Abstract
Molecular genetics have revolutionized the understanding of susceptibility to the broad spectrum of kidney diseases with light microscopic appearance of FSGS, particularly in populations with recent African ancestry. These disorders include idiopathic FSGS, HIV-associated nephropathy, severe lupus nephritis, sickle cell nephropathy, and the primary kidney disorder focal global glomerulosclerosis, which had historically been ascribed to systemic hypertension. FSGS was once thought to include a multitude of unrelated disorders with similar histologic appearance. However, variation in the apolipoprotein L1 gene locus is now known to account for the vast majority of such cases in African Americans as well as nearly all the excess risk for FSGS and related forms of progressive nondiabetic nephropathy in populations with recent African ancestry, relative to European ancestry. Inheriting two coding apolipoprotein L1 gene nephropathy risk variants is necessary for susceptibility to CKD; however, these variants alone are insufficient to produce disease. This work reviews the evidence supporting second hits or modifying factors that affect risk for apolipoprotein L1 gene-associated nephropathy and produce the protean manifestations of this common and complex syndrome. Targeting modifiable second factors will lead to preventive therapies for slowing progression of nondiabetic nephropathy in many patients possessing two apolipoprotein L1 gene risk variants. This model of genetic risk coupled with modifiable second hits will serve as a paradigm applicable to patients with CKD of various etiologies as well as a host of other complex disorders.
Keywords: CKD, genetic renal disease, virology, ethnicity, HIV nephropathy
Genetic Risk in Complex Disease
Extensive gene discovery efforts in a spectrum of common chronic disease states, including diabetes and metabolic syndrome, inflammatory, and kidney disorders, reveal that only a small subset of these complex diseases can be accounted for by standalone mutations at genetic loci that cause disease following Mendelian patterns of inheritance, diseases in which the affected gene product can be mechanistically related to pathogenesis (1). Moreover, common DNA sequence variants at these same loci often do not strongly associate with disease. Rather, population-based gene discovery studies have identified thousands of common tagging DNA sequence variants with statistically convincing associations with human traits, including predisposition to common diseases, but in which the individual or even cumulative quantitative contribution of the genetic loci tagged by these variants to relative risk of disease is small. As such, the odds ratios (ORs) for disease associations are typically low (well below 1.5 for individual disease risk variants, signifying <50% increase in risk of disease per inherited variant) with limited contributions to population disease risk, typically far <10% (2,3). As such, mechanistic relationships between associated variants and disease often remain obscure.
This realization challenges the development of preventive and therapeutic strategies for the overwhelming burden of noncommunicable diseases, including nephropathy, and motivates a shift to a paradigm comprising the interaction of genetic variation with second-hit triggers, which transform genetic risk to disease causation and may explain the gap seen in the heritability of disease (4–6). This paradigm also seems relevant for the nephropathy spectrum that is strongly associated with apolipoprotein L1 gene (APOL1) variants in populations with recent African ancestry. This disease spectrum exhibits the strongest genetic association with complex disease, to date, in an autosomal recessive mode of inheritance (7,8).
APOL1-Associated Nephropathy
CKD is a devastating illness estimated to affect 200 million persons worldwide. It is associated with markedly increased mortality from hypertension and cardio- and cerebrovascular complications and culminates in ESRD requiring RRT, dialysis, or transplantation in those patients surviving the early stages. ESRD is lethal in most parts of the world where RRTs are not available.
A major population disparity in CKD has long been documented in United States registry data (9,10). Relative to European Americans, African Americans disproportionately develop progressive diabetes-associated and nondiabetic CKD. In nondiabetic patients, sclerosing lesions affecting glomeruli and blood vessels and tubulointerstitial fibrosis are typically observed on kidney biopsy (11). Patients present with hypertension and variable degrees of proteinuria from absent to nephrotic range. Thus, although African Americans comprise 13% of the United States population, as a group, they comprise 33% of incident-patients of ESRD with younger ages of onset, face an 8.2% lifetime risk for ESRD (relative to 2.7% in non-Hispanic European-ancestry Americans), and are 3.5-fold more likely to require RRT, with a disproportionate number on dialysis compared with transplantation as a result of poorer transplantation rates and outcomes (12–14).
In 2010, a research breakthrough attributed a major component of this disparity to two coding variants in the APOL1 gene on chromosome 22q13 (7,8). These risk variants (G1, nonsynonymous coding variants S342G:I384M [leading to a change in two amino acids]; G2, del.N388/Y38 [deleting two amino acids]) were shown to be associated with ORs from 7.3- to 29-fold in ESRD, with this range of ORs in APOL1-associated disease corresponding to the spectrum of leading etiologies of nondiabetic CKD and accounting for up to 40% of the total disease burden in African Americans (7,8,15–21). Disease etiologies include focal global glomerulosclerosis (FGGS) historically ascribed to hypertension, nonmonogenic forms of FSGS, HIV-associated nephropathy (HIVAN), sickle cell kidney disease, and severe lupus nephritis (LN) (7,8,22–25). The APOL1 risk variants also account for younger age of onset of ESRD and may also be associated with poorer outcome of kidney grafts from African-ancestry donors (26–28).
APOL1 Risk Variants and Nephropathy: Initiators or Progression Factors?
The markedly elevated ORs from 7.3 to 29 observed for APOL1 association with ESRD in the presence of two risk alleles were previously unheard of for common DNA variants in chronic nonmonogenic disease. More recently, lower ORs ranging from 1.5 to 2.2 have been reported for APOL1 and milder kidney disease manifesting as microalbuminuria and/or reduced eGFRs (eGFR<60 ml/min per 1.73 m2) in population-based studies (29–31). These ORs are strikingly high when considered in the context of most complex genetic diseases, but they seem to support a more pronounced role for APOL1 in nephropathy progression to end-stage rather than initiation of CKD. This finding is partly on the basis of the lower ORs in mild nephropathy compared with ESRD (32).
An early study found that chromosome 22q risk variants were associated with LN ESRD in a small number of patients recruited from dialysis clinics; however, the effect was subsequently lost when ESRD cases were combined with genotypes of LN cases with milder phenotypes (33). This result led to the incorrect conclusion that LN did not reside in this spectrum of disease association. However, a subsequent national study in patients with LN ESRD and controls with systemic lupus erythematosus lacking nephropathy showed that progressive LN in African Americans was significantly associated with APOL1 (OR, approximately 2.8) (25). The vast majority of severe LN ESRD patients failed prior cytotoxic therapy, revealing the refractory and aggressive progression of this phenotype. Differences in APOL1 risk variant frequencies accounted for much of the poorer outcomes in LN in African Americans relative to European Americans and associated with more rapid progression to ESRD. The collapsing variant of LN is strongly APOL1-associated as well (24).
The African American Study of Kidney Disease and Hypertension (AASK) and Chronic Renal Insufficiency Cohort (CRIC) studies reported APOL1-mediated risk for nephropathy progression (23,34). These longitudinal studies observed that two APOL1 risk variants were associated with more rapid loss of kidney function in nondiabetic subjects in AASK and both diabetic and nondiabetic subjects in CRIC. The rates of CKD progression in CRIC were lowest for European Americans (who essentially lack APOL1 risk variants), intermediate for African Americans with zero or one APOL1 risk variants, and highest for African Americans with two APOL1 risk variants (34). Because subjects with and without type 2 diabetes mellitus (T2D) were at similar risk in CRIC, the role of diabetic kidney disease (DKD) relating to APOL1 was raised.
Glomerulosclerosis (FSGS and FGGS) and T2D-associated CKD are both relatively common proteinuric disorders in the African American population. The initial admixture linkage disequilibrium signal on chromosome 22q was absent in African Americans with T2D-associated ESRD in the Family Investigation of Nephropathy and Diabetes study and Wake Forest samples collected from patients with ESRD at dialysis facilities in the southeastern United States (35,36). Moreover, the presence of chromosome 22q nephropathy risk alleles could be used to genetically dissect patients with nondiabetic nephropathy from those patients with DKD (37,38). These observations lead us to believe that two APOL1 risk variants lead to increased risk for nephropathy progression, regardless of ambient blood glucose levels, but are unlikely associated with frank diabetic glomerulosclerosis. APOL1 protein is involved in autophagy, a lysosomal degradation pathway that removes protein aggregates and damaged organelles to maintain intracellular homeostasis, particularly during times of stress (39,40). Because it has been shown that autophagy is inherently dysregulated in the diabetic kidney, we speculate that the effects of APOL1 protein on autophagy may be limited in the setting of DKD (41,42). Although multiple second hits drive the diverse clinical presentation of APOL1-associated nephropathy, classic DKD seems unlikely to reside in this spectrum.
APOL1 risk variants rose to high allele frequency during one or more evolutionary adaptive selective sweeps in sub-Saharan African populations during the past approximately 10,000 years, wherein even a single G1 or G2 allele conferred protection against a certain species of the African sleeping sickness parasite Trypanosoma brucei rhodesiense. Although protection from this form of African sleeping sickness is no longer relevant to most carriers of these allelic variants, the allele frequency remains high, with 51% of African Americans having at least one risk allele and 13% having two parental risk alleles (7,8,11). The risk allele frequencies are even higher in certain sub-Saharan African nations, where hypertension and kidney disease are correspondingly rampant (43). Thus, it is estimated that 40% of CKD among African Americans and sub-Saharan continental African populations could be prevented if the processes linking the common risk genotype to disease could be targeted.
Modifying Factors in APOL1-Associated Nephropathy
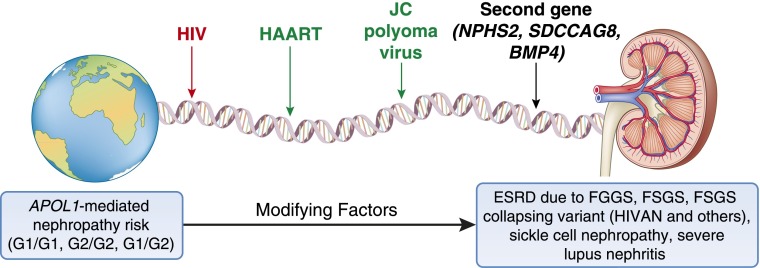
Several observations strongly support the presence of modifying factors in susceptibility to APOL1-associated nephropathy (Table 1). A key observation is that only a subset of individuals with genetic risk (two APOL1 nephropathy variants) develops kidney disease, such that although markedly elevated, the lifetime risk for progressive CKD remains low. Kopp et al. (21) reported that the lifetime risk for developing FSGS in individuals with APOL1-risk genotypes approximated 4%, whereas risk for HIVAN in individuals with HIV infection and risk genotypes was 50% before the widespread availability of highly active antiretroviral therapy (HAART). Although not precisely quantified, the risk for FGGS (putative hypertension-attributed nephropathy) falls between the 4% and 50% lifetime risks, respectively, for FSGS and HIVAN (44). Hence, it is important to understand why most individuals who carry this genetic risk for ESRD on the basis of their APOL1 genotypes do not manifest kidney disease. This realization has great potential to yield insights for prevention of kidney disease in those individuals who would otherwise progress. Thus, individuals with APOL1 genetic risk who do not develop kidney disease likely either possess a protective factor(s) and/or lack exposure to additional modifying risk factors. Identifiable and modifiable requisite nongenetic second-hit viral pathogens are important triggers for CKD in genetically susceptible individuals. One such second-hit or modifiable environmental factor that has been well studied is the HIV virus (45–48). Recent studies suggest the presence of additional modifying urinary tract viral infections, including polyomaviruses, and interactive second genes (Figure 1).
Table 1.
Evidence supporting second hits in the apolipoprotein L1 gene-associated nephropathy spectrum
| Disease Manifestation | Observation | Ref. |
|---|---|---|
| Lifetime risk for CKD | Low in those with two risk variants (range from a low of 4% in FSGS to a high of 50% in HIVAN) | 21 |
| Renal histology | Variable (suggesting different modifying factors in FSGS, FSGS collapsing variant, FGGS, and lupus nephritis) | 11 |
| Rapidly changing natural history in HIVAN | Risk of HIVAN markedly reduced after widespread use of HAART | 46 |
| Non-HIV interactive viral infections | JC polyoma virus potentially protective; search for additional interactive viruses ongoing | 59 |
| Interactive second genes | NPHS2, SDCCAG8, near BMP4, and others | 67 |
FGGS, focal global glomerulosclerosis; HIVAN, HIV-associated nephropathy; HAART, highly active antiretroviral therapy; NPHS2, podocin; SDCCAG8, serologically defined colon cancer antigen 8; BMP4, bone morphogenetic protein 4.
Figure 1.
Pathways leading from genetic susceptibility to clinical kidney disease. APOL1, apolipoprotein L1 gene; BMP4, bone morphogenetic protein 4; HAART, highly active antiretroviral therapy; HIVAN, HIV-associated nephropathy; NPHS2, podocin; SDCCAG8, serologically defined colon cancer antigen 8.
Another important aspect of the spectrum of APOL1-associated kidney diseases includes its multiple histologic variants. The search for major nephropathy variants was initially driven by the marked familial clustering of ESRD, particularly that seen in African American families (49). In the early 1990s, it was appreciated that they were a unique population group, because single families often clustered multiple etiologies of ESRD. This finding suggested the presence of an overarching nephropathy susceptibility allele coupled with different interactive factors (50). Some 20 years later, APOL1 was identified on the basis of a combination of admixture mapping and subsequent fine mapping of the linked region. It is clear that idiopathic FSGS and HIVAN (FSGS and collapsing variant) are the cornerstones of this spectrum, likely with different inciting factors, and that many patients of FGGS with low-level proteinuria represent additional manifestations of APOL1-mediated kidney disease (51,52). Other nondiabetic nephropathies, which fill in the majority of the spectrum, include severe and progressive LN and sickle-cell nephropathy (22,24,25). The pleiotropic glomerular, vascular, and tubule interstitial histopathologic features also support different modifying factors: HIV in collapsing FSGS, antinuclear antibodies in LN, and sickled red blood cells in sickle-cell nephropathy. Major modifying factors for FSGS and FGGS remain to be identified. A recent study has suggested the association of the G1 and G2 APOL1 risk variants with adverse cardiovascular outcomes independent of CKD (53). Additional analysis of these datasets and other available datasets should clarify whether this finding represents an association that is, indeed, independent of kidney involvement and whether a different set of modifying factors is involved. Taken together, it is likely that other as-yet-undetected factors affect the spectrum of kidney disease and related disorders in this APOL1 risk association spectrum.
HIV Infection: The Prototype Environmental Modifier of APOL1-Mediated Disease
HIVAN was initially thought to result from environmental exposure to retroviral infection (54). The marked familial aggregation of HIVAN in African American families, akin to that seen in hypertension-attributed ESRD and LN ESRD, was surprising (49,55,56). Moreover, relatives of index patients with HIVAN who had ESRD uniformly lacked HIV infection; they had other etiologies of ESRD. It is now appreciated that untreated or undertreated HIV-infected individuals with two APOL1 risk alleles are destined to experience inexorable rapid progression of HIVAN, which can be abrogated with appropriate antiretroviral therapy (46,47). In contrast, HIV-infected individuals whose APOL1 genotype status is nonrisk at both parental alleles are protected from this form of CKD, even when systemic viral replication is not controlled, although they may develop HIV immune complex nephropathy, a disorder unrelated to APOL1.
A striking example of the effect of APOL1-mediated risk for HIVAN is seen in the HIV-infected Ethiopian population (57). Whether residing in Ethiopia or emigrating to Israel, Ethiopians seemed to be protected from HIVAN when contrasted to Africans residing in the western and southern regions of the continent. The landmark observation that Ethiopian genomes lacked APOL1 risk variants, compared with other African populations that faced high rates of HIVAN, provided pinpoint classification of the issue. Thus, although other tagging risk variants in the 22q risk region were found to be present among Ethiopians, APOL1 risk variants are absent, and it is this absence of APOL1 risk variants that confers protection from HIVAN. Thus, HIV proved to be a risk factor for HIVAN only when precisely coupled with host genetic susceptibility. Either viral infection or genetic risk alone fails to equate to kidney disease; it is the gene-by-environment interaction that is of cardinal pathogenic importance. Biologic studies have since delineated pathways for the interaction of APOL1 and its allelic gene products as well as other APOL gene family members with viral response mediators (e.g., class II interferons) in circulating immune cells as well as the key kidney target cell, the podocyte, which is responsible for structural integrity of the glomerulus or filtering unit of the kidney (58).
Additional support that HIV infection is a modifiable environmental risk factor for APOL1-associated HIVAN comes from its rapidly changing natural history. Despite stable risk-variant frequencies in the HIV-infected United States population, the incidence rate of HIVAN has fallen dramatically with widespread use of HAART (9). Controlling the viral reservoir in the cells of the kidney with HAART is a likely explanation for this effect and provides great hope that identifying other viral pathogens maintaining similar renal reservoirs of infection (or possibly lymphotrophic viral infections as for HIV) may prove to be an effective treatment for CKD.
Non-HIV Viral Infections as Second Hits in APOL1-Associated Nephropathy
Driven by the striking observations in HIVAN, evidence was sought for other viral modifiers of risk in APOL1-associated nephropathy (59,60). The initial focus was on previously identified viruses with similarities to HIV, including maintaining renal reservoirs of infection and lymphotrophism. However, novel viruses residing in the urinary tract, identifiable using next-generation sequencing technologies, may prove to be critically important modifiable second hits.
A cohort of 865 siblings and children of African Americans with nondiabetic forms of ESRD potentially related to APOL1 is actively being followed for development of albuminuria, falling eGFR, or rising serum cystatin C concentrations in the Natural History of APOL1-Associated Nephropathy Study (30). A biorepository contains their DNA, urine, plasma, serum, and buffy coat samples. This repository allows testing for interactive environmental factors in APOL1-associated nephropathy. The cohort is markedly enriched for APOL1 risk variants: 23% of relatives possess two risk alleles, and 47% of relatives possess one risk allele, higher rates than in the general population. APOL1 associates with mild kidney disease phenotypes in these families.
Plasma testing for lymphotrophic Human Herpes Virus 6 and Cytomegalovirus and urine testing for JC and BK polyomaviruses with renal/urothelial reservoirs of infection were performed in this cohort using quantitative PCR (59). Viral infection results were linked with APOL1 genotypes to assess potential interactive effects on CKD. Although Human Herpes Virus 6 and Cytomegalovirus viral infections were rare in these nonimmune-suppressed subjects, 30% and 10% of individuals had JC and BK viral replication in urine, respectively. Furthermore, JC polyomavirus additively interacted with APOL1 risk (recessive model) and paradoxically reduced risk of CKD. This finding was unexpected; it was presumed that urinary tract viruses would increase CKD risk by interactions with APOL1.
JC polyomavirus is typically not nephropathy-associated in kidney transplantation, and most individuals only develop urinary tract infections with a single polyomavirus strain (either BK or JC but not both) (61). Infection with one virus seems to inhibit replication of the other. As such, we suspect that subjects with JC polyomavirus were unlikely to have urinary tract infections with other more nephrotoxic viruses and might, thereby, be protected from nephropathy. Alternatively, JC polyomavirus infection could directly alter gene transcription and reduce nephropathy risk. A report from Brazil also showed significantly higher rates of urine JC polyomavirus in healthy controls compared with subjects with ESRD (62). Brazilians have varying degrees of recent African ancestry, and these results are broadly consistent with our observation that African Americans without renal disease more often had JC viruria than African Americans with CKD. Unfortunately, interactive effects of APOL1 genotypes and JC viruria were not assessed in the work by Pires et al. (62). Isolated JC viruria is also associated with lower rates of acute renal allograft rejection after transplantation, further supporting protective effects of JC viruria on the kidney (63).
The urinary tract and kidneys have largely been ignored in the search for novel viral infections using methods such as next-generation sequencing of urine DNA and RNA. Correcting this gap in our knowledge and testing for interactions between urinary tract viruses and genetic risk may explain why only a subset of at-risk individuals develops APOL1 nephropathy. The foregoing arguments lead to a conceptual transformation invoking viral triggers as modifiable pathways and hence, key preventive and therapeutic targets for a major form of noncommunicable disease: CKD in individuals with well defined genetic susceptibility. Moreover, it is likely that different viral milieus (viromes) determine the varied clinical and histopathologic presentations in genetically susceptible individuals.
This model provides reason for great optimism. Falling rates of HIVAN with use of HAART prove that control of a viral pathogen can prevent kidney disease in those individuals at genetic risk. This finding contrasts with the failure of conventional therapies to halt progression of other forms of kidney disease associated with APOL1 risk alleles, such as the large number of patients historically misclassified as hypertension-attributed kidney disease (64). It is, thus, imperative to identify the inciting interactive viral triggers and their mechanisms of interaction with APOL1 risk moieties in the kidney and immune system.
APOL1 Second-Gene Interactions in Nephropathy
APOL1 gene-by-second gene epistatic interactions have only recently been investigated. Many genes have reproducible effects on risk for nondiabetic ESRD, and several genes seem to be major modifiers. This field has been hampered by the lack of a directly genotyped genome-wide association study (GWAS) in large numbers of African Americans with nondiabetic ESRD (65,66).
Gene–gene interaction analyses were reported on the basis of a pooled GWAS in African American nondiabetic ESRD patients and African American non-nephropathy controls (66). This analysis revealed that APOL1 was independently associated with nondiabetic ESRD, and gene–gene interaction analyses revealed several polymorphic genetic markers termed single-nucleotide polymorphisms (SNPs) that significantly interacted with APOL1. A replication study confirmed many of these interactions in a larger sample consisting of 1367 African American nondiabetic ESRD patients and 1504 controls, with validation in Natural History of APOL1-Associated Nephropathy Study participants (67). These polymorphic markers are termed reference SNPs (rs) and given accession numbers to allow researchers to work with specific variants. Eleven SNPs from the initial pooled GWAS replicated APOL1 interaction in the large ESRD case-control study; podocin (NPHS2; rs16854341; OR, 0.6; P<0.001), serologically defined colon cancer antigen 8 (SDCCAG8; rs2802723; OR, 1.8; P<0.001), and near the bone morphogenetic protein 4 (BMP4; rs8014363; OR, 1.6; P=0.001) genes all met Bonferroni-corrected significance.
Interaction effects were subsequently quantified (67). In addition to inheriting two APOL1 risk variants, each copy of the NPHS2 minor allele at SNP rs16854341 reduced the ESRD OR by approximately 50% (from 7.03 in those homozygous for the major allele to 3.52 for heterozygotes to 1.76 for minor allele homozygotes). Inheriting two APOL1 risk variants with zero, one, and two SDCCAG8 minor alleles changed the OR for ESRD from 5.1 to 7.3 to 10.5, and inheriting two APOL1 risk variants with zero, one, and two rs8014363 minor alleles near BMP4 changed the OR for association from 4.8 to 6.8 to 9.6, respectively. These results show the impressive effects on APOL1 association on the basis of genotypes of interactive genes.
NPHS2 and SDCCAG8 are known to be involved in nondiabetic nephropathy susceptibility, with critical roles in FSGS/glomerulosclerosis and Bardet Biedl syndrome, respectively; BMP pathway genes have recently been implicated in DKD (68–71). Significantly different effects for the APOL1 second-gene interaction on the basis of presence of two APOL1 risk variants (versus less than two) were seen for all 14 interactive genes (67). NPHS2 SNP rs16854341 showed significant interactive effects solely in carriers with two APOL1 risk variants (OR, 0.6), with a statistically significant different interactive effect in the zero/one APOL1 risk variant group (OR, 1.0; P=0.02 between strata). SDCCAG8 SNP rs2802723 displayed an OR of 1.4 for APOL1 interaction in carriers, with an OR opposite in direction in noncarriers (OR, 0.8; P=0.002 between strata).
Several interactive gene effects were replicated in independent samples from Natural History of APOL1-Associated Nephropathy participants (67). These subjects have mild renal phenotypes known to display weaker APOL1 association compared with ESRD. Nonetheless, rs16854341 in NPHS2 showed APOL1 interaction with albuminuria (P=0.05, dominant model) and a trend for interaction with CKD (P=0.08, recessive model). Similar results were seen for rs2802723 in SDCCAG8 with albuminuria (P=0.03, dominant model) and a trend for interaction with CKD (P=0.11, dominant model).
Conclusions
Appreciating the role of APOL1 was a major step toward an improved understanding of nondiabetic nephropathy in African Americans; >5 million African Americans are at risk for CKD, because approximately 13% of the approximately 42 million African Americans possess two APOL1 nephropathy variants. Unfortunately, no substantially effective treatments yet exist. Because only a subset of genetically high-risk individuals ultimately develops nephropathy, identification of modifiable environmental and interactive genetic variants affecting risk will refine genetic screening methodologies and lead to novel preventive and therapeutic measures with the potential for a major beneficial public health effect.
For viral mediators of nondiabetic ESRD, such as in HIVAN, preventive vaccines and antiviral therapies provide great hope for new therapies in the near future. Infectious pathogens and, in particular, common viral infections, represent a major research focus for such triggers. If viruses residing in the kidney interact with APOL1 to trigger (or protect from) nephropathy, they likely arrive through the urinary tract or bloodstream. HAART has dramatically reduced rates of HIVAN, suggesting that the renal reservoir of a virus is controlled by systemic antiretroviral treatment. In the future, identification of currently unsuspected non-HIV nephropathic viruses could allow for similar antiviral treatments or immunization to prevent initial infections in unexposed individuals. More broadly and assuming no untoward systemic effects, protective (nonpathologic) viruses could potentially be introduced to inhibit pathologic virus infection. It is estimated that we each acquire a suite of >10 such chronic viral infections comprising our virome (72). These triggers can act in concert with corresponding genetic variants to cause common chronic organ system disease, even in immunocompetent individuals. These implications extend well beyond the important public health challenge of APOL1 and ESRD. In a similar manner, for the majority of common diseases for which tag SNPs have been identified, virus discovery applied to affected and unaffected individuals with and without genetic risk could bridge the gap of missing heritability (73) and point to functional causal variants that interact with viral triggers to shed light on disease mechanisms and point to potential therapies. Correspondingly, an important role also exists for interactive second genes with APOL1 to both accurately assess and modify the risk for CKD. It is likely that the interplay between APOL1 and several modifiable environmental factors and interactive genes produces the variable clinical phenotypes in this devastating spectrum of kidney disease, a spectrum with poor response rates to conventional therapies targeting reductions in systemic BP and proteinuria. It has been just 3 years since APOL1 was first associated with nephropathy. Studies now focus on whether circulating (potentially filtered) APOL1 protein or intrinsic renal gene expression causes disease, intracellular trafficking of APOL1 protein, renal cell types involved in APOL1-associated nephropathy, and mechanisms of cell death. Detecting modifiable second hits will inform all these fields as the global nephrology community works to cure APOL1-associated forms of progressive glomerulosclerosis.
Disclosures
None.
Acknowledgments
This research was supported by National Institutes of Health Grants R01-DK084149 (to B.I.F.) and R01-DK070941 (to B.I.F.), The Ernest and Bonnie Beutler Research Program of Excellence in Genomic Medicine, Israel Science Foundation Grant Number 3002/09 (to K.S.), and United States–Israel Binational Science Foundation Grant 2013504 (to B.I.F. and K.S.).
However, opinions, conclusions, or recommendations arising out of supported research activities are those of the author or the grantee and should not be presented as implying that they are views of the Binational Science Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.McClellan J, King MC: Genetic heterogeneity in human disease. Cell 141: 210–217, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Manolio TA: Bringing genome-wide association findings into clinical use. Nat Rev Genet 14: 549–558, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Hall SS: Revolution postponed. Sci Am 303: 60–67, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Todd JA: D’oh! genes and environment cause Crohn’s disease. Cell 141: 1114–1116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virgin HW, Wherry EJ, Ahmed R: Redefining chronic viral infection. Cell 138: 30–50, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Renal Data System : USRDS 2012 Annual Data Report, Vol 1: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 10.Muntner P, Newsome B, Kramer H, Peralta CA, Kim Y, Jacobs DR, Jr., Kiefe CI, Lewis CE: Racial differences in the incidence of chronic kidney disease. Clin J Am Soc Nephrol 7: 101–107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR: The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 21: 1422–1426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM: Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol 22: 743–751, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakkera HA, O’Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, Chertow GM: Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol 16: 269–277, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D: Effect of donor ethnicity on kidney survival in different recipient pairs: An analysis of the OPTN/UNOS database. Transplant Proc 41: 4125–4130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosset S, Tzur S, Behar DM, Wasser WG, Skorecki K: The population genetics of chronic kidney disease: Insights from the MYH9-APOL1 locus. Nat Rev Nephrol 7: 313–326, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Friedman DJ, Pollak MR: Genetics of kidney failure and the evolving story of APOL1. J Clin Invest 121: 3367–3374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genovese G, Friedman DJ, Pollak MR: APOL1 variants and kidney disease in people of recent African ancestry. Nat Rev Nephrol 9: 240–244, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Pollak MR, Genovese G, Friedman DJ: APOL1 and kidney disease. Curr Opin Nephrol Hypertens 21: 179–182, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Freedman BI, Langefeld CD: The new era of APOL1-associated glomerulosclerosis. Nephrol Dial Transplant 27: 1288–1291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasser WG, Tzur S, Wolday D, Adu D, Baumstein D, Rosset S, Skorecki K: Population genetics of chronic kidney disease: The evolving story of APOL1. J Nephrol 25: 603–618, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, Orringer EP, Eckman JR, Telen MJ: MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 155: 386–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB, SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KM, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP: End-stage kidney disease in African Americans with lupus nephritis associates with APOL1. Arthritis Rheumatol 66: 390–396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanji Z, Powe CE, Wenger JB, Huang C, Ankers E, Sullivan DA, Collerone G, Powe NR, Tonelli M, Bhan I, Bernhardy AJ, Dibartolo S, Friedman D, Genovese G, Pollak MR, Thadhani R: Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol 22: 2091–2097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzur S, Rosset S, Skorecki K, Wasser WG: APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant 27: 1498–1505, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, Langefeld CD, Bowden DW, Hicks PJ, Stratta RJ, Lin JJ, Kiger DF, Gautreaux MD, Divers J, Freedman BI: The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 11: 1025–1030, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR: Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 22: 2098–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman BI, Langefeld CD, Turner J, Núñez M, High KP, Spainhour M, Hicks PJ, Bowden DW, Reeves-Daniel AM, Murea M, Rocco MV, Divers J: Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int 82: 805–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, Boerwinkle E, Parekh RS, Kao WH: APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol 24: 1484–1491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer ND, Freedman BI: APOL1 and progression of nondiabetic nephropathy. J Am Soc Nephrol 24: 1344–1346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman BI, Edberg JC, Comeau ME, Murea M, Bowden DW, Divers J, Alarcón GS, Brown EE, McGwin G, Jr., Kopp JB, Winkler CA, Nelson GW, Illei G, Petri M, Ramsey-Goldman R, Reveille JD, Vilá LM, Langefeld CD, Kimberly RP, PROFILE Study Group : The non-muscle Myosin heavy chain 9 gene (MYH9) is not associated with lupus nephritis in African Americans. Am J Nephrol 32: 66–72, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ, AASK Study Investigators. CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS, Family Investigation of Nephropathy and Diabetes Research Group : MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA: MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freedman BI, Langefeld CD, Lu L, Divers J, Comeau ME, Kopp JB, Winkler CA, Nelson GW, Johnson RC, Palmer ND, Hicks PJ, Bostrom MA, Cooke JN, McDonough CW, Bowden DW: Differential effects of MYH9 and APOL1 risk variants on FRMD3 association with diabetic ESRD in African Americans. PLoS Genet 7: e1002150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopalakrishnan I, Iskandar SS, Daeihagh P, Divers J, Langefeld CD, Bowden DW, Hicks PJ, Rocco MV, Freedman BI: Coincident idiopathic focal segmental glomerulosclerosis collapsing variant and diabetic nephropathy in an African American homozygous for MYH9 risk variants. Hum Pathol 42: 291–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kroemer G, Mariño G, Levine B: Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA: Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 283: 21540–21549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB: Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kume S, Thomas MC, Koya D: Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes 61: 23–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulasi II, Tzur S, Wasser WG, Shemer R, Kruzel E, Feigin E, Ijoma CK, Onodugo OD, Okoye JU, Arodiwe EB, Ifebunandu NA, Chukwuka CJ, Onyedum CC, Ijoma UN, Nna E, Onuigbo M, Rosset S, Skorecki K: High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract 123: 123–128, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Kopp JB: Rethinking hypertensive kidney disease: Arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens 22: 266–272, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hays T, Wyatt CM: APOL1 variants in HIV-associated nephropathy: Just one piece of the puzzle. Kidney Int 82: 259–260, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Kalayjian RC, Lau B, Mechekano RN, Crane HM, Rodriguez B, Salata RA, Krishnasami Z, Willig JH, Martin JN, Moore RD, Eron JJ, Kitahata MM: Risk factors for chronic kidney disease in a large cohort of HIV-1 infected individuals initiating antiretroviral therapy in routine care. AIDS 26: 1907–1915, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, Rajasekaran A, Tzur S, Racusen LC, Skorecki K: APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol 23: 343–350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estrella MM, Wyatt CM, Pearce CL, Li M, Shlipak MG, Aouizerat BE, Gustafson D, Cohen MH, Gange SJ, Kao WH, Parekh RS: Host APOL1 genotype is independently associated with proteinuria in HIV infection. Kidney Int 84: 834–840, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM, Jr.: The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis 21: 387–393, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Freedman BI, Iskandar SS, Appel RG: The link between hypertension and nephrosclerosis. Am J Kidney Dis 25: 207–221, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Freedman BI, Murea M: Target organ damage in African American hypertension: Role of APOL1. Curr Hypertens Rep 14: 21–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skorecki KL, Wasser WG: Hypertension-misattributed kidney disease in African Americans. Kidney Int 83: 6–9, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, Depalma SR, Gupta N, Gabriel SB, Taylor HA, Jr., Fox ER, Newton-Cheh C, Kathiresan S, Hirschhorn JN, Altshuler DM, Pollak MR, Wilson JG, Seidman JG, Seidman C: Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 114: 845–850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, Friedman EA: Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 310: 669–673, 1984 [DOI] [PubMed] [Google Scholar]
- 55.Freedman BI, Wilson CH, Spray BJ, Tuttle AB, Olorenshaw IM, Kammer GM: Familial clustering of end-stage renal disease in blacks with lupus nephritis. Am J Kidney Dis 29: 729–732, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Freedman BI, Soucie JM, Stone SM, Pegram S: Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis 34: 254–258, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Behar DM, Kedem E, Rosset S, Haileselassie Y, Tzur S, Kra-Oz Z, Wasser WG, Shenhar Y, Shahar E, Hassoun G, Maor C, Wolday D, Pollack S, Skorecki K: Absence of APOL1 risk variants protects against HIV-associated nephropathy in the Ethiopian population. Am J Nephrol 34: 452–459, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Taylor HE, Khatua AK, Popik W: The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol 88: 592–603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Divers J, Núñez M, High KP, Murea M, Rocco MV, Ma L, Bowden DW, Hicks PJ, Spainhour M, Ornelles DA, Kleiboeker SB, Duncan K, Langefeld CD, Turner J, Freedman BI: JC polyoma virus interacts with APOL1 in African Americans with nondiabetic nephropathy. Kidney Int 84: 1207–1213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kopp JB: JC viruria and kidney disease in APOL1 risk genotype individuals: Is this a clue to a gene × environment interaction? Kidney Int 84: 1069–1072, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng XS, Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Major EO, Randhawa P, Hardinger KL, Brennan DC: Inhibitory interactions between BK and JC virus among kidney transplant recipients. J Am Soc Nephrol 22: 825–831, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pires EP, Bernardino-Vallinoto CV, Alves DM, Migone SR, Machado LF, Ishak MO, Ishak R, Cayres-Vallinoto IM, Vallinoto AC: Prevalence of infection by JC and BK polyomaviruses in kidney transplant recipients and patients with chronic renal disease. Transpl Infect Dis 13: 633–637, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Rossi AP, Anderson KL, Brennan DC: JC polyoma virus and kidney disease. Kidney Int 85: 1242, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Appel LJ, Wright JT, Jr., Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD, Wang X, AASK Collaborative Research Group : Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, Needham AW, Lazarus R, Pollak MR: A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 78: 698–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bostrom MA, Kao WH, Li M, Abboud HE, Adler SG, Iyengar SK, Kimmel PL, Hanson RL, Nicholas SB, Rasooly RS, Sedor JR, Coresh J, Kohn OF, Leehey DJ, Thornley-Brown D, Bottinger EP, Lipkowitz MS, Meoni LA, Klag MJ, Lu L, Hicks PJ, Langefeld CD, Parekh RS, Bowden DW, Freedman BI, Family Investigation of Nephropathy and Diabetes (FIND) Research Group : Genetic association and gene-gene interaction analyses in African American dialysis patients with nondiabetic nephropathy. Am J Kidney Dis 59: 210–221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Divers J, Palmer ND, Lu L, Langefeld CD, Rocco MV, Hicks PJ, Murea M, Ma L, Bowden DW, Freedman BI: Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant 29: 587–594, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Dusel JA, Burdon KP, Hicks PJ, Hawkins GA, Bowden DW, Freedman BI: Identification of podocin (NPHS2) gene mutations in African Americans with nondiabetic end-stage renal disease. Kidney Int 68: 256–262, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Martini S, Nair V, Patel SR, Eichinger F, Nelson RG, Weil EJ, Pezzolesi MG, Krolewski AS, Randolph A, Keller BJ, Werner T, Kretzler M: From single nucleotide polymorphism to transcriptional mechanism: A model for FRMD3 in diabetic nephropathy. Diabetes 62: 2605–2612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaefer E, Zaloszyc A, Lauer J, Durand M, Stutzmann F, Perdomo-Trujillo Y, Redin C, Bennouna Greene V, Toutain A, Perrin L, Gérard M, Caillard S, Bei X, Lewis RA, Christmann D, Letsch J, Kribs M, Mutter C, Muller J, Stoetzel C, Fischbach M, Marion V, Katsanis N, Dollfus H: Mutations in SDCCAG8/NPHP10 cause Bardet-Biedl syndrome and are associated with penetrant renal disease and absent polydactyly. Mol Syndromol 1: 273–281, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW: Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141: 1135–1145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM: Finding the missing heritability of complex diseases. Nature 461: 747–753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]