Abstract
Background and objectives
Various dietary strategies have been investigated to slow kidney function decline. However, it is unknown whether a Mediterranean diet, which has been associated with improved cardiovascular risk, is associated with change in kidney function.
Design, setting, participants, & measurements
This study used the Northern Manhattan Study, a prospective, multiethnic, observational cohort of participants who were stroke free at baseline. Data were collected between 1993 and 2008. Serum creatinine measurements were taken a mean 6.9 years apart. A baseline dietary questionnaire was extrapolated into a previously used 9-point scoring system (MeDi). The primary outcome was incident eGFR<60 ml/min per 1.73 m2using the Modification of Diet in Renal Disease formula. A secondary outcome was the upper quartile of annualized eGFR decline (≥2.5 ml/min per 1.73 m2 per year). Conditional logistic regression models adjusted for demographics and baseline vascular risk factors.
Results
Mean baseline age was 64 years, with 59% women and 65% Hispanics (N=900); mean baseline eGFR was 83.1 ml/min per 1.73 m2. Incident eGFR<60 ml/min per 1.73 m2 developed in 14% . In adjusted models, every 1-point increase in the MeDi score, indicating increasing adherence to a Mediterranean diet, was associated with decreased odds of incident eGFR<60 ml/min per 1.73 m2 (odds ratio, 0.83; 95% confidence interval, 0.71 to 0.96) and decreased odds of being in the upper quartile of eGFR decline (odds ratio, 0.88; 95% confidence interval, 0.79 to 0.98).
Conclusions
A Mediterranean diet was associated with a reduced incidence of eGFR<60 ml/min per 1.73 m2 and upper quartile of eGFR decline in a multiethnic cohort.
Keywords: CKD, nutrition, progression of chronic renal failure
Introduction
CKD is a growing epidemic resulting in substantial morbidity, mortality, and economic costs (1,2). Although there has been significant progress in protecting against kidney disease and its progression through aggressive treatment of established traditional risk factors such as hypertension (3), diabetes (4), and proteinuria (5), kidney function still declines over time. Ascertaining other modifiable risk factors that may modulate CKD incidence or progression could assist prevention and treatment strategies.
One such risk factor is diet. There is mounting evidence that poor dietary patterns may lead to kidney disease (6,7). Until recently, dietary studies in CKD have focused mainly on protein intake, which have suggested a modest benefit with protein restriction (8,9). The effect of other dietary patterns on kidney function is unclear. The Mediterranean diet has received attention for ameliorating cardiovascular risk in observational studies (10,11) and randomized trials (12,13). Purported mechanisms of action, including improvements in BP (14,15), lipid profile (15), endothelial function (16), and inflammation (17), may benefit the kidney as well. Few studies have examined relationships between a Mediterranean-style diet and kidney function, but their results are inconclusive due to cross-sectional study design (18–21), limited demographics (6,18), or short duration (22). The purpose of this observational analysis was to characterize the effect of varying degrees of a Mediterranean-style diet on long-term kidney function in a community-based, prospective cohort. We hypothesized that increased adherence to a Mediterranean diet would be associated with a decreased loss of kidney function.
Materials and Methods
Selection of Prospective Cohort
The Northern Manhattan Study (NOMAS) is a prospective, multiethnic, community-based cohort of 3298 participants recruited from a geographic area of northern Manhattan, New York. The details of recruitment and assessment were previously described (23). Briefly, participants were enrolled between 1993 and 2001, and were eligible if they met the following criteria: no prior history of stroke, age >40 years, and residence in a household with a telephone in northern Manhattan. The institutional review boards of Columbia University and University of Miami approved the study.
Baseline Evaluation and Follow-Up
Demographic, laboratory, and medical history data were collected at baseline enrollment. Participants were followed annually starting in 1998. From 2003 to 2008, a subgroup of 1091 participants from the original cohort was recruited into a brain magnetic resonance imaging (MRI) substudy. In order to be eligible for this substudy, participants had to be clinically free of stroke, free of contraindications to MRI, aged at least 55 years, and able to sign informed consent. A new set of laboratory data, including serum creatinine, were collected upon enrollment.
Inclusion criteria for this analysis included all participants enrolled in the MRI substudy. The final sample size was 900, because participants were excluded if they did not have two serum creatinine measurements (n=81) or were lacking baseline dietary assessment (n=110).
Assessment of Kidney Function and Outcomes
Serum creatinine was measured using the Jaffé reaction. Kidney function was estimated using the Modification of Diet in Renal Disease (MDRD) formula for eGFR as follows:
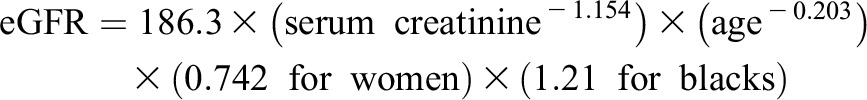 |
Few participants (n=41) had serum creatinine measurements that were standardized to the isotope dilution mass spectrometry reference and the eGFR was calculated by the following formula (24):
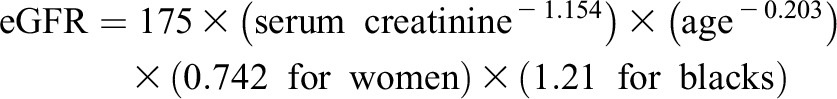 |
Participants had their eGFR assessed at the time of original enrollment, as well as at the time of enrollment into the MRI substudy. The MDRD formula was used instead of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (25) because of a lack of standardized creatinine values in all participants.
The primary outcome of incident eGFR<60 ml/min per 1.73 m2 was defined as participants who started with an eGFR≥60 ml/min per 1.73 m2 at baseline, and had an eGFR<60 ml/min per 1.73 m2 on follow-up. Absolute change in kidney function was evaluated as an annualized rate of change in the eGFR. We also created a dichotomized variable using a cutoff of the upper quartile of annualized eGFR decliners within this cohort, a threshold used previously that portends a higher risk of cardiovascular disease (26). For the primary outcome of incident eGFR<60 ml/min per 1.73 m2, 97 participants had an eGFR<60 ml/min per 1.73 m2 at baseline and were excluded (n=803 for this analysis). These participants, however, were included in the outcomes involving continuous annualized eGFR decline and upper quartile of annualized eGFR decline.
Measurement of Other Covariates
Demographic variables and well established baseline risk factors for CKD progression were assessed as previously described and included in multivariable regression models (23). Race/ethnicity was based on self-identification. Educational status was dichotomized based on completion of high school. Insurance status was classified as having Medicaid/no insurance versus other (Medicare or private insurance). Hypertension was considered present if BP exceeded 140/90 based on an average of two measurements during initial evaluation, self-reported history of hypertension, or baseline antihypertensive medication use. Cigarette smoking status was categorized as never smoked, prior smoking, or current smoking within the last year. Fasting HDL cholesterol and calculated LDL cholesterol were included. Diabetes mellitus was present if participants had a self-reported history, used oral hypoglycemic agents or insulin, or fasting blood sugar was measured to be at least 126 mg/dl. Physical activity was based on exercise questionnaires. Body mass index was calculated as follows: (weight in kilograms/ [height in meters×height in meters]). Analyses were also adjusted for baseline use of either angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs).
Diet
At baseline, participants were administered a modified Block National Cancer Institute food frequency questionnaire (27) to assess dietary patterns over the preceding year. Responses were converted to a Mediterranean Diet (MeDi) score as previously described (11,28,29). Kilocalorie intake was regressed and residuals of daily gram intake were calculated for the following components: dairy, meat, fruit, vegetables (excluding potatoes), legumes, cereals, and fish (28). Participants were assigned a value of 1 for each beneficial category (fruit, vegetables, legumes, cereals, and fish) in which consumption was at least as high as the sex-specific median. A value of 1 was assigned if daily intake was below the sex-specific median for the detrimental food groups (dairy and meat). In addition, 1 point each was added for moderate alcohol consumption and a dietary ratio of monounsaturated fats to saturated fats that was at or above the median. Moderate alcohol consumption was defined as drinking two or fewer drinks per day (30). In total, there were nine components of the MeDi score, which ranged from 0–9, with higher scores representing greater similarity to a Mediterranean diet. Approximately 84% of the entire NOMAS cohort had sufficient data to calculate a MeDi score.
Statistical Analyses
The distribution of baseline characteristics was calculated by the median MeDi score for the cohort (<5 versus ≥5), and was tested for differences using the Wilcoxon rank-sum test for continuous variables and the chi-squared test for categorical variables. The primary outcome was the change in eGFR from ≥60 ml/min per 1.73 m2 at baseline to <60 ml/min per 1.73 m2 at follow-up. Secondary outcomes included annualized eGFR change, as well as a binary outcome for upper quartile of annualized eGFR decline within this cohort (≥2.5 ml/min per 1.73 m2 per year). The MeDi score was used continuously, dichotomized at the median score, and was also used as quartiles as distributed in the NOMAS cohort (0–3, 4, 5, and 6–9). Conditional logistic regression models that account for strata of time differences between the two measures of creatinine were fitted for the outcome of incident eGFR<60 ml/min per 1.73 m2, logistic regression modeling was used for the upper quartile of decline, and linear regression analyses were performed for the continuous annual change in eGFR outcome. Three models were fitted: (1) unadjusted, (2) adjusted for demographics, and (3) fully adjusted with comorbidities, medications, and laboratory results. Interaction terms between continuous MeDi scores and all covariates were tested for the primary outcome, with stratified analyses performed for interaction terms with a P value <0.05. All analyses were performed using SAS 9.3 software (Cary, NC).
Results
Baseline Characteristics
The sample size included 900 participants (Table 1), with a mean follow-up period of 6.9 years (SD 2.3 years) between serum creatinine measurements. The mean age was 64 years (SD 8.1 years); 65% of participants were Hispanic, 17% were black, and 15% were white. The mean baseline eGFR was 83.1 ml/min per 1.73 m2 (SD 19.9 ml/min per 1.73 m2), with a mean annualized decline of 1.1 ml/min per 1.73 m2 (SD 2.8 ml/min per 1.73 m2). The median MeDi score was 4 (interquartile range, 3–6). Participants with a MeDi score ≥5 were more likely to be physically active compared with those with MeDi score <5 (P=0.003), but otherwise there were no statistically significant differences in characteristics between participants with a MeDi score ≥5 versus <5. We also assessed for differences in characteristics and risk factors between the 900 participants included in this study and the 191 individuals that were not included because of the unavailability of a MeDi score. We found that both groups were similar with respect to all variables, including annual change in eGFR, with the exception that the study group had a slightly lower HDL (45.7 versus 47.3 mg/dl).
Table 1.
Baseline characteristics
| Characteristic | MeDi Score <5 (n=455) | MeDi Score ≥5 (n=445) | P Value |
|---|---|---|---|
| Mean age, yr | 64.2 (8.2) | 64.1 (7.9) | 0.58 |
| Male sex | 177 (39) | 193 (43) | 0.17 |
| Race/ethnicity | |||
| White | 69 (15) | 68 (15) | Ref |
| Black | 83 (18) | 74 (17) | 0.67 |
| Hispanic | 291 (64) | 295 (66) | 0.88 |
| Other | 12 (3) | 8 (2) | n/a |
| High school education | 208 (46) | 196 (44) | 0.62 |
| Medicare or private insurance | 249 (55) | 219 (49) | 0.10 |
| Smoking status | |||
| Never | 215 (47) | 210 (47) | Ref |
| Past | 163 (36) | 172 (39) | 0.60 |
| Current | 77 (17) | 63 (14) | 0.37 |
| Physical activity (any exercise versus not) | 232 (51) | 270 (61) | 0.003 |
| ACE inhibitor or ARB usage | 80 (18) | 76 (17) | 0.84 |
| Hypertension | 312 (69) | 309 (69) | 0.78 |
| Diabetes | 90 (20) | 80 (18) | 0.49 |
| Mean BMI, kg/m2 | 28.2 (5.0) | 27.8 (4.5) | 0.37 |
| Mean LDL, mg/dl | 130.3 (34.5) | 130.1 (34.5) | 0.78 |
| Mean HDL, mg/dl | 45.6 (14.4) | 45.8 (13.7) | 0.75 |
| Mean eGFR at baseline, ml/min per 1.73 m2 | 82.4 (21.1) | 83.9 (18.7) | 0.06 |
Results are presented as n (%) or mean (SD). Baseline characteristics are for the entire study sample (N=900). For the primary outcome of incident eGFR <60 ml/min per 1.73 m2, 97 participants had an eGFR <60 ml/min per 1.73 m2 at baseline and were excluded (n=803 for this analysis). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; MeDi, Mediterranean Diet scoring system; Ref, reference; n/a, not applicable.
Primary Analyses
Table 2 represents the odds of incident eGFR<60 ml/min per 1.73 m2. Among those with an eGFR≥60 at baseline (n=803), 115 participants (14%) developed eGFR<60 ml/min per 1.73 m2. In fully adjusted models, higher MeDi scores protected against kidney function decline. Every 1-point increase in the MeDi score was associated with a 17% reduced odds of the eGFR falling to <60 ml/min per 1.73 m2 at follow-up (adjusted odds ratio [OR], 0.83; 95% confidence interval [95% CI], 0.71 to 0.96). In addition, a MeDi score ≥5 (versus <5) was associated with a 50% lower odds of incident eGFR<60 ml/min per 1.73 m2 (adjusted OR, 0.50; 95% CI, 0.31 to 0.81). In fully adjusted analyses, associations with each of the upper two quartiles of MeDi scores did not reach statistical significance (Table 2). A sensitivity analysis excluding participants with incident eGFR<60 ml/min per 1.73 m2 who had only small annual eGFR changes (≤1 ml/min per 1.73 m2 per year; n=10) yielded similar results (data not shown). Another sensitivity analysis excluded participants with an eGFR between 60 and 69 ml/min per 1.73 m2, which also did not change results (data not shown).
Table 2.
Association between MeDi score and incident eGFR<60 ml/min per 1.73 m2
| MeDi Score | OR (95% CI) | P Value |
|---|---|---|
| Continuous (per 1-point increase in MeDi score) | ||
| Unadjusted | 0.81 (0.72 to 0.92) | 0.002 |
| Demographic adjusteda | 0.83 (0.73 to 0.95) | <0.01 |
| Fully adjustedb | 0.83 (0.71 to 0.96) | 0.02 |
| MeDi score ≥5 (versus <5) | ||
| Unadjusted | 0.49 (0.33 to 0.75) | 0.001 |
| Demographic adjusteda | 0.49 (0.32 to 0.75) | 0.001 |
| Fully adjustedb | 0.50 (0.31 to 0.81) | 0.004 |
| MeDi quartiles (unadjusted) | ||
| 0–3 | Ref | |
| 4 | 1.30 (0.78 to 2.16) | 0.31 |
| 5 | 0.52 (0.28 to 0.95) | 0.03 |
| 6–9 | 0.60 (0.34 to 1.07) | 0.08 |
| MeDi quartiles (demographic adjusted) | ||
| 0–3 | Ref | |
| 4 | 1.44 (0.85 to 2.46) | 0.18 |
| 5 | 0.50 (0.27 to 0.93) | 0.03 |
| 6–9 | 0.67 (0.37 to 1.21) | 0.18 |
| MeDi quartiles (fully adjusted) | ||
| 0–3 | Ref | |
| 4 | 1.61 (0.88 to 2.97) | 0.12 |
| 5 | 0.51 (0.26 to 1.02) | 0.06 |
| 6–9 | 0.76 (0.39 to 1.46) | 0.41 |
OR, odds ratio; 95% CI, 95% confidence interval.
Adjusted for age (years), sex, race (black, white, Hispanic, or other), education (high school completion versus not), and insurance status (Medicare/private insurance versus Medicaid/no insurance).
Adjusted for age (years), sex, race/ethnicity (black, white, Hispanic, or other), education (high school completion versus not), insurance status (Medicare/private insurance versus Medicaid/no insurance), physical activity (any exercise versus not), BMI (kg/m2), diabetes (yes or no), smoking status (never, past, or current), hypertension (yes or no), LDL (mg/dl), HDL (mg/dl), baseline eGFR (ml/min per 1.73 m2), and ACE inhibitor/ARB usage (yes or no).
Secondary Analyses
We determined associations between MeDi scores and absolute changes in kidney function. We dichotomized the cohort at the upper quartile of annualized eGFR decline (≥2.5 ml/min per 1.73 m2 per year), which is higher than expected with normal aging. In fully adjusted analyses (Table 3), every 1-point increase in the MeDi score was associated with a 12% reduced odds of the eGFR declining ≥2.5 ml/min per 1.73 m2 per year (OR, 0.88; 95% CI, 0.79 to 0.98). In fully adjusted analyses, associations with each of the upper two quartiles of MeDi scores either reached statistical significance or showed a strong trend (Table 3).With annualized eGFR change as the outcome, there was no statistically significant association in fully adjusted analysis with the continuous MeDi score (Table 4). By contrast, a trend for a reduced rate of decline in eGFR of 0.31 ml/min per 1.73 m2 per year (P=0.06) was found for participants with a MeDi score ≥5 versus <5 in fully adjusted analysis.
Table 3.
Association between MeDi score and upper quartile of eGFR decline
| MeDi Score | OR (95% CI) | P Value |
|---|---|---|
| Continuous (per 1-point increase in MeDi score) | ||
| Unadjusted | 0.88 (0.80 to 0.96) | 0.01 |
| Demographic adjusteda | 0.89 (0.80 to 0.98) | 0.02 |
| Fully adjustedb | 0.88 (0.79 to 0.98) | 0.02 |
| MeDi score ≥5 (versus <5) | ||
| Unadjusted | 0.65 (0.48 to 0.88) | 0.01 |
| Demographic adjusteda | 0.65 (0.47 to 0.88) | 0.01 |
| Fully adjustedb | 0.58 (0.41 to 0.83) | 0.003 |
| MeDi quartiles (unadjusted) | ||
| 0–3 | Ref | |
| 4 | 0.81 (0.53 to 1.23) | 0.32 |
| 5 | 0.52 (0.33 to 0.82) | 0.004 |
| 6–9 | 0.63 (0.41 to 0.95) | 0.03 |
| MeDi quartiles (demographic adjusted) | ||
| 0–3 | Ref | |
| 4 | 0.86 (0.55 to 1.33) | 0.49 |
| 5 | 0.51 (0.32 to 0.81) | 0.004 |
| 6–9 | 0.66 (0.43 to 1.02) | 0.06 |
| MeDi quartiles (fully adjusted) | ||
| 0–3 | Ref | |
| 4 | 1.01 (0.62 to 1.66) | 0.96 |
| 5 | 0.49 (0.29 to 0.82) | 0.01 |
| 6–9 | 0.67 (0.41 to 1.10) | 0.11 |
Adjusted for age (years), sex, race/ethnicity (black, white, Hispanic, or other), education (high school completion versus not), and insurance status (Medicare/private insurance versus Medicaid/no insurance).
Adjusted for age (years), sex, race/ethnicity (black, white, Hispanic, other), education (high school completion versus not), insurance status (Medicare/private insurance versus Medicaid/no insurance), physical activity (any exercise versus not), BMI (kg/m2), diabetes (yes or no), smoking status (never, past, or current), hypertension (yes or no), LDL (mg/dl), HDL (mg/dl), baseline eGFR (ml/min per 1.73 m2), and ACE inhibitor/ARB usage (yes or no).
Table 4.
Association between MeDi score and annualized change in eGFR
| MeDi Score | Parameter Estimate (95% CI)a | P Value |
|---|---|---|
| Continuous (per 1-point increase in MeDi score) | ||
| Unadjusted | 0.13 (0.01 to 0.24) | 0.03 |
| Demographic adjustedb | 0.09 (−0.02 to 0.21) | 0.12 |
| Fully adjustedc | 0.07 (−0.03 to 0.17) | 0.17 |
| MeDi score ≥5 (versus <5) | ||
| Unadjusted | 0.36 (−0.01 to 0.72) | 0.06 |
| Demographic adjustedb | 0.31 (−0.05 to 0.67) | 0.10 |
| Fully adjustedc | 0.31 (−0.01 to 0.64) | 0.06 |
| MeDi quartiles (unadjusted) | ||
| 0–3 | Ref | |
| 4 | 0.08 (−0.47 to 0.62) | 0.79 |
| 5 | 0.30 (−0.25 to 0.85) | 0.28 |
| 6–9 | 0.52 (−0.01 to 1.05) | 0.05 |
| MeDi quartiles (demographic adjusted) | ||
| 0–3 | Ref | |
| 4 | −0.11 (−0.66 to 0.43) | 0.68 |
| 5 | 0.25 (−0.29 to 0.80) | 0.36 |
| 6–9 | 0.34 (−0.19 to 0.87) | 0.21 |
| MeDi quartiles (fully adjusted) | ||
| 0–3 | Ref | |
| 4 | −0.37 (−0.86 to 0.12) | 0.13 |
| 5 | 0.11 (−0.37 to 0.60) | 0.65 |
| 6–9 | 0.20 (−0.28 to 0.67) | 0.42 |
Parameter estimate data are presented as the reduced rate of decline in eGFR in ml/min per 1.73 m2 per year.
Adjusted for age (years), sex, race/ethnicity (black, white, Hispanic, or other), education (high school completion versus not), and insurance status (Medicare/private insurance versus Medicaid/no insurance).
Adjusted for age (years), sex, race/ethnicity (black, white, Hispanic, or other), education (high school completion versus not), insurance status (Medicare/private insurance versus Medicaid/no insurance), physical activity (any exercise versus not), BMI (kg/m2), diabetes (yes or no), smoking status (never, past, or current), hypertension (yes or no), LDL (mg/dl), HDL (mg/dl), baseline eGFR (ml/min per 1.73 m2), and ACE inhibitor/ARB usage (yes or no).
These associations did not differ by baseline eGFR (≥60 versus <60 ml/min per 1.73 m2; P for interaction, P>0.05), and these results were similar when only participants with a baseline eGFR≥60 ml/min per 1.73 m2 were considered (data not shown). Results were materially unchanged if the CKD-EPI formula for estimating GFR was used (data not shown).
Interactions between MeDi Scores and Other Risk Factors
There was an interaction between MeDi scores and diabetes for incident eGFR<60 ml/min per 1.73 m2 (P for interaction, P=0.01). Stratified analyses revealed that the Mediterranean diet may be more beneficial in non-diabetics. Every 1-point increase in MeDi score was associated with a 25% reduced odds of incident eGFR<60 ml/min per 1.73 m2 (adjusted OR, 0.75; 95% CI, 0.63 to 0.89; P=0.001) among non-diabetics, whereas there was no association among participants with diabetes (adjusted OR, 1.21; 95% CI, 0.87 to 1.69; P=0.25). The effect of the MeDi score on incident eGFR<60 ml/min per 1.73 m2 also differed by use of ACE inhibitor/ARB (P for interaction, P=0.04). The MeDi score was associated with reduced odds of eGFR<60 ml/min per 1.73 m2 at follow-up among those not taking these medications (adjusted OR per 1-unit increase in the MeDi score, 0.76; 95% CI, 0.63 to 0.90; P=0.002), but not among those who were taking ACE inhibitors/ARBs (adjusted OR, 1.06; 95% CI, 0.80 to 1.39; P=0.69).
Analyses of Individual Components of the MeDi Score
Relationships between each MeDi component and the primary outcome were tested individually, and only vegetable intake associated with incident eGFR<60 ml/min per 1.73 m2 (adjusted OR, 0.59; 95% CI, 0.37 to 0.94) (Supplemental Table 1).
Discussion
In this prospective, multiethnic cohort, we found that dietary patterns with increasing similarity to a Mediterranean diet were favorably associated with kidney function. For participants with a MeDi score at or above the median (≥5), there was an approximate 50% decreased odds of developing incident eGFR<60 ml/min per 1.73 m2. This is one of the few studies that has shown an association between a dietary pattern and reduced incidence of eGFR<60 ml/min per 1.73 m2 in participants with relatively preserved kidney function. The effect of the MeDi score on decline of eGFR also did not differ by baseline eGFR (≥60 versus <60 ml/min per 1.73 m2), raising the possibility that the effect of a Mediterranean diet may extend to those with prevalent CKD as well.
This study adds to the growing literature (observational studies and randomized control trials) demonstrating health benefits associated with Mediterranean-style diets (11–13). Prior studies examining Mediterranean-style diets and kidney function have been inconsistent, potentially because of important limitations that we attempted to address. Cross-sectional analyses have suggested a beneficial association with albuminuria, creatinine clearance, and CKD (18–21). By contrast, reported outcomes from the Nurses’ Health Study (NHS) and the Prevención con Dieta Mediterránea (PREDIMED) randomized clinical trial (which used the Mediterranean diet for primary prevention of cardiovascular disease) (12) differed from ours. In the NHS, there was an association between diet and eGFR decline; however, the benefit was significant for the Dietary Approaches to Stop Hypertension (DASH)–style diet and not for the prudent diet that was more similar to a Mediterranean diet (6). It is interesting to note that prudent and DASH diets were correlated (r=0.76). Differences in outcomes between the NHS and our study may be due to differences in cohort composition and study design. For example, NHS participants were mainly older, white, and female, whereas NOMAS is a multiethnic cohort of men and women; food frequency questionnaires used in NHS and NOMAS differed; and NHS had repeated dietary assessments over time, which NOMAS did not. Finally, a secondary analysis of the PREDIMED trial at 1 year of follow-up did not show a significant difference in eGFR between the Mediterranean diet and control arms (22); however, the duration was nearly 6 years shorter than in the NOMAS cohort and may not have been long enough to determine an effect of diet on kidney function. There were also differences between the PREDIMED and NOMAS trials. PREDIMED had more men, a higher prevalence of diabetes, and a slightly lower baseline eGFR compared with NOMAS. Moreover, the high percentage of Hispanics in NOMAS makes it difficult to directly compare the cohorts, because there are important race/ethnic differences in kidney disease progression.
There is biologic plausibility that a Mediterranean-style diet may protect against declining kidney function. One mechanism may be endothelial dysfunction, which has been associated with both CKD and cardiovascular risk, and has been favorably affected by a Mediterranean diet (16,31–33). Endothelial dysfunction is also closely linked to inflammation, which contributes to CKD. Recruitment of inflammatory cells into the kidney starts with their interaction with selectins found on endothelial cells, which were reduced with a Mediterranean diet in a controlled study in humans (31). Inflammatory biomarkers, lipid profile, and BP have all been favorably affected by a Mediterranean diet (as a whole or its components) in several randomized trials, which may also translate into beneficial kidney effects (32,34–38).
It is also possible that lower animal protein and red meat intake characteristic of this type of diet may benefit kidney function (39,40). Although meat consumption was not associated with incident eGFR<60 ml/min per 1.73 m2, this study may have been inadequately powered to detect a relationship. The reduction of dietary red meat consumption may have led to increased vegetable consumption, which favorably affected kidney function in this study. Similar findings have been reported elsewhere, with improvement in metabolic acidosis and phosphorus metabolism as possible mechanisms of action (41–43).
The benefit of MeDi was more apparent among non-diabetics and among those who did not take ACE inhibitors/ARBs. It is unclear why MeDi would preferentially benefit non-diabetics other than that any effect in participants with diabetes may be overwhelmed by other deleterious renal risk factors such as proteinuria. The lack of an effect among participants taking ACE inhibitors/ARBs may be due to the possibility that both MeDi and ACE inhibitors/ARBs could work through overlapping mechanisms such as lowering intraglomerular pressure or by decreasing inflammation. For instance, the amino acid profile associated with an animal protein diet (compared with a vegetable protein diet) has been associated with increased eGFR (44). Therefore, it is possible that the higher vegetable intake of a Mediterranean diet may exert beneficial effects through improved glomerular hemodynamics, which is a similar effect to that of ACEI/ARBs. In addition, 32% of participants with diabetes in this study were taking ACE inhibitors/ARBs (versus 14% of non-diabetics), which may also have diminished the Mediterranean diet benefit in participants with diabetes.
Ultimately, randomized trials are needed to confirm or refute the findings of these types of observational studies. Large-scale Mediterranean diet trials have successfully been conducted in the general population (12,13). Although adherence to dietary interventions can vary, several trials have demonstrated that high compliance (even over several years) can be feasible (9,12,13). This could conceivably be extended to a CKD population, where numerous dietary trials involving protein restriction have been performed. Moreover, recent randomized trials involving high fruit and vegetable intake have shown safety and potential benefit in even advanced CKD, increasing the feasibility of a Mediterranean diet trial in this population (41,42).
Strengths of this study include the duration of follow-up, a well characterized cohort, a valid food frequency questionnaire, adjustment for multiple confounders, and multiethnic composition that permits generalizability of results. However, there are also limitations. First, the subgroup of participants within NOMAS who had follow-up creatinine values were likely healthier than the overall cohort, thus introducing a survival bias; this should, however, bias results toward the null. Second, there was no assessment of proteinuria. However, given that <20% of participants had diabetes, significant proteinuria would not be expected to be common. Third, only baseline food consumption data were available (which are limited because of self-reporting), and we could not adjust for dietary changes during follow-up. However, MeDi scores may not change significantly over time either. Analyses of the Washington Heights-Inwood Columbia Aging Project cohort, which was recruited from a geographic area similar to NOMAS, showed that MeDi scores remained relatively stable over 6–8 years of repeated measurement (29). Fourth, although we adjusted for physical activity, we cannot eliminate the possibility that the benefit seen with higher MeDi scores is related to an overall healthier lifestyle, as opposed to diet alone. Finally, we could not determine exactly how closely dietary patterns would need to resemble a true Mediterranean diet in order to potentially have beneficial kidney effects. Our categorical analyses showed a protective effect with MeDi scores ≥5, and our continuous models demonstrated benefit with every 1-point increase in the MeDi score.
In conclusion, in a multiethnic cohort of men and women, we demonstrated that a Mediterranean-style diet was associated with lower risk of incident eGFR<60 ml/min per 1.73 m2 and upper quartile of eGFR decline. If these data are confirmed in clinical trials, they will offer a novel and important approach in the fight against kidney disease. The growing body of literature suggesting that a Mediterranean-style diet has a kidney-protective effect supports the need for a randomized controlled trial in patients at risk for kidney disease and its progression.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the NOMAS staff, particularly Project Manager Janet DeRosa.
M.S.V.E. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data analysis was performed by Y.P.M., K.C., and Y.G.
This work is supported by grants from the National Institute of Neurological Disorders and Stroke (R37-NS29993) and the National Institute on Aging (R01-AG028506).
The abstract was presented as an oral presentation at the 2013 American Society of Nephrology Annual Meeting, held November 5–10, in Atlanta, Georgia, and was also reported in Kidney News (2013;5[12]:10–11).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01080114/-/DCSupplemental.
See related editorial, “Where What Is Not Stated or Required May Be the Most Illuminating,” on pages 1826–1828.
References
- 1.US Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, AIPRD Study Group : Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group : Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290: 2159–2167, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 6.Lin J, Fung TT, Hu FB, Curhan GC: Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis 57: 245–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang A, Van Horn L, Jacobs DR, Jr, Liu K, Muntner P, Newsome B, Shoham DA, Durazo-Arvizu R, Bibbins-Domingo K, Reis J, Kramer H: Lifestyle-related factors, obesity, and incident microalbuminuria: The CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis 62: 267–275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouque D, Laville M: Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev (3): CD001892, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G, Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A: Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 337: a1344, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardener H, Wright CB, Gu Y, Demmer RT, Boden-Albala B, Elkind MS, Sacco RL, Scarmeas N: Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: The Northern Manhattan Study. Am J Clin Nutr 94: 1458–1464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D,Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA, PREDIMED Study Investigators : Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368: 1279–1290, 2013 [DOI] [PubMed] [Google Scholar]
- 13.de Lorgeril M, Salen P, Martin J-L, Monjaud I, Delaye J, Mamelle N: Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 99: 779–785, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A: Olive oil, the Mediterranean diet, and arterial blood pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr 80: 1012–1018, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Tzima N, Pitsavos C, Panagiotakos DB, Skoumas J, Zampelas A, Chrysohoou C, Stefanadis C: Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis 6: 22, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rallidis LS, Lekakis J, Kolomvotsou A, Zampelas A, Vamvakou G, Efstathiou S, Dimitriadis G, Raptis SA, Kremastinos DT: Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am J Clin Nutr 90: 263–268, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V: Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation 117: 169–175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Jiménez-Moleón JJ, Lindholm B, Cederholm T, Arnlöv J, Risérus U, Sjögren P, Carrero JJ: Mediterranean diet, kidney function, and mortality in men with CKD. Clin J Am Soc Nephrol 8: 1548–1555, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazaraki A, Tsioufis C, Dimitriadis K, Tsiachris D, Stefanadi E, Zampelas A, Richter D, Mariolis A, Panagiotakos D, Tousoulis D, Stefanadis C: Adherence to the Mediterranean diet and albuminuria levels in Greek adolescents: Data from the Leontio Lyceum ALbuminuria (3L study). Eur J Clin Nutr 65: 219–225, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Nettleton JA, Steffen LM, Palmas W, Burke GL, Jacobs DR, Jr: Associations between microalbuminuria and animal foods, plant foods, and dietary patterns in the Multiethnic Study of Atherosclerosis. Am J Clin Nutr 87: 1825–1836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Zeimbekis A, Kastorini CM, Stefanadis C: Adherence to the Mediterranean diet is associated with renal function among healthy adults: The ATTICA study. J Ren Nutr 20: 176–184, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Díaz-López A, Bulló M, Martínez-González MÁ, Guasch-Ferré M, Ros E, Basora J, Covas MI, del Carmen López-Sabater M, Salas-Salvadó J, PREDIMED (Prevención con Dieta Mediterránea) Reus Study Investigators : Effects of Mediterranean diets on kidney function: A report from the PREDIMED trial. Am J Kidney Dis 60: 380–389, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, Paik MC: Homocysteine and the risk of ischemic stroke in a triethnic cohort: The NOrthern MAnhattan Study. Stroke 35: 2263–2269, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L: A data-based approach to diet questionnaire design and testing. Am J Epidemiol 124: 453–469, 1986 [DOI] [PubMed] [Google Scholar]
- 28.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D: Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 348: 2599–2608, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA: Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 59: 912–921, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, Shea S, Paik MC: The protective effect of moderate alcohol consumption on ischemic stroke. JAMA 281: 53–60, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Fuentes F, López-Miranda J, Sánchez E, Sánchez F, Paez J, Paz-Rojas E, Marín C, Gómez P, Jimenez-Perepérez J, Ordovás JM, Pérez-Jiménez F: Mediterranean and low-fat diets improve endothelial function in hypercholesterolemic men. Ann Intern Med 134: 1115–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D: Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 292: 1440–1446, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Tharaux PL, Chatziantoniou C, Casellas D, Fouassier L, Ardaillou R, Dussaule JC: Vascular endothelin-1 gene expression and synthesis and effect on renal type I collagen synthesis and nephroangiosclerosis during nitric oxide synthase inhibition in rats. Circulation 99: 2185–2191, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Mena MP, Sacanella E, Vazquez-Agell M, Morales M, Fitó M, Escoda R, Serrano-Martínez M, Salas-Salvadó J, Benages N, Casas R, Lamuela-Raventós RM, Masanes F, Ros E, Estruch R: Inhibition of circulating immune cell activation: A molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr 89: 248–256, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles E, Arós F, Conde M, Lahoz C, Lapetra J, Sáez G, Ros E, PREDIMED Study Investigators : Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann Intern Med 145: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Vincent-Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, Portugal H, Planells R, Grolier P, Amiot-Carlin MJ, Vague P, Lairon D: The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr 82: 964–971, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Singh RB, Dubnov G, Niaz MA, Ghosh S, Singh R, Rastogi SS, Manor O, Pella D, Berry EM: Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): A randomised single-blind trial. Lancet 360: 1455–1461, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Perona JS, Cañizares J, Montero E, Sánchez-Domínguez JM, Catalá A, Ruiz-Gutiérrez V: Virgin olive oil reduces blood pressure in hypertensive elderly subjects. Clin Nutr 23: 1113–1121, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Lin J, Hu FB, Curhan GC: Associations of diet with albuminuria and kidney function decline. Clin J Am Soc Nephrol 5: 836–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC: The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 138: 460–467, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Goraya N, Simoni J, Jo CH, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goraya N, Simoni J, Jo C, Wesson DE: Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int 81: 86–93, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR: Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 6: 257–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontessis PA, Bossinakou I, Sarika L, Iliopoulou E, Papantoniou A, Trevisan R, Roussi D, Stipsanelli K, Grigorakis S, Souvatzoglou A: Renal, metabolic, and hormonal responses to proteins of different origin in normotensive, nonproteinuric type I diabetic patients. Diabetes Care 18: 1233, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


