shown by blue and red arrows, respectively. (B) The schematics of the model. The key assumption of the model is that the proton transfer rate upon ET between the hemes (step 2) is much higher along the pumping channel 3 than along the chemical channel 5, in other words step 3 occurs before step 5.
As the cycle advances, an electron is supplied (by cyt c) to the heme a3–CuB binuclear center (BNC) via CuA and heme a redox centers of the enzyme. As a result, one of the metal centers of BNC is reduced, and the overall charge of the BNC becomes one charge unit more negative. The driving force of electron transfer is about 20 meV*.7 In response to the increased negative charge of the BNC, the proton from Glu242 has now a driving force to move closer to the BNC. There are two pathways leading from Glu242 to two possible sites: one is leading to the BNC itself, the second is leading to His291 Nδ1 position 13. The assumption (kinetic gating, or other type of gating 14) is made that the rate of proton transfer to His291 is much higher than that of transfer to the BNC (the basis for this key assumption is discussed later in the paper). Therefore, the protonation of His291 occurs before that of the BNC. The fast proton transfer from Glu242 to His291 occurs by Grotthuss mechanism 15 via Arg438 and PRDa3, details of the transfer are discussed below. The driving force for this transfer is about 100 meV.7 With this transition, His291 becomes protonated. Subsequently, the arrival of the chemical proton to BNC, either via D channel or K channel, expels the proton from His291 to the P-side of the membrane, resulting in a translocation of one proton across the membrane.
The two-step pumping mechanism, in which the pumped proton is first loaded to the Proton Loading Site (PLS) from the N-side of the membrane and then expelled by the chemical proton arriving to BNC to the P-side of the membrane, is part of all most recently discussed models. The main difference between the models is in the nature of the PLS site, whose identity is still not known; however, recent experimental and theoretical studies point to one of the residues in the group His291, Prop A or D of heme a3, or a group (including a water molecule) nearby 10,11,16,17. The proton translocation from Glu242 to His291 in response to the reduction of BNC (i.e. the assumption that PLS is His291, see however discussion in Refs. 18-20) is a distinguishing point of the mechanism discussed in this paper; otherwise the mechanism considered is quite generic.
In this work, we compare CcO enzymes taken from different organisms to check for similarities or differences in the properties of key residues of the described mechanism. For quantitative comparisons, we examine pKa values of the bovine Glu242 and His291 analogs for so-called 7,8,21,22 OORO and OORR states of CcO. In this notation of the redox state “O” or “R” letters mean formally oxidized (O) or reduced (R) state of each CcO redox center: CuA, heme a, heme a3 and CuB, respectively. Thus, the two last letters specify redox state of BNC, e. g. BNC in OORR state is reduced in respect to the OORO state of CcO.
For a more complete picture of the proton pumping mechanism, we also included into consideration the protonatable sites of hemes, proton-entrance23 and proton-exit pathways22. Since the proton-exit pathways are not known with certainty, we include the elements of the proposed 22 exit pathways. The location of the key residues in the enzyme is shown in Fig.2.
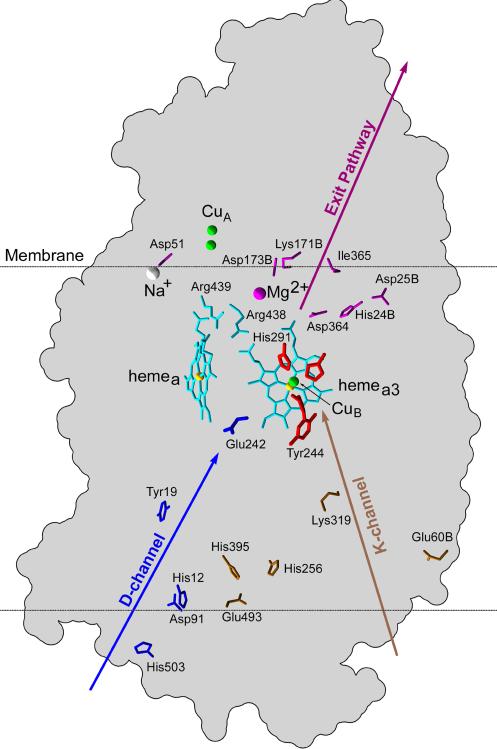
Fig.2.
Location of key residues in bovine cytochrome c oxidase. The residues are mainly distributed in subunit A, but those located in subunit B are marked with the letter “B” at the end of its label. D- and K-channel residues are shown in blue and brown color, respectively. Possible key residues of exit pathways are in purple color. Ligands of CuB co-factor are in red color and the hemes along with the salt-bridged Arginines are shown in cyan color.
CcO structural comparison for the species considered is given in the next section. Continuum electrostatic pKa calculations and results are described in the section 3. Conclusions are summarized in section 4.
3. Systems studied
Four CcO structures from different organisms have been studied.
1. Bovine Heart Mitochondrion CcO
For mitochondrial enzyme calculations, we use 2OCC pdb crystal structure resolved by Yoshikawa et al. 12 with 2.30 Å resolution, released 01-13-99. The catalytic core involves subunits I and II out of the total 13 subunits.
2. Rhodobacter Sphaeroides Bacterial CcO
Rhodobacter sphaeroides is a photosynthetic bacterium capable of aerobic and anaerobic respiration. For this organism, two structures (with and without Glu242 analog) have been considered. Namely, 2GSM pdb crystal structure resolved by Qin et al.24 with 2.00 Å resolution, released 10-10-06. The 2GSM structure has only 2 core subunits. The other, 1M57 pdb crystal structure resolved by Svensson-Ek et al.25 with 3.00 Å resolution, released 08-28-02; it has 4 subunits. The Glu242 analog in this structure is mutated to non-titratable glutamine. The mutation of the critically important residue Glu242, which shuttles protons along the D-channel, to a residue that cannot function as a proton transfer group naturally renders the mutant 1M57 to be non-pumping. However, the other minor structural changes, in particular around the heme a3 propionates, that the mutation induces are considered to be also mechanistically significant; it is interesting to see what kind of pKa changes these structural changes introduce. We therefore include this non-pumping mutant into comparison with the wild type enzymes.
3. Paracoccus Denitrificans Bacterial CcO
Paracoccus denitrificans is a soil bacterium capable of reducing nitrate to molecular nitrogen under anaerobic conditions but also capable of aerobic respiration. We examine 1AR1 pdb crystal structure resolved by Ostermeier et al.26 with 2.70 Å resolution, released 02-11-98. The structure has 2 subunits.
To unify reference on residues across multiple structures we accept residue labeling corresponding to the bovine CcO structure, i.e. bovine residue number used for other structures means its functional analog. The few residues, analogs for which can not be determined in other structures, will be mentioned explicitly.
The residue statistics of CcO in different organisms is summarized in Table 1. Although the catalytic core of each enzyme is generally constituted by a unique sequence of amino acids, the cofactors and metal centers are essentially the same. Moreover, the mutual location of the cofactors, metal centers and some other functional groups responsible for the enzyme function is remarkably conserved across different structures (see Fig.3).
Table 1.
Some comparative characteristics of CcO redox cofactors, and the number of titratable residues in the enzymes from different organisms.
| life form | Bovine | R. Sphaeroides | P. Denitrificans | ||
|---|---|---|---|---|---|
| structure | 2OCC | 2GSM*) | 1M57 | 1AR1 | |
| Redox cofactors | 1 | Cu A | Cu A | Cu A | Cu A |
| 2 | heme a | heme a | heme a | heme a | |
| 3 | heme a3 | heme a3 | heme a3 | heme a3 | |
| 4 | Cu B | Cu B | Cu B | Cu B | |
| Metal centers | 1 | Mg 2+ | Mg 2+ | Mg 2+ | Mg 2+ |
| 2 | Na + | Ca 2+ | Ca 2+ | Ca 2+ | |
| Titratable residues**) | Arg | 14 | 21 | 24 | 18 |
| Asp**) | 25 | 16 | 17 | 25 | |
| Cys | 1 | - | - | 4 | |
| Glu**) | 23 | 30 | 32 | 24 | |
| His**) | 15 | 16 | 15 | 15 | |
| Lys | 15 | 15 | 16 | 17 | |
| Tyr | 29 | 32 | 33 | 35 | |
2GSM structure has also metal center Cd2+ added for technological reasons
residues ligated to co-factor or metal center, are excluded from the statistics
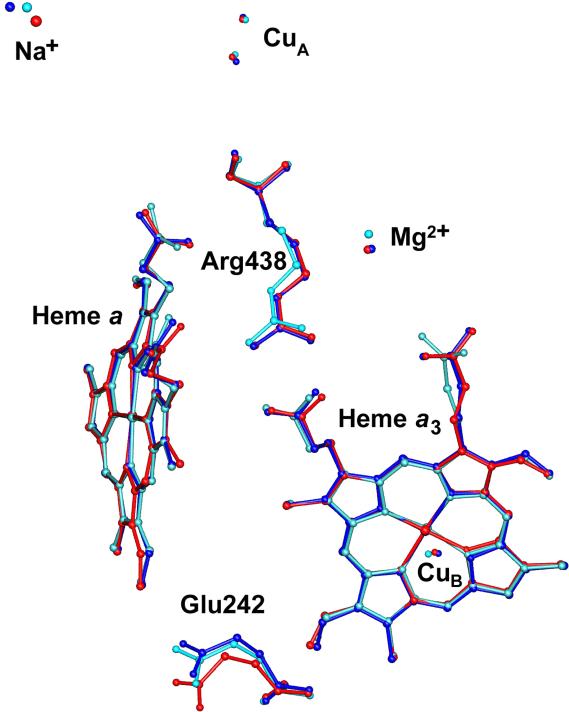
Fig.3.
Superimposed CcO Catalytic Centers: Bovine (red), R. sphaeroides (blue), P. denitrificans (cyan). The key residue labels correspond to the bovine structure. The structure matching has been done by VMD program and corresponds to the best fitting of heme porphyrin rings.
To quantify the similarity in the spatial arrangement of the putative proton pumping residues, we calculated their rmsd with respect to the bovine enzyme. The analysis has been performed by using the VMD program27; the structure matching were achieved by best fitting of heme porphyrin rings. The list of key residues (shown in Fig.2), their analogs and corresponding rmsd's are given in the Table 2.
Table 2.
RMSD of the key residues with respect to bovine 2OCC structure
| Organism | Bovine | R. Sphaeroides | P. Denitrificans | |||||
|---|---|---|---|---|---|---|---|---|
| strucuture | 2OCC | 2GSM | 1M57 | 1AR1 | ||||
| Redox cofactors | CuA | Cu228B | Cu3003 | 2.61 | Cu1003B | 2.54 | Cu270B | 2.53 |
| Cu229B | Cu3004 | 2.43 | Cu1004B | 2.61 | Cu271B | 2.47 | ||
| heme a | Hea515A | Hea2001A | 1.01 | Hea1001A | 0.61 | Hea562A | 0.28 | |
| heme a3 | Hea516A | Hea2002A | 0.92 | Hea1002A | 0.36 | Hea563A | 0.62 | |
| CuB | Cu517A | Cu3005 | 0.15 | Cu1005A | 0.24 | Cu559A | 0.72 | |
| Tyr244A | Tyr288A | 0.27 | Tyr288A | 0.53 | Tyr280A | 0.39 | ||
| His291A | His334A | 0.21 | His334A | 0.33 | His326A | 0.56 | ||
| Metal centers | Mg2+ | Mg518A | Mg3006 | 0.14 | Mg2006 | 0.24 | Mg560A | 0.82 |
| Na+ | NA519A | Ca3007 | 2.96 | Ca1007A | 2.55 | Ca561A | 1.99 | |
| Protonatable key sites | D-channel entrance residues | His503A | His549A | 0.63 | His549A | 1.17 | His541A | 1.15 |
| Asp91A | Asp132A | 0.9 | Asp132A | 1.15 | Asp124A | 0.69 | ||
| His12A | His26A | 1.09 | His26A | 1.74 | His28A | 1.41 | ||
| Tyr19A | Tyr33A | 1.68 | Tyr33A | 1.72 | Tyr35A | 0.65 | ||
| Glu242A | Glu286A | 0.91 | Gln286A | 1.23 | Glu278A | 0.73 | ||
| K-channel entrance residues | His395A*) | |||||||
| Glu493A | Glu539A | 0.95 | Glu539A | 0.93 | Glu531A | 1.31 | ||
| His256A | His300A | 0.36 | His300A | 0.44 | His292A | 0.57 | ||
| Lys319A | Lys362A | 0.33 | Lys362A | 0.26 | Lys354A | 0.61 | ||
| Glu60B*) | ||||||||
| Glu62B | Glu101B | 2.54 | Glu101B | 1.8 | Glu78B | 1.31 | ||
| Arginine salt bridge | Arg438A | Arg481A | 0.16 | Arg481A | 0.47 | Arg473A | 0.65 | |
| Arg439A | Arg482A | 0.16 | Arg482A | 0.6 | Arg474A | 0.26 | ||
| Possible exit pathways | His24B | His55B | 6.83 | His55B | 7.03 | His36B | 5.76 | |
| Asp25B | Asp58B | 5.33 | Asp58B | 5.38 | Asp35B | 5.49 | ||
| Asp51A**) | Glu182A | 0 | Glu182A | 0.61 | Glu174A | 1.42 | ||
| His93A | 0 | His93A | 0.23 | His85A | 0.41 | |||
| Asp364A | Asp407A | 0.52 | Asp407A | 0.82 | Asp399A | 0.68 | ||
| Ile365A**) | Arg408A | 0 | Arg408A | 0.87 | Arg400A | 0.42 | ||
| Lys171B | Lys227B | 0.66 | Lys227B | 1.27 | Lys191B | 0.7 | ||
| Asp173B | Asp229B | 1.11 | Asp229B | 0.51 | Asp193B | 0.51 | ||
These residues are included because of their possible role in the bovine enzyme; however, there are no their counterparts in the bacterial enzymes.
Asp51A and Ile365A residues of bovine enzyme have no direct analogues in the enzymes of other organisms; the closest possible candidates are shown, rmsdes for those residues have been calculated with respect to 2GSM structure.
The location of the key titratable residues identified in the previous studies7,8,22 is seen to be mainly conserved (rmsd within 3.0Å); this may indicate that these residues and their location are indeed essential for the enzyme function, including its proton pumping mechanism. The exceptions are only for the residues of proton entrance and possible exit pathways which were not firmly established22. The absence of close analogs of some of the residues in different organisms most likely indicate that they are not essential for the CcO function.
To provide physically more reliable arguments, we calculated pKa values of all key residues listed in the Table 2.
4. Methods and Results
The number of titratable sites in the CcO is variable across the multiple structures (see Table 1). For the analysis of the behavior of the titratable groups, the continuum electrostatic calculations were employed as described in ref. 7 In the calculations, the X-ray atomic coordinates and the corresponding partial atomic charges, which are placed in a low dielectric medium (ε = 4), describe the protein. The external solvent is defined as a high dielectric continuum (ε = 80). The effect from the internal solvent molecules is also described by the dielectric of 80 except for the water molecules ligated to the metal centers Mg2+, Na+ or Ca2+. (For a discussion of dielectric properties of internal cavities and their possible dielectric constants, see Refs. 21,28.) The ligands to heme a3 and CuB are also treated implicitly by adding their charges to the corresponding metal centers. The calculations for all structures were performed on chains A and B. In addition, we included the membrane that shields the membrane-spanning helices of subunit A from the solvent. The membrane was modeled as a low dielectric slab (ε = 4) of 40 Å.
The coordinates of hydrogen atoms were added and optimized by the AMBER7 package.29 For this optimization, all titratable groups were in their standard protonation state (charged state for acidic and basic amino acids, neutral state for polar and hydrophobic amino acids, and reduced state for all redox-active centers).
The site-site interaction energies in the protein-membrane-solvent system were obtained by solving the Poisson equation as implemented in the MULTIFLEX program30,31 with a three-step grid focusing procedure.32,33 A solvent probe radius of 1.4 Å was used to define the dielectric boundaries. The external mobile ions and ionic strength were not included in the computation, because their influence on the pumping mechanism and catalytic function of CcO was found to be minor.7 The titration curves and bias energies of the protonatable groups of CcO were subsequently obtained by applying the Monte Carlo method as implemented in the KARSBERG program.34
Most of the charges used in this application have been successfully used previously in calculations of redox potentials and protonation patterns of other proteins. 7,32,33 Basically, we refer to the CHARMM22 partial atomic charges and radii,35 when they are available, while charges of the residues in their nonstandard protonation states are taken from our previous publications.32,33 Several above water molecules included in the calculation explicitly were assigned the TIP3P charges.36 The atomic partial charges for all four redox-active cofactors in their reduced state were computed as Mulliken charges using the ZINDO program.37 In these quantum-chemical calculations, the redox centers were taken together with their ligated residues (bis-histidine ligated heme a, mono-histidine coordinated heme a3, the CuA center consisting of the two Cu atoms, two His and two Cys, and the CuB center containing three ligated His and Tyr244 covalently bound to the His240). The atomic charges for the oxidized states of redox cofactors were obtained from the corresponding reduced states by evenly distributing a +1 charge between the metal ion and atoms directly coordinated to it.
The results of pKa calculations at pH=7 for OORO and OORR redox state of CcO are summarized in Table 3. It is seen that in different organisms the pKa values of His291 analogs are significantly lower than 7.0 when CuB cofactor is oxidized, while the values become dramatically higher than pH once CuB is reduced. Physically this means that His291 residue is rapidly protonated once the additional electron is supplied to BNC. The proton should be provided by one of closest sites which proton affinity in the OORR state is lower than one of His291, i.e. Glu242, Arg438 or Arg439 residue analogs. The most favorable candidate is Glu242 since in all structures it has lowest pKa value. After donating a proton, Glu242 is quickly reprotonated, as its pKa still high enough to pull a proton from the N-side of the membrane; thus Glu242 is acting as a proton intermediate. Therefore, one of the key elements of the mechanism is that upon BNC reduction, the proton translocation occurring from Glu242 to His291 is conserved in all considered organisms of CcO.
Table 3.
pKa values of CcO key residues in different organisms. The location of residues in bovine CcO is shown in Fig.2.
| Bovine | R. Sphaeroides | P. Denitrificans | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2OCC | 2GSM | 1M57 | 1AR1 | |||||||||
| residue | pKaa) | residue | pKaa) | residue | pKaa) | residue | pKaa) | |||||
| OORO | OORR | OORO | OORR | OORO | OORR | OORO | OORR | |||||
| D-channel entrance residues | His-D 503A | 7.9 | 7.9 | His-D 549A | 7.7 | 7.7 | His-D 549A | 7.3 | 7.3 | His-D 541A | 8.5 | 8.5 |
| His-E 503A | 7.3 | 7.3 | His-E 549A | 6.9 | 7.0 | His-E 549A | 6.9 | 6.9 | His-E 541A | 8.6 | 8.7 | |
| Asp 91A | 3.7 | 3.7 | Asp 132A | 4.4 | 4.4 | Asp 132A | 6.2 | 6.2 | Asp 124A | 5.4 | 5.4 | |
| His-D 12A | 1.9 | 1.9 | His-D 26A | 4.9 | 4.8 | His-D 26A | 6.8 | 6.8 | His-D 28A | 5.9 | 6.0 | |
| His-E 12A | 12.1 | 12.2 | His-E 26A | 9.2 | 9.2 | His-E 26A | 7.3 | 7.3 | His-E 28A | 8.5 | 8.5 | |
| Tyr 19A | 17.4 | 17.5 | Tyr 33A | 16.4 | 16.5 | Tyr 33A | 16.4 | 16.5 | Tyr 35A | 19.4 | 19.5 | |
| Glu 242A | 14.7 | 15.0 | Glu 286A | 12.7 | 12.9 | Gln 286A | N/A | N/A | Glu 278A | 12.5 | 12.7 | |
| K-channel entrance residues | His-D 395A | 7.3 | 7.3 | |||||||||
| His-E 395A | 7.9 | 7.9 | ||||||||||
| Glu 493A | 6.6 | 6.6 | Glu 539A | −1.1 | −1.0 | Glu 539A | 1.4 | 1.5 | Glu 531A | 0.1 | 0.2 | |
| His-D 256A | 4.5 | 4.5 | His-D 300A | 4.2 | 4.2 | His-D 300A | 5.4 | 5.4 | His-D 292A | 3.9 | 3.9 | |
| His-E 256A | 9.6 | 9.5 | His-E 300A | 9.8 | 9.8 | His-E 300A | 8.6 | 8.6 | His-E 292A | 10.1 | 10.1 | |
| Lys 319A | −2.5 | −2.0 | Lys 362A | −2.5 | −2.1 | Lys 362A | −0.9 | −0.6 | Lys 354A | 0.4 | 0.8 | |
| Glu 60B | 14.4 | 14.5 | ||||||||||
| Glu 62B | 12.8 | 13.0 | Glu 101B | −26.6b) | −26.6b) | Glu 101B | 8.4 | 8.6 | Glu 78B | 4.1 | 4.3 | |
| CuB ligands | Tyr 244A | 19.6 | 23.5 | Tyr 288A | 17.4 | 21.4 | Tyr 288A | 18.4 | 22.0 | Tyr 280A | 18.5 | 22.5 |
| His 291A | 2.5 | 18.1 | His 334A | −2.1 | 14.0 | His 334A | 2.3 | 16.5 | His 326A | 1.9 | 16.2 | |
| Heme propionate | Heme a: A | 4.5 | 4.0 | Heme a: A | −1.0 | −1.1 | Heme a: A | −2.7 | −2.9 | Heme a: A | −1.5 | −1.8 |
| Heme a: D | −5.8 | −5.6 | Heme a: D | −7.0 | −6.2 | Heme a: D | −7.7 | −7.1 | Heme a: D | −6.3 | −5.6 | |
| Heme a3: A | 2.8 | 0.8 | Heme a3: A | 2.3 | 0.5 | Heme a3: A | 1.5 | −0.2 | Heme a3: A | 6.1 | 4.2 | |
| Heme a3: D | −1.5 | −3.4 | Heme a3: D | −2.4 | −3.5 | Heme a3: D | −0.1 | −1.3 | Heme a3: D | −1.1 | −2.4 | |
| Arginine salt bridge | Arg 438A | 15.5 | 16.3 | Arg 481A | 15.1 | 13.0 | Arg 481A | 14.0 | 13.5 | Arg 473A | 14.8 | 14.4 |
| Arg 439A | 18.0 | 17.1 | Arg 482A | 15.6 | 13.8 | Arg 482A | 15.5 | 14.0 | Arg 474A | 15.2 | 14.3 | |
| Possible exit pathway | His-D 24B | 7.0 | 7.0 | His-D 55B | 8.6 | 8.6 | His-D 55B | 8.3 | 8.3 | His-D 36B | 7.5 | 7.5 |
| His-E 24B | 7.0 | 7.0 | His-E 55B | 5.5 | 5.5 | His-E 55B | 6.0 | 5.9 | His-E 36B | 6.5 | 6.5 | |
| Asp 25Bc) | 8.2 | 8.2 | Asp 58Bc) | 3.7 | 3.7 | Asp 58Bc) | 8.3 | 8.5 | Asp 35Bc) | 3.5 | 3.7 | |
| Asp 51A | 4.8 | 4.6 | Glu 182A | 5.2 | 5.0 | Glu 182A | 6.3 | 6.3 | Glu 174A | 4.9 | 4.8 | |
| His-D 93A | 12.7 | 12.7 | His-D 93A | 13.7 | 13.7 | His-D 85A | 13.6 | 13.5 | ||||
| His-E 93A | 6.1 | 6.0 | His-E 93A | 7.7 | 7.7 | His-E 85A | 8.4 | 8.3 | ||||
| Asp 364A | 11.4 | 13.2 | Asp 407A | 11.7 | 13.5 | Asp 407A | 12.5 | 14.0 | Asp 399A | 7.9 | 9.8 | |
| Ile 365A | N/A | N/A | Arg 408A | 13.5 | 13.3 | Arg 408A | 11.6 | 11.9 | Arg 400A | 11.9 | 11.6 | |
| Lys 171B | 14.1 | 13.7 | Lys 227Bd) | 10.8 | 10.3 | Lys 227Bd) | 6.7 | 6.1 | Lys 191B | 8.9 | 8.3 | |
| Asp 173B | −2.9 | −3.7 | Asp 229B | −4.7 | −5.3 | Asp 229B | −0.6 | −1.4 | Asp 193B | 0.5 | 0.2 | |
In the notation of states, O and R mean formally oxidized (O) or reduced (R) state of each CcO redox center: CuA, heme a, heme a3 and CuB, respectively.
The low pKa of Glu101B is an artifact due to the presence of Cd2+ ion located just 2A apart from this residue; ions Cd2+ are not natural, and were introduced to 2GSM structure in preparation procedure.
The pKa scattering for Asp25B analogs is a result of the presence of 2 more protonatable residues (Arg and Tyr) at a distance of a hydrogen bond in these structures. In this case, the pKa of the residue describes only the partial proton population of the residue in the group Asp-Arg-Tyr; the protonation of the whole group would be more correclty to consider.
The difference between 2GSM and 1M57 results here is due to the difference in the distances between the protonatable site of Lys227B and Mg2+ ion; it is 6A in 1M57 structure, while it is 7.5 A in 2GSM structure.
Another important point is that heme propionates remain deprotonated in both OORO and OORR states of the enzyme due to significant proton repulsion with salt-bridged positive residues Arg438 and Arg439. A possibility of protonation of both heme a3 propionates has been often discussed in the literature, e.g. recently in Ref. 14; however, the computational method used in our calculations does not predict such a possibility.
Table 3 lists essentially all the residues that have been discussed in the past as possibly having some relevance to proton translocation in CcO. Just a casual inspection of the calculated pKa's of these groups shows that mostly their proton transfer properties (reflected in their pKa's) if not identical, are very similar quantitatively†. This is quite remarkable, because the enzymes outside the two central subunits are in fact rather different. Moreover, the sequence similarity between the structures of the different organisms is below 50%. Given the time of genetic separation of the considered species, i.e. length of their separate evolution, the precision of preservation of the properties of some of the key residues of the enzyme in completely different cellular environments is remarkable.
5. Conclusions
Three different forms of cytochrome c oxidase (bovine, Rhodobacter sphaeroides and Paracoccus denitrificans), all belonging to the same A-family, have been studied by a comparative analysis of the putative proton pumping functional groups. Though a constitution of all structures is generally different, a remarkable conservation of the key residues in different organisms is observed. The spatial distribution of the proton pumping residues also is matching with high precision (rmsd < 3.0 Å, see Table 2).
To perform further analysis we calculated pKa values of titratable residues which in our earlier studies7,8 have been identified as possible proton pumping elements (see list in the Table 2). The proton translocation from Glu242 to His291 upon BNC reduction proposed for mitochondrial bovine heart CcO also most likely occurs in the bacterial enzyme, because the energetic characteristics of proton translocation between these groups are almost identical in all enzymes studied. Of course, our present conclusions, as well as the assumption of the His291 role, are based only on calculations; the accuracy of these and similar calculations arguably is difficult to estimate. However, even if the computational scheme used here is not sufficiently accurate (for example to distinguish different candidates for the Proton Loading Site 10,11), the observed quantitative similarity of the energy characteristics of the key residues in enzymes of different organisms is rather remarkable, and points to a strongly preserved, unique mechanism of proton pumping.
It is interesting to notice similarity of the pKa values of the key residues between the wild type enzymes and 1M57 non-pumping mutant (with the exception of Glu242, which is mutated in 1M57 structure.) There were suggestions that the structural changes in this mutant, in particular around heme propionates, are of mechanistic significance, and could mimic what occurs when Glu-242 is deprotonated25. The current work suggests that there is no major difference in the pK values of key residues. Also, there were reports of spectroscopic changes due to this mutation38; the data indicate that the pK values may not be so sensitive.
The similarity in pKa values of key proton-translocation residues between the two bacterial and the bovine enzymes suggest the similarity in their mechanism; this may also be used to argue against the model of proton pump proposed by Yoshikawa and coworkers, who suggest that the mammalian oxidase does not pump protons in the same way as do the two bacterial enzymes 39.
The need to conserve the details of the mechanism may be due to a relative complexity and non-trivial physical principles of the mechanism which requires subtle balance of both energetic and kinetic properties of the system. As shown recently40,41, even a mutation of a seemingly unimportant residue (i.e. not blocking proton transfer) in the D-channel far removed from the catalytic site can render the enzyme incapable of proton pumping (but still able to reduce oxygen). Thus in some sense, there is no much freedom in variation of the parameters for the enzyme to be functional, so that once found, the mechanism is necessarily preserved in further evolutional steps. The mechanism by which key residues of the enzyme retained their proton-translocation properties in evolution, and stay fine-tuned to perform the proton pumping function, despite the significant differences accumulated in their overall structure, remain unclear and will require further studies. It will be also interesting in this respect to compare oxidases from B- and C- families.
Acknowledgements
One of the authors (AAS) wishes to acknowledge many useful discussions of the subject of the paper with Prof. James Fee of the Scripps Research Institute. We also are grateful to our referees, who provided a number of valuable suggestions. This work was supported in part by the research grants from NSF (PHY 0646273) and NIH (GM54052).
Footnotes
This means that in fact an electron is initially equilibrated between the two hemes, and the transfer is incomplete. However, this electron transfer is directly coupled with a proton transfer to the proton loading site, PLS, located near BNC, as explained later in the text. The proton transfer to PLS significantly increases the redox potential of BNC (about 0.5 mV) thereby stabilizing the electron at BNC, which in turn further increases the driving force for proton transfer to PLS. Therefore, one can say that the electron and the proton drive each other at this step to the more stable (intermediate) state of the enzyme where they occupy BNC and PLS, respectively.
It should be noted too that for residues that do not directly participate in proton translocation, it is their charge (i.e. protonation state) but not the specific value of pKa that may be important, as long as the variations of pKa do not affect the protonation state of the residue.
References
- 1.Gennis RB. Coupled proton and electron transfer reactions in cytochrome oxidase. Front Biosci. 2004;9:581–591. doi: 10.2741/1237. [DOI] [PubMed] [Google Scholar]
- 2.Wikstrom M. Proton translocation by bacteriorhodopsin and heme-copper oxidases. Curr Opin Struct Biol. 1998;8:480–488. doi: 10.1016/s0959-440x(98)80127-9. [DOI] [PubMed] [Google Scholar]
- 3.Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxidants & Redox Signaling. 2008;10(1):1–29. doi: 10.1089/ars.2007.1705. [DOI] [PubMed] [Google Scholar]
- 4.Brzezinski P, Gennis RB. Cytochrome c oxidase: exciting progress and remaining mysteries. Journal of Bioenergetics and Biomembranes. 2008;40(5):521–531. doi: 10.1007/s10863-008-9181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smirnova IA, Zaslavsky D, Fee JA, Gennis RB, Brzezinski P. Electron and proton transfer in the ba(3) oxidase from Thermus thermophilus. Journal of Bioenergetics and Biomembranes. 2008;40(4):281–287. doi: 10.1007/s10863-008-9157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang H-Y, Hemp J, Chen Y, Fee JA, Gennis RB. The cytochrome ba3 oxygen reductase from Thermus thermophilus uses a single input channel for proton delivery to the active site and for proton pumping. 2010 doi: 10.1073/pnas.0905264106. (to be published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popovic DM, Stuchebrukhov AA. Electrostatic Study of the Proton Pumping Mechanism in Bovine Heart Cytochrome cOxidase. J Am Chem Soc. 2004;126:1858–1871. doi: 10.1021/ja038267w. [DOI] [PubMed] [Google Scholar]
- 8.Popovic DM, Stuchebrukhov AA. Proton pumping mechanism and catalytic cycle of cytochrome c oxidase: Coulomb pump model with kinetic gating. FEBS Lett. 2004;566:126–130. doi: 10.1016/j.febslet.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Quenneville J, Popovic DM, Stuchebrukhov AA. Combined DFT/Continuum electrostatics studies of proton pumping mechanism of cytochrome c oxidase. In: Solomon EI, King RB, Scott RA, editors. Computational Inorganic and Bioinorganic Chemistry. Wiley; 2009. [Google Scholar]
- 10.Belevich I, Bloch DA, Belevich N, Wikstrom M, Verkhovsky MI. Exploring the proton pump mechanism of cytochrome c oxidase in real time. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2685–2690. doi: 10.1073/pnas.0608794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugitani R, Medvedev ES, Stuchebrukhov AA. Theoretical and computational analysis of the membrane potential generated by cytochrome c oxidase upon single electron injection into the enzyme. Biochimica Et Biophysica Acta-Bioenergetics. 2008;1777(9):1129–1139. doi: 10.1016/j.bbabio.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei MJ, Libeu CP, Mizushima T, Yamaguchi H, Tomizaki T, Tsukihara T. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280(5370):1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 13.Zheng XH, Medvedev DM, Swanson J, Stuchebrukhov AA. Computer simulation of water in cytochrome c oxidase. Biochimica Et Biophysica Acta-Bioenergetics. 2003;1557(1-3):99–107. doi: 10.1016/s0005-2728(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 14.Kaila VRI, Verkhovsky MI, Hummer G, Wikstrom M. Glutamic acid 242 is a valve in the proton pump of cytochrome c oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(17):6255–6259. doi: 10.1073/pnas.0800770105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuchebrukhov AA. Mechanisms of proton transfer in proteins: Localized charge transfer versus delocalized soliton transfer. Physical Review E. 2009;79(3) doi: 10.1103/PhysRevE.79.031927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe MA, Ferguson-Miller S. A chemically explicit model for the mechanism of proton pumping in heme-copper oxidases. Journal of Bioenergetics and Biomembranes. 2008;40(5):541–549. doi: 10.1007/s10863-008-9182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisliakov AV, Sharma PK, Chu ZT, Haranczyk M, Warshel A. Electrostatic basis for the uniderectionality of hte primary proton transfer in cytochrome c oxidase. Proc Natl Acad Sci USA. 2008;105:7726–7731. doi: 10.1073/pnas.0800580105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song YF, Michonova-Alexova E, Gunner MR. Calculated proton uptake on anaerobic reduction of cytochrome c oxidase: Is the reaction electroneutral? Biochemistry. 2006;45(26):7959–7975. doi: 10.1021/bi052183d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadda E, Chakrabarti N, Pomes R. Acidity of a Cu-bound histidine in the binuclear center of cytochrome C oxidase. Journal of Physical Chemistry B. 2005;109(47):22629–22640. doi: 10.1021/jp052734+. [DOI] [PubMed] [Google Scholar]
- 20.Stuchebrukhov AA, Popovic DM. Comment on “acidity of a Cu-bound histidine in the binuclear center of cytochrome c oxidase”. Journal of Physical Chemistry B. 2006;110(34):17286–17287. doi: 10.1021/jp057310u. [DOI] [PubMed] [Google Scholar]
- 21.Popovic DM, Quenneville J, Stuchebrukhov AA. DFT/Electrostatic Calculations of pKa Values in Cytochrome c Oxidase. J Phys Chem B. 2005;109:3616–3626. doi: 10.1021/jp046535m. [DOI] [PubMed] [Google Scholar]
- 22.Popovic DM, Stuchebrukhov AA. Proton Exit Channels in Bovine Cytochrome c Oxidase. J Phys Chem B. 2005;109:1999–2006. doi: 10.1021/jp0464371. [DOI] [PubMed] [Google Scholar]
- 23.Gennis RB. Multiple proton-conducting pathways in cytochrome oxidase and a proposed role for the active-site tyrosine. Biophys Biochim Acta. 1998;1365:241–248. [Google Scholar]
- 24.Qin L, Hiser C, Mulichak A, Garavito RM, Ferguson-Miller S. Identification of conserved lipid/detergent-binding sites in a high-resolution structure of the membrane protein cytochrome c oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(44):16117–16122. doi: 10.1073/pnas.0606149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson-Ek M, Abramson J, Larsson G, Tornroth S, Brzezinski P, Iwata S. The X-ray crystal structures of wild-type and EQ(I-286) mutant cytochrome c oxidases from Rhodobacter sphaeroides. Journal of Molecular Biology. 2002;321(2):329–339. doi: 10.1016/s0022-2836(02)00619-8. [DOI] [PubMed] [Google Scholar]
- 26.Ostermeier C, Harrenga A, Ermler U, Michel H. Structure at 2.7 angstrom resolution of the Paracoccus denitrificans two-subunit cytochrome c oxidase complexed with an antibody F-V fragment. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(20):10547–10553. doi: 10.1073/pnas.94.20.10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics. 1996;14(1):33–&. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 28.Leontyev IV, Stuchebrukhov AA. Dielectric relaxation of cytochrome c oxidase: Comparison of the microscopic and continuum models. Journal of Chemical Physics. 2009;130(8):085103. doi: 10.1063/1.3060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Case DA, Pearlman DA, Caldwell JW, Cheatham TE, III, Wang J, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, Cheng AL, Vincent JJ, Crow-ley M, Tsui V, Gohlke H, Radmer RJ, Duan Y, Pitera J, Massova I, Seibel GL, Singh UC, Weiner PK, Kollman PA. AMBER 7 Users’ Manual. University of California; San Francisco: 2002. [Google Scholar]
- 30.Bashford D, Gerwert K. Electrostatic calculations of the pKa values of ionizable groups in bacteriorhodopsin. J Mol Biol. 1992;224:473–486. doi: 10.1016/0022-2836(92)91009-e. [DOI] [PubMed] [Google Scholar]
- 31.Bashford D. An Object-Oriented Programming Suite for Electrostatic Effects in Biological Molecules. In: Ishikawa Y, Oldehoeft RR, Reynders JVW, Tholburn M, editors. Scientific Computing in Object-Oriented Parallel Environments. Volume 1343, volume 1343 of Lecture Notes in Computer Science. ISCOPE97, Springer; Berlin: 1997. pp. 233–240. [Google Scholar]
- 32.Popovic DM, Zaric SD, Rabenstein B, Knapp EW. Artificial cytochrome b: Computer modeling and evaluation of redox potentials. Journal of the American Chemical Society. 2001;123(25):6040–6053. doi: 10.1021/ja003878z. [DOI] [PubMed] [Google Scholar]
- 33.Popovic DM, Zmiric A, Zaric SD, Knapp EW. Energetics of radical transfer in DNA photolyase. Journal of the American Chemical Society. 2002;124(14):3775–3782. doi: 10.1021/ja016249d. [DOI] [PubMed] [Google Scholar]
- 34.Rabenstein B. Monte Carlo Methods for Simulation of Protein Folding and Titration. Karlsberg online manual http://liechemiefu-berlin/karlsberg/1999.
- 35.Mackerell AD, Bashford D, Bellott M, Dunbrack R, Evanseck J, Field M, Fischer S, Gao J, Guo H, Ha S, Joseph-McCarthy D, Kuchnir L, Kuczera K, Lau FTK, Mattos C, Michnick S, Ngo T, Nguyen DT, Prodhom B, Reiher WE, III, Roux B, Schlenkrich M, Smith JC, Stote R, Straub J, Watanabe M, Wiorkiewicz-Kuczera J, Yin D, Karplus M. All-Atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. Comparison of Simple Potential Functions for Simulating Liquid Water. Journal of Chemical Physics. 1983;79(2):926–935. [Google Scholar]
- 37.Zerner MC, Ridely JE, Bacon A, McKelvey J, Edwards W, Head JD, Culberson C, Cory MG, Zheng X, Parkinson W, Yu Y, Cameron A, Tamm T, Pearl G, Broo A, Albert K, ZINDO, Gainesville FL. distributed by Accelrys: 9685. Scranton Rd, San Diego, CA: 2002. pp. 92121–3752. [Google Scholar]
- 38.Konstantinov AA, Siletsky S, Mitchell D, Kaulen A, Gennis RB. The roles of the two proton input channels in cytochrome c oxidase from Rhodobacter sphaeroides probed by the effects of site-directed mutations on time-resolved electrogenic intraprotein proton transfer. Proc Natl Acad Sci USA. 1997;94:9085–9090. doi: 10.1073/pnas.94.17.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukihara T, Shimokata K, Katayama Y, Shimada H, Muramoto K, Aoyoma H, Mochizuki M, Shinzawa-Itoh K, Yamashita E, Yao M, Ishimura Y, Yoshikawa S. The low-spin heme of cytochrome c oxidase as the driving element of the proton-pumping process. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15304–15309. doi: 10.1073/pnas.2635097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durr KL, Koepkee J, Hellwig P, Muller H, Angerere H, Peng G, Olkhova E, Richter OMH, Ludwig B, Michele H. A D-Pathway Mutation Decouples the Paracoccus denitrificans Cytochrome c Oxidase by Altering the Side-Chain Orientation of a Distant Conserved Glutamate. Journal of Molecular Biology. 2008;384(4):865–877. doi: 10.1016/j.jmb.2008.09.074. [DOI] [PubMed] [Google Scholar]
- 41.Lepp H, Salomonsson L, Zhu JP, Gennis RB, Brzezinski P. Impaired proton pumping in cytochrome c oxidase upon structural alteration of the D pathway. Biochimica Et Biophysica Acta-Bioenergetics. 2008;1777(7-8):897–903. doi: 10.1016/j.bbabio.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]