Abstract
The kidneys can be injured in diverse ways by many drugs, both legal and illegal. Novel associations and descriptions of nephrotoxic effects of common and emerging drugs of abuse have appeared over the past several years. Anabolic androgenic steroids, illicitly used by athletes and others for decades to increase muscle mass and decrease body fat, are emerging as podocyte toxins given recent descriptions of severe forms of FSGS in long-term abusers. Synthetic cannabinoids, a new group of compounds with marijuana-like effects, recently became popular as recreational drugs and have been associated with an atypical form of AKI. 3,4-Methylenedioxymethamphetamine, commonly known as ecstasy, is a widely used synthetic recreational drug with mood-enhancing properties and a constellation of toxicities that can result in death. These toxic effects include hyperthermia, hypotonic hyponatremia due to its arginine vasopressin secretagogue–like effects, rhabdomyolysis, and cardiovascular collapse. Cocaine, a serotonin-norepinephrine-dopamine reuptake inhibitor that serves as an illegal stimulant, appetite suppressant, and anesthetic, also causes vasoconstriction and rhabdomyolysis. Recent adulteration of much of the world’s supply of cocaine with levamisole, an antihelminthic agent with attributes similar to but distinct from those of cocaine, appears to have spawned a new type of ANCA-associated systemic vasculitis. This review discusses the nephrotoxic effects of these common and emerging drugs of abuse, of which both community and health care providers should become aware given their widespread abuse. Future investigation into pathogenetic mechanisms associated with these drugs is critical and may provide a window into ways to lessen and even prevent the nephrotoxic effects of these drugs of abuse and perhaps allow a deeper understanding of the nephrotoxicities themselves.
Keywords: drug nephrotoxicity, anabolic steroids, synthetic cannabinoids, methamphetamines, cocaine
Introduction
The kidneys can be injured in diverse ways by many drugs, both legal and illegal. Susceptibility of the kidneys to such insults is primarily due to their high degree of filtration and their metabolism by the kidneys to potentially toxic byproducts (1,2). Over the past several years, novel associations and descriptions of the nephrotoxic effects of common and emerging drugs of abuse have appeared. Here we review the nephrotoxic effects of anabolic androgenic steroids, synthetic cannabinoids, methamphetamines (ecstasy), and cocaine and its levamisole-adulterated counterpart. It is important to point out that this is not a comprehensive review of all renal syndromes associated with drugs of abuse. Other notable nephrotoxic drugs of abuse include older syndromes of heroin nephropathy, toluene-induced renal tubular acidosis, and a more recently described syndrome of AKI seen with bath salts. There is probably a general paucity of data in the literature, partly because of under-reporting of incidents; thus, it is important to recognize this when examining data presented herein with regards to clinicopathologic characteristics and treatment recommendations. The importance of the specific nephrotoxic drugs of abuse reviewed here is highlighted by the widespread use of these drugs and the ever-growing global burden of illicit drug use and dependence (3).
Anabolic Androgenic Steroids
Background
Anabolic androgenic steroids (AASs) are a family of hormones that include testosterone as well as its naturally occurring and synthetic derivatives, which have been illicitly used by athletes and other individuals who desire to increase muscle mass and decrease body fat since the 1950s. Damage from years of androgen excess, often 50–100 times physiologic levels (4), may become an increasingly important cause of CKD. A wide range of prevalence estimates generally suggest that at least 3% of young men in Western countries use AASs at some time in their lives (5). In selected populations, such as weightlifters, up to 44% of those surveyed admit to AAS abuse (6). More relevant for the nephrologist is the estimate of how many AAS users become long-term abusers who are more likely to develop clinically significant kidney disease. Studies suggest that approximately 30% of AAS users develop dependence and would therefore be at a higher risk for developing the medical consequences of protracted abuse (7).
Confounding the effort to better define the medical effects of AAS abuse is the high incidence of polypharmacy in AAS abusers. Almost universally, AAS users ingest numerous “nutraceuticals” and dietary supplements, none of which are regulated by the US Food and Drug Administration. Bodybuilders report high protein consumption, sometimes in excess of 500 g/d, and the renal effects of this high metabolic burden are unknown (8). Nonandrogenic anabolic hormones, such as human growth hormone (9) and insulin (10), are used with moderate frequency by AAS users to further augment muscle gain. Opioid abuse is common in AAS users, and opioid and androgen dependence may share common neurologic pathways in the brain (4). Cocaine abuse also appears to be common in AAS users, with one survey showing 11.3% of AAS users reporting cocaine use within the past month, compared with 4.7% of non-AAS users with otherwise similar characteristics (11).
Clinical and Nephropathologic Findings in AAS Abuse
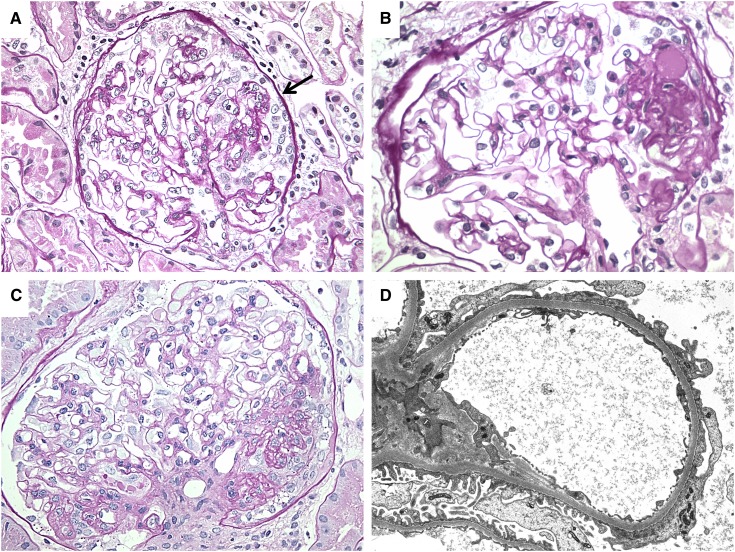
The cardiac, neuroendocrine, and neuropsychiatric effects of AAS abuse are well documented in other reviews (4,5). Renal effects of AAS abuse in humans are primarily described in case reports (12–14) and a single small series (8). The series featured 10 long-term AAS abusers who presented with variable elevations in serum creatinine (mean, 3.0 mg/dl) and substantial proteinuria (mean, 10.1 g/d; range, 1.3–26.3 g/d). Three of the 10 patients in the series had full nephrotic syndrome, and two additional patients had nephrotic-range proteinuria and hypoalbuminemia but lacked edema. Renal biopsy in these patients revealed FSGS. Four of the 10 patients had perihilar lesions of FSGS, a finding classically seen in hyperfiltration-induced forms of FSGS, but three patients showed collapsing lesions, which are uncommon in the setting of postadaptive forms of FSGS. Electron microscopy showed a mean of 69% podocyte foot process effacement, and five of eight patients had >50% effacement, which is also uncommon in postadaptive FSGS (8,15,16). Figure 1 provides examples of the nephropathologic findings seen in the setting of AAS abuse. These unusual biopsy features suggest that, in addition to the expected metabolic strain that elevated lean body mass and high protein intake places on glomeruli, androgens may be exerting a toxic effect on podocytes.
Figure 1.
Light microscopic and ultrastructural findings in anabolic androgenic steroids abusers with FSGS. (A) A glomerulus shows collapsing focal sclerosis with retraction of the glomerular tuft and hyperplasia of overlying epithelial cells (arrow) (periodic acid-Schiff; original magnification, ×400). (B) A discrete lesion of segmental sclerosis is identified at the glomerular vascular pole (periodic acid-Schiff; original magnification, ×400) (C) A markedly hypertrophied glomerulus showing the unusual combination of a perihilar lesion of segmental sclerosis with collapsing features (periodic acid-Schiff; original magnification, ×400). (D) Electron micrograph showing >50% podocyte foot process effacement (original magnification, ×8000).
Treatment
Cessation of AAS is clearly the mainstay of treatment in cases of AAS-associated toxicity. Additional treatment aimed at reducing hyperfiltration injury, such as renin-angiotensin system blockade along with lifestyle modification (including a reduction of strenuous exercise and weight loss), has been reported to help reduce proteinuria and stabilize renal function (8). No evidence supports the use of immunosuppressive therapies, including corticosteroids. Notably, lifestyle modifications are difficult to sustain for some patients who experience AAS dependence. In the published series of 10 patients, one patient refused to stop AAS altogether, and two stopped for a period of time but then restarted use after experiencing depressive symptoms and body image issues. In one patient, nephrotic syndrome relapsed after resumption of AAS use (8).
Pathogenesis of AAS-Associated Renal Injury
The mechanism of renal injury in the setting of AAS abuse is not well established and is likely multifactorial. Hyperfiltration injury is an important factor; patients with markedly elevated lean body mass require an increase in single-nephron glomerular filtration, similar to patients with obesity-related glomerulopathy (16). Numerous animal models have investigated the influence of sex hormones on the development of renal disease, and they generally show a protective role for estrogens, while androgens either are permissive or accelerate injury (17). Doublier and colleagues recently developed a mouse model of glomerulosclerosis associated with high testosterone levels. They demonstrated that podocytes express both androgen and estrogen receptors and that in vitro, testosterone can cause podocyte apoptosis, which is blocked by the addition of flutamide (18). In skeletal and cardiac muscle cells, testosterone signals through the mammalian target of rapamycin pathway (19,20). Likewise, the importance of mammalian target of rapamycin signaling in podocytes and in essential podocyte functions, including autophagy (21) and compensatory hypertrophy (22), is increasingly recognized. Future studies aimed at improving understanding of the specific role of androgen signaling in these key podocyte processes are needed to better understand the mechanism of AAS-induced kidney disease.
Synthetic Cannabinoids
Background
The synthetic cannabinoids are a group of compounds that have cannabis- or marijuana-like effects. These compounds were originally developed in laboratories for research and drug development; recently, however, they have become popular as recreational drugs. As such, they are solubilized and sprayed onto herbal mixtures and then marketed in the form of incense preparations, bath additives, or air fresheners under a variety of names, such as “Spice” or “K2”. These drugs have become widely used for a variety of reasons. They are relatively inexpensive and have been readily available—online and at convenience stores, gas stations, and “head shops”. They are often marketed as herbal or natural products and therefore perceived as being safe. Effects of these products are supposed to mimic those of marijuana, and many are purported to give a more intense high than marijuana itself. That they are not detected by routine urine drug screens makes them attractive to people who are required to undergo random testing.
Although recreational use of synthetic cannabinoids is relatively new, these drugs have very quickly become widely available (23–25). Eight percent of University of Florida students surveyed in 2010 admitted to having used a synthetic cannabinoid on at least one occasion (26), and 11.4% of 12th graders in the United States surveyed in 2011 admitting to having used a synthetic cannabinoid within the previous 12 months (27).
Δ9-Tetrahydrocannabinol, the principal psychoactive compound in marijuana, was first synthesized in 1964 (28), and two major cannabinoid receptors were cloned in the 1990s. The cannabinoid type 1 (CB1) receptor, thought to be present mainly in the central nervous system, is responsible for the psychotropic effects of marijuana and other cannabinoids (29). The CB2 receptor is found in abundance in the immune system, particularly the spleen (30). Agonists of these receptors stimulate Gi/o protein–mediated signal transduction pathways. More recent studies have demonstrated the presence of low concentrations of CB1 receptors in almost all tissues, and CB2 receptors have been found in parts of the brain as well as other organs and tissues. Endogenous cannabinoids have been identified, and the endogenous cannabinoid system is an area of intense investigation in an effort to develop novel therapies for a broad spectrum of diseases. While a detailed discussion of the cannabinoid receptors and the endogenous cannabinoid system is beyond the scope of this paper, further information can be found in the reviews of Howlett and colleagues (31) and Pacher and Kunos (32).
The synthetic cannabinoids are a group of chemically unrelated compounds that have in common their ability to bind to the cannabis receptors. Several classification schemes have been used, based on chemical structure and the laboratory in which they were discovered. Major categories of the synthetic cannabinoids include (23) the classic cannabinoids (with a dibenzopyran ring), nonclassic cannabinoids (cycloheylphenols), naphthylmethylindoles, naphthopyrroles, naphthylmethylindenes, naphthylindoles, phenylacetylindoles, methanandamine, and other synthetic analogues to the endogenous eicosanoids.
Compounds from most of the classes listed above have been isolated from recreational herbal preparations (23), and in March 2011, five synthetic cannabinoids were temporarily classified as Schedule I drugs under the Controlled Substances Act (25). Synthetic cannabinoids that are used recreationally are an ever-changing group of compounds, with new drugs appearing as others are declared illegal.
Renal Manifestations of Synthetic Cannabinoid Use
Synthetic cannabinoids became of interest to nephrologists because of reports from local emergency departments and the lay press of AKI among users of these drugs (33,34). Bhanushali and colleagues reported four cases of AKI among users of synthetic cannabinoids from northeast Alabama (35). In response to reports of three cases of AKI among synthetic cannabinoid users in Natrona County, Wyoming, the Wyoming Department of Health launched a collaborative investigation among public health officials from several states, clinicians, and the Arkansas K2 Research Consortium, which identified a total of 16 cases (36). Kazory and Aiyer have reported an additional case (37). Findings from these publications are summarized below and in Table 1.
Table 1.
Clinical findings among 21 users of synthetic cannabinoid (also known as ”Spice”) with AKI
| Variable | Value |
|---|---|
| Mean age (yr) | 20 (15–33) |
| Male patients, n (%) | 20 (95) |
| Presenting symptoms, n (%) | |
| Nausea and vomiting | 21 (100) |
| Abdominal, flank, and/or back pain | 15 (71) |
| Mean peak serum creatinine (mg/dl) | 7.7 (3.2–21) |
| Renal ultrasonograph findings (n=17) (n) | |
| Normal | 5 |
| Increased cortical echogenicity | 12 |
| Bilateral symmetrical enlargement | 1 |
| Renal biopsy findings (n=13) (n) | |
| Acute tubular necrosis | 10 |
| Acute interstitial nephritis | 3 |
All synthetic cannabinoid users reported were young (median age, 20 years), and all but one were male. Presenting symptoms included nausea and vomiting in all patients and abdominal flank or back pain in 15 (71%). Two patients reported diarrhea. There was a broad spectrum of urinalysis findings; only two patients had normal urinalyses. Renal ultrasonography findings were reported in 17 patients. In five, the findings were unremarkable; 12 showed increased echogenicity without hydronephrosis, including one patient with the additional finding of bilateral symmetrical renal enlargement. Renal biopsies were performed in 13 cases. Because these data were obtained from several publications and were based on chart review, diagnostic criteria used in the histopathologic diagnoses were not standardized; however, 10 of the biopsies showed acute tubular necrosis and three were consistent with acute interstitial nephritis. Five patients required dialysis. Apart from the use of two ibuprofen tablets in one patient with flank pain (37), no potential precipitating factors for AKI could be identified. Creatine phosphokinase values, available in only four patients, were not markedly elevated (median, 255 U/L; range, 144–357 U/L). Although follow-up serum creatinine values were not available for most of the patients, renal function improved in at least some of them.
No specific “brand” of synthetic cannabinoid could be implicated, and, where stated, nine distinct street products were named. In a minority of cases, the product or clinical samples were available for analysis (36). A common finding in the analytes was the synthetic cannabinoid XLR-11, present in five products, two urine samples, and three blood or serum samples. No synthetic cannabinoid could be detected in four blood or serum samples, one urine sample, and one product.
Proposed Mechanisms of Nephrotoxic Effects of Synthetic Cannabinoids
The pathogenesis of AKI among users of synthetic cannabinoids is unknown. The predominance of men with AKI is not surprising because men make up almost 80% of the users of these drugs (38). Given the history of nausea and vomiting before presentation in every case, ischemic acute tubular necrosis secondary to hypovolemia is a plausible mechanism; however, in the five cases where vital signs were stated, no tachycardia or hypotension was present on presentation. Moreover, where reported, renal function worsened, despite aggressive hydration (37). Although it is conceivable that some cannabinoid mixtures contain a noncannabinoid nephrotoxic contaminant, an alternative explanation is that some of the synthetic cannabinoids may be inherently nephrotoxic. Low levels of CB1 and CB2 receptors have been demonstrated in renal podocytes, endothelial cells, mesangial cells, and proximal tubules (39–41). A pathogenic role involving activation of the CB1 receptor has been demonstrated in experimental models of kidney diseases, such as diabetic nephropathy and cisplatin nephrotoxicity (39,42). Thus, it is possible that some synthetic cannabinoids cause derangements in the endocannabinoid system of the kidneys leading to AKI.
Treatment
Treatment for AKI secondary to synthetic cannabinoids has been largely supportive, including fluid resuscitation, given the presenting symptoms of nausea and vomiting and, where indicated, dialysis. Because three of the patients described in the literature had evidence of acute interstitial nephritis, a short course of steroids may also be indicated in such instances.
Methamphetamines (Ecstasy)
Background
Ecstasy (3,4-methylenedioxymethamphetamine [MDMA]) is a widely used, Schedule I, synthetic recreational drug first synthesized in 1914 (43,44). Estimates are that approximately 39% of college students in the United States have used ecstasy in the past year (45). Typically, the drug is taken in nightclub environments or “rave” parties and has mood-enhancing properties, such as causing energy, empathy, and euphoria. Another similar drug used in “party pills,” N-benzylpiperazine, leads to similar effects and has similar toxicities (46). The pattern of toxicity related to ecstasy is idiosyncratic and not usually due to overdose. In fact, first-time users of “typical” dosages may experience serious life-threatening consequences, such as recently seen in two deaths at a music festival in New York City in 2013 (47).
Most deaths secondary to ecstasy use occur in women and can be accompanied by acute hyperthermia, hyponatremia, cardiovascular collapse, rhabdomyolysis, and even permanent neurologic damage (48,49). The direct mechanisms that underlie the toxic actions are difficult to elucidate because ecstasy is not the only drug involved and pills may be adulterated with other compounds. However, likely effects are due to a combination of a serotonin syndrome as well as the drug’s sympathomimetic effects and ability to lead to the release of arginine vasopressin (AVP) (50). In recent years, ecstasy has been associated with a spectrum of nephrotoxic effects, including AKI and hyponatremia (51). These effects are usually seen with the more commonly encountered central nervous system (CNS) effects (Table 2).
Table 2.
Major clinical manifestations of ecstasy
| Central nervous system |
| Mild: Hyperactivity, dry mouth, increased thirst, restlessness, palpitations, dizziness, drowsiness, difficulty concentrating, anxiety, tremor |
| Serious: acute panic attacks, delirium, psychosis |
| Chronic: serotonin depletion leading to depressed mood, potential neurotoxicity with cognitive deficits |
| Cardiovascular |
| Acute: hypertension, tachycardia, arrhythmias, ischemia, and sudden death |
| Chronic: cardiomyopathy |
| Renal |
| Acute: AKI and hyponatremia |
| Musculoskeletal |
| Acute: rhabdomyolysis |
Clinical Manifestations of Ecstasy Use and Underlying Pathogenetic Mechanisms
The clinical syndromes associated with ecstasy use are myriad (Table 2). The drug is usually taken for its effects of euphoria and increased sociability. Adverse effects or toxicities related to its use generally involve greater degrees of CNS and autonomic nervous system activity.
Ecstasy and its related compounds and metabolites lead to release of serotonin, dopamine, and norepinephrine into the CNS (52). Furthermore, reuptake of these neurotransmitters is also inhibited. Ecstasy has also been documented to lead to the release of AVP, perhaps through the serotonin system (52,53).
MDMA undergoes metabolism by both N-dealkylation and O-demethylation followed by catechol-O-methyltransferase catalyzed methylation (52). The pathway involving O-demethylation exhibits genetic variance in its activity level through the cytochrome P450 isoenzyme CYP2D6 (54). The significance of this genetic variation is that some people will have slower metabolism and be at risk for greater toxicity (54). Because the metabolites of MDMA also form inhibitor complexes with the CYP2D6 enzyme, there is the potential for greater toxicity with multiple dosings (54). Finally, other drugs that are metabolized through this pathway can increase the risk for toxicity. Thus, the user’s genetic profiles, as well as interactions resulting from polydrug use, are likely key factors that modulate the individual response to MDMA and clinical outcomes.
AKI has been described in numerous case reports from ecstasy users (55). The absolute incidence of this complication cannot be determined but is probably low. In most cases, AKI is associated with nontraumatic rhabdomyolysis in the setting of hyperthermia, extreme exertion, and volume depletion (51). Elevations of creatinine phosphokinase are usually pronounced (>100,000 U/L). Although direct nephrotoxic effects of the drug cannot be excluded, there is only one case report of transient proximal tubular dysfunction in an ecstasy user (56). Thus, AKI is probably not due to a primary nephrotoxic effect.
The most common renal complication of ecstasy use is symptomatic hyponatremia (51). In these cases, the sodium level at presentation is typically <130 mEq/L and can be as low as 100 mEq/L. Of note, for public health concerns, most cases occurred in young women who ingested a single dose of ecstasy; in many cases this was the first use of the drug (51). Hyponatremia with ecstasy is dilutional in nature. Excessive water or other hypotonic beverage intake is a likely culprit. A common occurrence at “rave” parties is the use of “chill out” areas, which feature copious quantities of water or sports beverages. These beverages are consumed readily because of the hot atmosphere and intense physical activity associated with these parties. The effects of amphetamines, which increase the sensation of thirst as well as cause xerostomia, may increase the intake of water as well. However, merely the high intake of fluids is probably not enough to lead to hyponatremia because the kidney can usually excrete free water rapidly enough to match intake (51). Thus, impairment of renal free water excretion due to elevated AVP levels is the other major factor that ultimately leads to hyponatremia.
Ecstasy is a potent inducer of the secretion of AVP (57–60). Henry and colleagues demonstrated that small doses of MDMA (40 mg; common “street dose” is 100 mg) led to a significant increase in AVP levels (from 1.14–1.88 pmol/L to 2.46–9.16 pmol/L) within 1–2 hours after dosing. As expected, this rise in AVP led to a decrease in serum sodium levels, with a concomitant rise in urine osmolality (53). A more recent study documented a slight fall in serum sodium levels in “rave” attendees who used ecstasy compared with a control group of nonusers (61).
Acutely, the fall in serum sodium can lead to the devastating complication of cerebral edema. This may be signaled by symptoms such as mental status changes, seizures, and coma. Ultimately, in severe, untreated cases, brainstem herniation may occur, resulting in death. In ecstasy users, there is a preponderance of female patients who have developed life-threatening symptoms (>85% of patients with symptomatic hyponatremia) (51). The susceptibility of women to the effects of hyponatremia (not specifically in users of ecstasy) may be related to estrogen’s effect on inhibiting the cerebral membrane Na-K-ATPase activity. The pump is one of the primary defense mechanisms against the osmotic shifts induced by severe hyponatremia (62).
Treatment
In general, therapy for acute ecstasy-induced adverse events relies on supportive care, including aggressive cooling, correction of electrolytes, and intravenous fluids. Treatment of severe, symptomatic hyponatremia is a medical emergency, and 100–200 ml of 3% saline should be administered as soon as possible. The goal is to reduce the serum sodium concentration by 3–5 mEq/L, which should acutely lower intracranial pressure and improve symptoms (63).
In milder cases of ecstasy-induced hyponatremia, spontaneous free-water diuresis occurs and therapy may be limited to fluid restriction (51). In these cases, AVP levels fall quickly and the ability of the kidney to excrete free water returns. It is imperative to continue close monitoring and, if any deterioration is noted, hypertonic saline should be administered at once.
The only way to prevent the deleterious effects of ecstasy is avoidance of the drug. Educational efforts are needed so that young individuals understand the toxic effects of this substance of abuse.
Cocaine and Its Levamisole-Adulterated Counterpart
Background
Cocaine, one of the oldest and most addictive psychoactive substances in existence, is extracted from Erythroxylum coca leaves. It acts biologically as a serotonin-norepinephrine-dopamine reuptake inhibitor (triple reuptake inhibitor) to serve as a stimulant, appetite suppressant, and anesthetic. The two forms of cocaine that are abused are the soluble hydrochloride salt, or powdered, form and the insoluble base or freebase, also commonly known as “crack” (64). Euphoric effects of cocaine can occur through intraoral topical administration along the gums and oral mucosa, inhalation, nasal insufflation, intravenous injection, or even vaginal or anal suppository placement. A recent study estimated that 14–21 million (0.3%–0.5% of the population aged 15–64 years) users exist worldwide, and the highest prevalence of cocaine dependence is in North America (3,65).
Clinical Renal Manifestations of Cocaine Use
Nephrotoxic effects of cocaine are numerous and thought to be related to changes in renal hemodynamics and glomerular matrix synthesis, degradation and oxidative stress, and induction of renal atherogenesis (66,67). Cocaine is one of many abused drugs that can cause rhabdomyolysis, and this is probably the most common reason for AKI associated with cocaine use (68,69). With regard to altering renal hemodynamics, it is known that cocaine causes vascular smooth muscle constriction and inhibits reuptake of serotonin, norepinephrine, and dopamine to promote hypertension and tachycardia (66). Reports implicate this may be due in part to increased production of endothelin-1, which acts on its cognate receptors located on vascular smooth muscle cells of renal resistance vessels to decrease renal blood flow and GFR (70–72). Interestingly, however, a recent, large epidemiologic report examining the association of illicit drug use, including cocaine use, and CKD in the United States found no association with CKD (73). A possible explanation for this disconnect may be related to the fact that cocaine use is associated with acute and severe hypertension that is only transient in nature and is not considered to result in chronic hypertension (74). Severe and acute hyponatremia associated with cocaine exposure has been reported, possibly due to stimulation of AVP and subsequent development of a syndrome of inappropriate antidiuretic hormone secretion (75,76). Although rare, there are also case reports of cocaine-associated kidney infarction, presumably due to thrombotic or embolic disease, vasospasm, cardiogenic shock, or other forms of occlusive large vessel disease, such as dissection, aneurysmal rupture, trauma, or vasculitis (77–79). Finally, although not closely examined in recent literature, pregnant mothers who abuse cocaine place their fetuses at increased risk in many ways, including untoward and detrimental effects on the urinary system (80–82).
Recent Emergence of Levamisole-Adulterated Cocaine
An important and interesting association between cocaine use and the development of a systemic syndrome with renal and nonrenal manifestations has come to light in recent years after the discovery that drug dealers were cutting pure cocaine with levamisole (83–85). Levamisole is a nematode-specific nicotinic acetylcholine receptor antagonist that is used as an antihelminthic agent in animals. It was also used as an immunomodulator for minimal-change glomerulopathy and rheumatoid arthritis and was even approved by the Food and Drug Administration in 1991 as adjuvant therapy for colorectal cancer. In 1999, however, it was withdrawn from the market in the United States because of adverse effects, most notably agranulocytosis (86,87). Of note, it is still under investigation as a possible therapy for aplastic anemia (88). An increased incidence of agranulocytosis was first noticed among cocaine abusers in 2008, and toxicologic analysis of urine revealed the presence of cocaine and levamisole (89). By 2009, levamisole was detected in 69% of cocaine entering the United States that was seized by law enforcement officials (86,90). A mini-epidemic of complications related to levamisole-adulterated cocaine use ensued (83,84,91). It is thought that cocaine is cut with levamisole because levamisole is a white, odorless powder that may potentiate euphoria. Furthermore, levamisole is metabolized to aminorex, an anorectic and amphetamine-like stimulant (87).
The most striking association with levamisole-adulterated cocaine use, based on two key case series, is ANCA-associated vasculitis characterized by constitutional symptoms, arthralgias, and cutaneous necrotizing vasculitis (Figure 2), with or without pulmonary hemorrhage and/or pauci-immune focal necrotizing and crescentic GN. Serologically, almost all patients have antimyeloperoxidase (MPO)-ANCA and at least half of all patients also have antiproteinase 3 (PR3)-ANCA. In fact, positivity for both MPO- and PR3-ANCA is now becoming pathognomonic for levamisole-adulterated cocaine exposure (85). In addition, antinuclear autoantibodies, lupus anticoagulant, and low complement levels are detected in most patients. Although testing for autoantibodies to other neutrophil granule constituents is not routine, it appears that autoantibodies are also formed to human neutrophil elastase, cathepsin G, and lactoferrin (83–85). Furthermore, urine detection of cocaine and levamisole is an important diagnostic step. Interestingly, the earliest report of levamisole-induced nephropathy in 1978 described a patient with rheumatoid arthritis treated with levamisole who developed a pruritic rash; leukopenia; thrombocytopenia; circulating immune complexes; proteinuria; and a kidney biopsy with granular mesangial deposits of IgA, IgG, IgM, and C3 and skin biopsy with granular IgM and C3 deposits located in the dermal-epidermal junction, all of which resolved after drug cessation (92). Therefore, it is important to recognize that levamisole has also been associated with immune complex GN.
Figure 2.
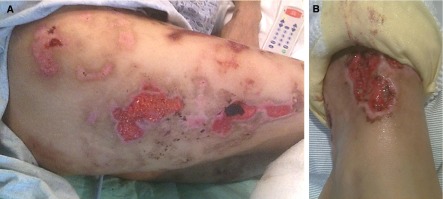
Representative cutaneous manifestations of levamisole–adulterated cocaine abuse. Large regions of necrotic, raised-edge, weeping ulcerations on the (A) thighs and (B) ankle of a patient with levamisole–adulterated cocaine-induced ANCA vasculitis.
Treatment and Future Directions
Treatment of the nephrotoxic effects of cocaine, including levamisole–adulterated cocaine-induced ANCA vasculitis, primarily involves immediate cessation of the causative agent, BP control, and supportive care focused on apparent nephrotoxic effects. Additional treatment modalities for levamisole–adulterated cocaine-induced ANCA vasculitis often include immunosuppression based on disease severity. However, data on efficacy are limited primarily to a single center, Massachusetts General Hospital, where it appears that treatment typically mirrors that of patients with idiopathic ANCA vasculitis and is necessary and effective in severe cases (83). Furthermore, any potential treatment strategy is limited by struggles with adherence in individuals whose main priorities focus on the use and acquisition of cocaine (85). More targeted therapeutic options are needed, which will require in-depth mechanistic insights. Mechanistic animal studies could prove to be quite useful. Furthermore, it could also be proposed that levamisole or one of its byproducts, in the setting of cocaine use, could specifically react with MPO and/or PR3 to create neoantigens and spur autoimmunity.
Conclusions
There is an ever-growing global burden of illicit drug use and dependence (3), and multiple reports of late implicate specific illicit drugs as nephrotoxins that were not previously known. Anabolic androgenic steroids, synthetic cannabinoids (also known as “Spice” or “K2”), ecstasy (formally known as MDMA), and cocaine and its levamisole-adulterated counterpart are common or emerging drugs of abuse with severe nephrotoxic effects about which both the community and health care providers should become more aware.
The clinicopathologic characteristics of the illicit drugs reviewed herein can easily be confounded by impurities or adulterants, dose, frequency, and concomitant polypharmacy. In fact, as an example, had levamisole not been discovered in cocaine preparations, investigators and clinicians alike might assume that cocaine itself was causing the untoward effects that are actually due to levamisole. Although rapid recognition, detection, and diagnosis of the associations described with these nephrotoxic drugs of abuse are paramount, it should also be emphasized that the features of untoward renal effects (e.g., timing, duration, and severity of exposure) can easily be modified by confounding impurities, dose, frequency, and concurrent use of other legal and illegal drugs. These confounders, which often remain unknown to medical providers, make prompt diagnosis challenging. Regardless, treatment primarily consists of immediate discontinuation of the offending agent and supportive care depending on disease severity. Future investigation into pathogenetic mechanisms associated with these drugs is critical and may provide a window into ways to impede and even prevent the nephrotoxic effects of these drugs of abuse and perhaps allow a deeper understanding of the nephrotoxicities themselves.
Disclosures
D.T.B. is a participant in a multicenter study sponsored by Genzyme (A Sanofi Company). J.L.N. has served as a rituximab-specific advisory board member for Genentech and is currently participating in the Genentech-sponsored RAVER study and receiving clinical research funding from Alexion Pharmaceuticals.
Acknowledgments
W.F.P. and J.L.N. wish to thank the patients; the clinic manager, Karen Laliberte; the clinical research coordinator, Andrew P. Murphy; and the clinic nurses dedicated to their care in the Massachusetts General Hospital Vasculitis and Glomerulonephritis Clinic, including Donna Hagstrom, Kate Cosgrove, Eleanor Coughlin, Laura Chambers White, Chelsea Barrett, Stefanie Navarro, Luke Cogswell, and Naira Arrellano.
W.F.P. is supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant no. 1-F32-DK097891-02.
Part of this work was presented at the American Society of Nephrology’s Kidney Week, November 5–10, 2013, Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Perazella MA: Renal vulnerability to drug toxicity. Clin J Am Soc Nephrol 4: 1275–1283, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Loghman-Adham M, Kiu Weber CI, Ciorciaro C, Mann J, Meier M: Detection and management of nephrotoxicity during drug development. Expert Opin Drug Saf 11: 581–596, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJ, Vos T: Global burden of disease attributable to illicit drug use and dependence: Findings from the Global Burden of Disease Study 2010. Lancet 382: 1564–1574, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Kanayama G, Hudson JI, Pope HG, Jr: Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: A looming public health concern? Drug Alcohol Depend 98: 1–12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanayama G, Hudson JI, Pope HG, Jr: Illicit anabolic-androgenic steroid use. Horm Behav 58: 111–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope HG, Jr, Kanayama G, Hudson JI: Risk factors for illicit anabolic-androgenic steroid use in male weightlifters: A cross-sectional cohort study. Biol Psychiatry 71: 254–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG, Jr: Anabolic-androgenic steroid dependence: An emerging disorder. Addiction 104: 1966–1978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herlitz LC, Markowitz GS, Farris AB, Schwimmer JA, Stokes MB, Kunis C, Colvin RB, D’Agati VD: Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 21: 163–172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan BP, Kanayama G, Hudson JI, Pope HG, Jr: Human growth hormone abuse in male weightlifters. Am J Addict 20: 9–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ: Weightlifting’s risky new trend: A case series of 41 insulin users. Curr Sports Med Rep 11: 176–179, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ: The Anabolic 500 survey: Characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy 31: 757–766, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Winnett G, Cranfield L, Almond M: Apparent renal disease due to elevated creatinine levels associated with the use of boldenone. Nephrol Dial Transplant 26: 744–747, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Harrington P, Ali G, Chan A: The development of focal segmental glomerulosclerosis secondary to anabolic steroid abuse. BMJ Case Rep 2011: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartung R, Gerth J, Fünfstück R, Gröne HJ, Stein G: End-stage renal disease in a bodybuilder: a multifactorial process or simply doping? Nephrol Dial Transplant 16: 163–165, 2001 [DOI] [PubMed] [Google Scholar]
- 15.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD: Obesity-related glomerulopathy: An emerging epidemic. Kidney Int 59: 1498–1509, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Silbiger S, Neugarten J: Gender and human chronic renal disease. Gend Med 5[Suppl A]: S3–S10, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, Berho M, Elliot SJ, Karl M: Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E, Estrada M: Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc 45: 1712–1720, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, Lavandero S, Uhlén P, Estrada M: Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol 202: 299–307, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S, Dong Z: Emerging role of autophagy in kidney function, diseases and aging. Autophagy 8: 1009–1031, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Monitoring Centre for Drugs and Drug Addiction: Understanding the ‘Spice’ phenomenon. Lisbon, Portugal: European Monitoring Center for Drugs and Drug Addicton, 2009 [Google Scholar]
- 24.American Association of Poison Control Centers: Synthetic marijuana data. October 31, 2013. Available at: https://aapcc.s3.amazonaws.com/files/library/Synthetic_Marijuana_Data_for_Website_10.31.2013.pdf. Accessed October 31, 2013
- 25.National Forensic Laboratory Information System Special Report: Synthetic cannabinoids and synthetic cathinones reported in NFLIS, Springfield, VA, U.S. Drug Enforcement Administration, Office of Diversion Control, 2011 [Google Scholar]
- 26.Hu X, Primack BA, Barnett TE, Cook RL: College students and use of K2: An emerging drug of abuse in young persons. Subst Abuse Treat Prev Policy 6: 16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE: Monitoring the Future National Survey Results on Drug Use, 1975-2012, Volume I: Secondary School Students, Ann Arbor, MI, Institute for Social Research, University of Michigan, 2013 [Google Scholar]
- 28.Gaoni Y, Machoulam R: Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86(8): 1646–1647, 1964 [Google Scholar]
- 29.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI: Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Munro S, Thomas KL, Abu-Shaar M: Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA: International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB₁ and CB₂. Pharmacol Rev 62: 588–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacher P, Kunos G: Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J 280: 1918–1943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers L: Spiked Spice sickening users in DeKalb County. The Gadsden Times; May 11, 2012 [Google Scholar]
- 34.Morton T: Wyoming Spice smokers hospitalized with potential kidney failure. Star-Tribune March 2, 2012 [Google Scholar]
- 35.Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D: AKI associated with synthetic cannabinoids: A case series. Clin J Am Soc Nephrol 8: 523–526, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) : Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep 62: 93–98, 2013 [PMC free article] [PubMed] [Google Scholar]
- 37.Kazory A, Aiyer R: Synthetic Marijuana and acute kidney injury: An unforeseen association. Clin Kidney J 6(3): 330–333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winstock AR, Barratt MJ: Synthetic cannabis: A comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend 131: 106–111, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, Rastaldi MP, Perin PC, Gruden G: Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 59: 1046–1054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkin KA, McAinch AJ, Grinfeld E, Hryciw DH: Role for cannabinoid receptors in human proximal tubular hypertrophy. Cell Physiol Biochem 26: 879–886, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC: Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 100: 1538–1546, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukhopadhyay P, Pan H, Rajesh M, Bátkai S, Patel V, Harvey-White J, Mukhopadhyay B, Haskó G, Gao B, Mackie K, Pacher P: CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol 160: 657–668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Downing J: The psychological and physiological effects of MDMA on normal volunteers. J Psychoactive Drugs 18: 335–340, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Steele TD, McCann UD, Ricaurte GA: 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”): pharmacology and toxicology in animals and humans. Addiction 89: 539–551, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Peroutka SJ: Incidence of recreational use of 3,4-methylenedimethoxymethamphetamine (MDMA, “ecstasy”) on an undergraduate campus. N Engl J Med 317: 1542–1543, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Johnstone AC, Lea RA, Brennan KA, Schenk S, Kennedy MA, Fitzmaurice PS: Benzylpiperazine: A drug of abuse? J Psychopharmacol 21: 888–894, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Johnson J, Hermann P. “Molly” ecstasy drug linked to 3 deaths in week. 2013. http://e.standard.net/stories/2013/09/06/molly-ecstasy-drug-linked-3-deaths-week. December 21, 2013 [Google Scholar]
- 48.Lyles J, Cadet JL: Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev 42: 155–168, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Hall AP, Henry JA: Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: Overview of pathophysiology and clinical management. Br J Anaesth 96: 678–685, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Steinkellner T, Freissmuth M, Sitte HH, Montgomery T: The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol Chem 392: 103–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell GA, Rosner MH: The agony of ecstasy: MDMA (3,4-methylenedioxymethamphetamine) and the kidney. Clin J Am Soc Nephrol 3: 1852–1860, 2008 [DOI] [PubMed] [Google Scholar]
- 52.de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J: Human pharmacology of MDMA: Pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26: 137–144, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Henry JA, Fallon JK, Kicman AT, Hutt AJ, Cowan DA, Forsling M: Low-dose MDMA (“ecstasy”) induces vasopressin secretion. Lancet 351: 1784, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Rietjens SJ, Hondebrink L, Westerink RH, Meulenbelt J: Pharmacokinetics and pharmacodynamics of 3,4-methylenedioxymethamphetamine (MDMA): Interindividual differences due to polymorphisms and drug-drug interactions. Crit Rev Toxicol 42: 854–876, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Fahal IH, Sallomi DF, Yaqoob M, Bell GM: Acute renal failure after ecstasy. BMJ 305: 29, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon C, Zaritsky A, Dharnidharka VR: Transient proximal tubular renal injury following Ecstasy ingestion. Pediatr Nephrol 18: 820–822, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Ajaelo I, Koenig K, Snoey E: Severe hyponatremia and inappropriate antidiuretic hormone secretion following ecstasy use. Acad Emerg Med 5: 839–840, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Holden R, Jackson MA: Near-fatal hyponatraemic coma due to vasopressin over-secretion after “ecstasy” (3,4-MDMA). Lancet 347: 1052, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Fallon JK, Shah D, Kicman AT, Hutt AJ, Henry JA, Cowan DA, Forsling M: Action of MDMA (ecstasy) and its metabolites on arginine vasopressin release. Ann N Y Acad Sci 965: 399–409, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Forsling ML, Fallon JK, Shah D, Tilbrook GS, Cowan DA, Kicman AT, Hutt AJ: The effect of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its metabolites on neurohypophysial hormone release from the isolated rat hypothalamus. Br J Pharmacol 135: 649–656, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Dijken GD, Blom RE, Hené RJ, Boer WH, Consortium N, NIGRAM Consortium : High incidence of mild hyponatraemia in females using ecstasy at a rave party. Nephrol Dial Transplant 28: 2277–2283, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Ayus JC, Achinger SG, Arieff A: Brain cell volume regulation in hyponatremia: Role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol 295: F619–F624, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Peate WF: Hyponatremia in marathon runners. N Engl J Med 353: 427–428, author reply 427–428, 2005 [DOI] [PubMed] [Google Scholar]
- 64.National Institute on Drug Abuse. Cocaine. May 1999. Available at: http://www.drugabuse.gov/publications/research-reports/cocaine-abuse-addiction. Accessed November 15, 2013
- 65.Pomara C, Cassano T, D’Errico S, Bello S, Romano AD, Riezzo I, Serviddio G: Data available on the extent of cocaine use and dependence: biochemistry, pharmacologic effects and global burden of disease of cocaine abusers. Curr Med Chem 19: 5647–5657, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Jaffe JA, Kimmel PL: Chronic nephropathies of cocaine and heroin abuse: A critical review. Clin J Am Soc Nephrol 1: 655–667, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Lange RA, Hillis LD: Cardiovascular complications of cocaine use. N Engl J Med 345: 351–358, 2001 [DOI] [PubMed] [Google Scholar]
- 68.Bosch X, Poch E, Grau JM: Rhabdomyolysis and acute kidney injury. N Engl J Med 361: 62–72, 2009 [DOI] [PubMed] [Google Scholar]
- 69.van der Woude FJ: Cocaine use and kidney damage. Nephrol Dial Transplant 15: 299–301, 2000 [DOI] [PubMed]
- 70.Samuels P, Steinfeld JD, Braitman LE, Rhoa MF, Cines DB, McCrae KR: Plasma concentration of endothelin-1 in women with cocaine-associated pregnancy complications. Am J Obstet Gynecol 168: 528–533, 1993 [DOI] [PubMed] [Google Scholar]
- 71.Kon V, Yoshioka T, Fogo A, Ichikawa I: Glomerular actions of endothelin in vivo. J Clin Invest 83: 1762–1767, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sáez CG, Olivares P, Pallavicini J, Panes O, Moreno N, Massardo T, Mezzano D, Pereira J: Increased number of circulating endothelial cells and plasma markers of endothelial damage in chronic cocaine users. Thromb Res 128: e18–e23, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Akkina SK, Ricardo AC, Patel A, Das A, Bazzano LA, Brecklin C, Fischer MJ, Lash JP: Illicit drug use, hypertension, and chronic kidney disease in the US adult population. Transl Res 160: 391–398, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grossman E, Messerli FH: Drug-induced hypertension: An unappreciated cause of secondary hypertension. Am J Med 125: 14–22, 2012 [DOI] [PubMed] [Google Scholar]
- 75.Karim MR, Jawairia M, Rahman S, Balsam L, Rubinstein S: Cocaine-associated acute severe hyponatremia. Clin Nephrol 75[Suppl 1]: 11–15, 2011 [PubMed] [Google Scholar]
- 76.Espinoza LR, Perez Alamino R: Cocaine-induced vasculitis: Clinical and immunological spectrum. Curr Rheumatol Rep 14: 532–538, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Bemanian S, Motallebi M, Nosrati SM: Cocaine-induced renal infarction: Report of a case and review of the literature. BMC Nephrol 6: 10, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoefsloot W, de Vries RA, Bruijnen R, Bosch FH: Renal infarction after cocaine abuse: A case report and review. Clin Nephrol 72: 234–236, 2009 [DOI] [PubMed] [Google Scholar]
- 79.De Giorgi A, Fabbian F, Pala M, Bonetti F, Babini I, Bagnaresi I, Manfredini F, Portaluppi F, Mikhailidis DP, Manfredini R: Cocaine and acute vascular diseases. Curr Drug Abuse Rev 5: 129–134, 2012 [DOI] [PubMed] [Google Scholar]
- 80.Mitra SC: Effect of cocaine on fetal kidney and bladder function. J Matern Fetal Med 8: 262–269, 1999 [DOI] [PubMed] [Google Scholar]
- 81.Mitra SC, Seshan SV, Salcedo JR, Gil J: Maternal cocaine abuse and fetal renal arteries: A morphometric study. Pediatr Nephrol 14: 315–318, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Kashiwagi M, Chaoui R, Stallmach T, Hürlimann S, Lauper U, Hebisch G: Fetal bilateral renal agenesis, phocomelia, and single umbilical artery associated with cocaine abuse in early pregnancy. Birth Defects Res A Clin Mol Teratol 67: 951–952, 2003 [DOI] [PubMed] [Google Scholar]
- 83.McGrath MM, Isakova T, Rennke HG, Mottola AM, Laliberte KA, Niles JL: Contaminated cocaine and antineutrophil cytoplasmic antibody-associated disease. Clin J Am Soc Nephrol 6: 2799–2805, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graf J, Lynch K, Yeh CL, Tarter L, Richman N, Nguyen T, Kral A, Dominy S, Imboden J: Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum 63: 3998–4001, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Pendergraft WF, 3rd, Niles JL: Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol 26: 42–49, 2014 [DOI] [PubMed] [Google Scholar]
- 86.Chang A, Osterloh J, Thomas J: Levamisole: A dangerous new cocaine adulterant. Clin Pharmacol Ther 88: 408–411, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Karch SB, Mari F, Bartolini V, Bertol E: Aminorex poisoning in cocaine abusers. Int J Cardiol 158: 344–346, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Li X, Shao Y, Ge M, Shi J, Huang J, Huang Z, Zhang J, Nie N, Zheng Y: A promising immunosuppressive strategy of cyclosporine alternately combined with levamisole is highly effective for moderate aplastic anemia. Ann Hematol 92: 1239–1247, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Zhu NY, Legatt DF, Turner AR: Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 150: 287–289, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Centers for Disease Control and Prevention (CDC) : Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep 58: 1381–1385, 2009 [PubMed] [Google Scholar]
- 91.Graf J: Rheumatic manifestations of cocaine use. Curr Opin Rheumatol 25: 50–55, 2013 [DOI] [PubMed] [Google Scholar]
- 92.Hansen TM, Petersen J, Halberg P, Permin H, Ullman S, Brun C, Larsen S: Levamisole-induced nephropathy. Lancet 2: 737, 1978 [DOI] [PubMed] [Google Scholar]