Abstract
Background and objectives
Soluble urokinase plasminogen activator receptor (suPAR) was initially proposed as a pathogenic and predictive biomarker of primary FSGS, but the findings were controversial. This study aimed to clarify the clinical implications of suPAR.
Design, setting, participants, & measurements
The study enrolled 109 patients with biopsy-proven primary FSGS who were administered prednisone between January 2011 and May 2013 and followed up for 6–24 months (median duration of follow-up, 12 months). Ninety-six healthy volunteers, 20 patients with minimal-change disease (MCD), and 22 patients with membranous nephropathy (MN) served as controls. Serum suPAR levels were measured using ELISA.
Results
suPAR levels in patients with FSGS (median, 3512 [interquartile range (IQR), 2232–4231] pg/ml) were significantly higher than in healthy controls (median, 1823 [IQR, 1563–2212] pg/ml; P<0.001), patients with MCD (median, 1678 [IQR, 1476–2182] pg/ml; P<0.001), and patients with MN (median, 1668 [IQR, 1327–2127] pg/ml; P<0.001). With 3000 pg/ml used as a threshold, suPAR levels were elevated in 48.6% of patients with FSGS, in contrast to 5% of patients with MCD and 4.5% of those with MN. suPAR levels were independently associated with steroid response in patients with FSGS (odds ratio, 85.02; P=0.001). Patients who were sensitive to steroids had significantly higher suPAR levels than nonsensitive patients (median, 3426 [IQR, 2670–5655] pg/ml versus 2523 [IQR, 1977–3460] pg/ml; P=0.001). A suPAR level of 3400 pg/ml was chosen as the optimal cutoff value for steroid response. At the 6-month follow-up in 84 patients with FSGS, suPAR levels were significantly decreased in those with suPAR level ≥3400 pg/ml (median, 4553 [IQR, 3771–6120] pg/ml versus 3149 [IQR, 2278–3953]; P=0.002) but were unchanged in patients with suPAR level <3400 pg/ml (median, 2359 [IQR, 2023–2842] pg/ml versus 2490 [IQR, 1916–3623] pg/ml; P=0.09).
Conclusions
suPAR is specifically elevated in some patients with FSGS, which differs from the finding in patients with MCD and MN. A suPAR assay may help predict steroid response in patients with primary FSGS.
Keywords: urokinase plasminogen activator receptor, focal segmental, glomerulosclerosis, steroid, follow-up
Introduction
FSGS is a clinical-pathologic syndrome with a common glomerular lesion that leads to ESRD in both pediatric and adult patients (1). FSGS can be triggered by multiple initiating events, including viral and other infections, allergies, drugs, and other kidney insults (such as reflux disease or increased intraglomerular pressure) (1–4). Podocytes were identified as the cardinal cellular target of FSGS (5). Th2 polarization and elevated levels of IL-13, a Th2 cytokine, as well as cardiotrophin-like cytokine 1 (6), were found in primary FSGS (7,8). FSGS can recur immediately after transplant and responds to plasmapheresis or immunoadsorption; proteinuria is induced in experimental animals by infusion of patient plasma, which led to the speculation that there may be causative circulating factors (9,10). Recently, Wei and colleagues identified high levels of soluble urokinase plasminogen activator receptor (suPAR) in two thirds of patients with FSGS before renal transplantation and showed that higher levels of suPAR increased the recurrent risks after transplantation (11). In addition, their studies showed that the induction of urokinase receptor (uPAR) signaling in podocytes led to foot process effacement and proteinuria via αvβ3 integrin (11,12). They proposed that suPAR might be a pathogenic cause of FSGS. The importance of suPAR was further verified in their other two additional primary FSGS cohorts (13). Therefore, suPAR appeared to be a prominent candidate for a circulating mediator of primary FSGS.
However, studies by other groups challenged the specificity, pathogenicity, and potential role of suPAR in primary FSGS (14–16). Here, we sought to improve understanding of the contribution of suPAR in patients with FSGS to help evaluate its clinical implications. We assayed serum suPAR levels in 109 patients with primary FSGS and analyzed the association of suPAR levels with steroid response and therapeutic efficacy during follow-up.
Materials and Methods
Participants
We selected 109 patients who had complete clinical, laboratory, and pathologic data and were part of a primary FSGS cohort from January 2011 to May 2013 at the National Clinical Research Center of Kidney Diseases, Jinling Hospital. The inclusion criteria were biopsy-proven FSGS with proteinuria ≥3.5 g per 24 hours and serum albumin <3.0 g/dl. To eliminate the contribution of severe renal dysfunction to elevated suPAR levels and to safely treat the patients with a full dose of prednisone, we also included an eGFR≥40 ml/min per 1.73 m2 in the criteria, as per published reports (17,18). The exclusion criteria included the following: secondary FSGS, viral infections and inflammatory diseases, cancers, tissue regeneration, atherosclerotic lesions, brain ischemia, and a family history of kidney diseases. We enrolled 96 healthy controls and 42 disease controls with biopsy-proven minimal-change disease (MCD) (n=20) or membranous nephropathy (MN) (n=22). The blood samples were obtained on the day of renal biopsy from all the patients. Each participant gave informed consent for clinical, laboratory, and family data collection for blood sampling. The study complied with the Declaration of Helsinki principles and was approved by the ethics committee of Jinling Hospital.
The enrolled 109 patients with FSGS were newly diagnosed and did not receive any immunosuppressant therapy within 2 months before serum collection for the study. Prednisone as the initial therapy was administered orally at a dose of 1 mg/kg per day (maximum dose, 60 mg) once daily or three times per day (19–22). The duration of full-dose prednisone treatment was generally 8 weeks, with a taper extending for an additional 20–24 weeks. For patients who were steroid dependent or resistant after 8 weeks of treatment, low-dose prednisone alone or in combination with tacrolimus (FK506) was administered (21–24). Fifteen patients were treated with angiotensin-converting enzyme inhibitors, and 39 patients were treated with angiotensin-receptor blockers.
Follow-up
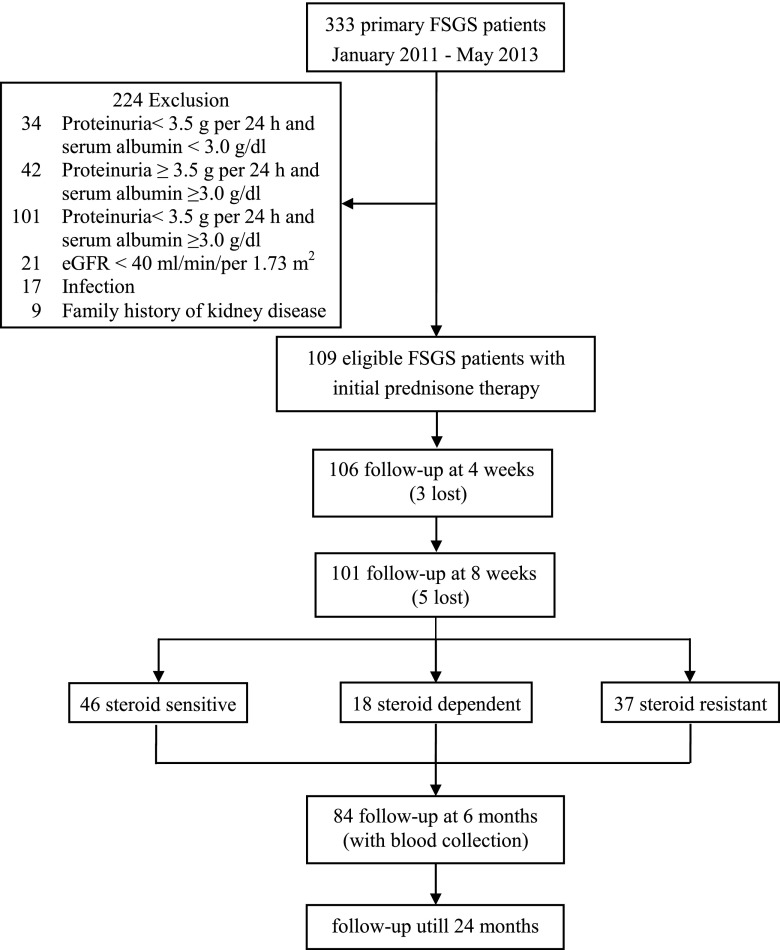
The follow-up time points were set at 1, 2, 3, 6, 9, 12, 18, and 24 months for patients with FSGS. Among the 109 patients, 101 patients had complete follow-up data for 6 months, and we obtained blood samples from 84 patients at the 6-month follow-up (Figure 1).
Figure 1.
Flow chart showing patients with FSGS selection and follow-up. A total of 109 eligible patients with FSGS were enrolled in the study and followed up. At the 6-month follow-up, we completed the clinical data collections from 101 patients but obtained blood samples from only 84 patients.
Definitions
Complete remission was defined as proteinuria≤0.4 g per 24 hours (according to the standard requirements of the kit) (22,25), with normal serum creatinine (0.51–1.24 mg/dl) and serum albumin >3.5 g/dl. Partial remission was defined as proteinuria<3.5 g per 24 hours but >0.4 g per 24 hours, with stable serum creatinine and no more than 25% change from baseline. Relapse was defined by nephrotic-range proteinuria after remission. Steroid sensitivity was defined as complete remission after 8 weeks of prednisone. Steroid dependence was defined as relapse upon tapering of steroid therapy or within 4 weeks of withdrawal of the steroid. Steroid resistance was defined as the absence of complete remission within 8 weeks (22,26).
Renal Histopathology
Histopathologic examination of renal biopsy specimens was performed with light, immunofluorescence, and electron microscopy. Light microscopic assessment of the glomeruli for FSGS lesions was performed according to the Columbia classification described by D'Agati et al. (27). Immunofluorescence and electron microscopy were used to help exclude secondary causes of FSGS. The evaluation of acute and chronic tubulointerstitial injury followed that described in our previous study (26). Chronic tubulointerstitial injury scores were as follows: absent, mild, moderate, and severe based on the percentage of affected cortical area (28).
eGFR Calculation
The eGFR was estimated using Schwartz formula for patients <18 years of age (29) and the CKD-Epidemiology Collaboration (CKD-EPI) formula for those ≥18 years of age (30).
Serum Storage and suPAR Assay
Serum aliquots from fasting blood were stored in −80°C refrigerators until analysis. Repeated freeze-thaw cycles were avoided. A commercially available ELISA kit (Quantikine Human uPAR Immunoassay; R&D, Minneapolis, MN) was used for the serum suPAR assay, and the manufacturer’s protocols were followed. All samples were measured in duplicate.
Statistical Analyses
The statistical software SPSS, version 18.0 (SPSS, Inc., Chicago, IL), was used for the statistical analyses. A descriptive statistical analysis was performed for all studied variables. Normally distributed data were analyzed using the Kolmogorov–Smirnov test. Data were expressed as the mean±SD, median and interquartile range (IQR) (25th and 75th percentiles), or the number (percentage) of patients for categorical variables. Differences in means with normal distribution were compared using a t test, and data with a non-normal distribution were compared using nonparametric tests or the Fisher exact and chi-squared test for categorical variables. Paired variables were compared using the paired samples t test or Wilcoxon matched-paired sign-rank test. Linear regression analyses were performed to explore the relationship between suPAR levels and other clinical parameters in all patients, and logistic regression analyses were used to identify steroid response and clinical measures in patients with FSGS. suPAR levels were log-transformed in regression analyses. Receiver–operating characteristic (ROC) curve analysis and the area under the curve (AUC) statistics provided a composite score for predicting an event. The ROC curve was generated by plotting sensitivity against 1−specificity. Two-sided P value <0.05 was considered to represent statistically significant differences in all analysis.
Results
Participant Characteristics
Our study included 109 patients with primary FSGS, 20 with MCD, 22 with MN, and 96 healthy controls. The clinical characteristics of the study participants are provided in Table 1. Patients with FSGS were older than those with MCD (P<0.01) and younger than those with MN (P<0.001) but were not significantly different in age from the healthy controls. The sex ratios in the disease groups were equivalent. All of the patients had similar degrees of nephrotic proteinuria, and they had higher levels of cholesterol and triglyceride and lower levels of serum albumin than the healthy controls (P<0.001). Compared with patients with FSGS and those with MN, patients with MCD had the lowest serum urea nitrogen and creatinine and the highest eGFR (P<0.001), but there were no significant differences between the FSGS and MN groups in these parameters. All participants had normal levels of C-reactive protein (<6.0 mg/l). Levels of hemoglobin and C-reactive protein (CRP) did not significantly differ among the groups.
Table 1.
Baseline characteristics of the study participants
| Characteristic | Control (n=96) | FSGS (n=109) | MCD (n=20) | MN (n=22) | P Value | P Values | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls versus FSGS | Controls versus MCD | Controls versus MN | FSGS versus MCD | FSGS versus MN | MCD versus MN | ||||||
| Age (yr) | 28±8 | 28±14 | 19±6 | 40±19 | <0.001 | 0.03 | <0.001 | 0.005 | <0.001 | <0.001 | |
| Male/female patients (n/n) | 73/23 | 83/26 | 17/3 | 19/3 | 0.21 | ||||||
| 24-hr urine protein (g/24 hr) | <0.40 | 7.52±2.95 | 6.39±2.23 | 6.07±3.67 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Serum albumin (g/dl) | 4.45±0.36 | 2.14±0.36 | 2.58±0.94 | 2.28±0.32 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Serum urea nitrogen (mg/dl) | 14.2±3.99 | 21.85±10.00 | 14.42±4.76 | 18.47±7.27 | <0.001 | <0.001 | <0.001 | 0.001 | 0.04 | ||
| Serum creatinine (mg/dl) | 0.74±0.14 | 1.03±0.38 | 0.75±0.15 | 0.89±0.40 | <0.001 | <0.001 | 0.004 | 0.002 | 0.04 | ||
| Serum uric acid (μmol/L) | 367±98 | 391±106 | 435±106 | 375±97 | 0.06 | ||||||
| Serum cholesterol (mmol/L) | 4.94±1.13 | 10.96±4.01 | 9.74±3.14 | 9.23±2.62 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Triglyceride (mg/dl) | 121±97 | 282±139 | 215±124 | 289±187 | <0.001 | <0.001 | 0.003 | <0.001 | |||
| Hemoglobin (g/dl) | 13.2±1.8 | 13.2±1.9 | 14.2±1.7 | 13.4±2.1 | 0.06 | ||||||
| eGFR (ml/min per 1.73 m2) | 125±21 | 100±31 | 123±20 | 109±31 | <0.001 | <0.001 | 0.004 | 0.04 | |||
| CRP (mg/L) | 0.10, 0.10–0.10 | 0.10, 0.10–0.10 | 0.10, 0.10–0.10 | 0.10, 0.10–0.10 | 0.29 | ||||||
Normal distribution continuous variables: mean±SD; non-normal distribution continuous variables: median, interquartile range; categorical variables: frequencies. CRP, C-reactive protein; MCD, minimal change disease; MN, membranous nephropathy.
Serum suPAR Levels in All Participants and Correlated Factors of suPAR Levels
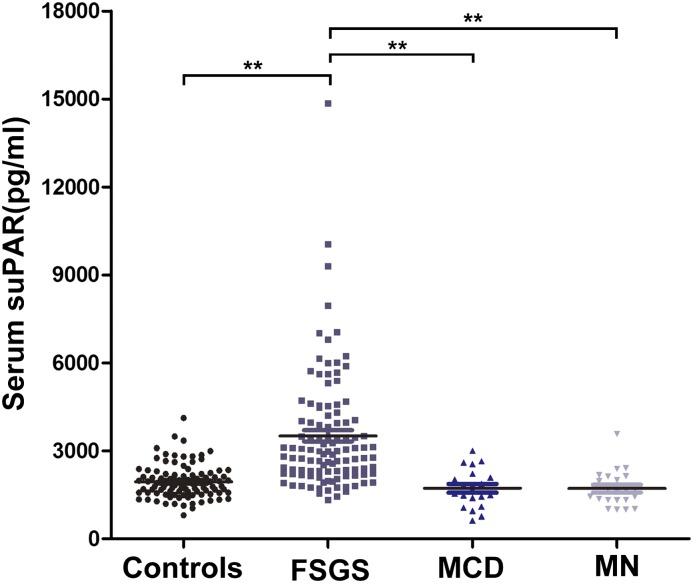
suPAR levels of all patients at baseline are shown in Figure 2. suPAR levels in patients with primary FSGS (median, 3512 [IQR, 2232–4231] pg/ml) were significantly higher than in the healthy controls (median, 1823 [IQR, 1563–2212] pg/ml; P<0.001), patients with MCD (median, 1678 [1476–2182] pg/ml; P<0.001) and patients with MN (median, 1668 [IQR, 1327–2127] pg/ml; P<0.001). The suPAR levels did not significantly differ among the healthy controls, patients with MCD (P=0.92), and patients with MN (P=0.90). A cutoff value of 3000 pg/ml was set on the basis of the mean suPAR levels of the healthy controls (mean+1.96 SD) (31). Using the cutoff of 3000 pg/ml, circulating suPAR concentration was elevated in 48.6% of the patients with primary FSGS, but only in 4.2% of the healthy controls (four of 96), 5% (one of 20) of patients with MCD, and 4.5% (one of 22) of patients with MN.
Figure 2.
Serum soluble urokinase plasminogen activator receptor (suPAR) levels in all participants. **P<0.001 for patients with FSGS versus healthy controls, patients with minimal-change disease (MCD), and patients with membranous nephropathy (MN). Serum suPAR levels in patients with primary FSGS were significantly higher than in healthy controls (P<0.001), patients with MCD (P<0.001), and patients with MN (P<0.001). Serum suPAR levels did not significantly differ among the healthy controls and patients with MCD (P=0.92) and MN (P=0.90). The median for each group is represented by the horizontal line.
To explore the factors that are correlated with suPAR levels, we used univariate linear regression analysis and multivariate stepwise linear regression analysis. The independent variables were sex, age, eGFR, 24-hour urine protein, serum albumin, and FSGS absent or present. In the multivariable model, only FSGS qualified for the final model, which revealed that FSGS was independently associated with suPAR levels (regression coefficient, 0.29; 95% confidence interval [95% CI], 0.22 to 0.36; P<0.001) (Table 2). Because we pooled the eGFR results from patients <18 years of age and those from patients age ≥18 years, we also performed separate linear regression analyses for eGFR calculated with the CKD-EPI (adult patients) and Schwartz (young patients) formulas. We obtained the same result: that suPAR was independently correlated with FSGS (Supplemental Tables 1 and 2).
Table 2.
Linear regression analysis of correlates of soluble urokinase plasminogen activator receptor in all patients
| Variable | suPAR | |||
|---|---|---|---|---|
| Univariable Model | Multivariable Model | |||
| Regression Coefficient (95% CI) | P Value | Regression Coefficient (95% CI) | P Value | |
| Gender (male) | −0.13 (−0.23 to −0.04) | 0.004 | ||
| Age | 0.001 (−0.001 to 0.003) | 0.43 | ||
| eGFR | <0.001 (−0.001 to 0.000) | 0.37 | ||
| 24-hr urine protein | 0.02 (0.003 to 0.027) | 0.02 | ||
| Serum albumin | −0.001 (−0.009 to 0.006) | 0.74 | ||
| FSGS (yes) | 0.29 (0.22 to 0.36) | <0.001 | 0.29 (0.22 to 0.36) | <0.001 |
suPAR, soluble urokinase receptor plasminogen activator; 95% CI, 95% confidence interval.
For patients with FSGS, the frequencies of histologic variants were as follows: 49.5% tip variant (54 of 109), 29.4% not-otherwise-specified variant (32 of 109), 5.5% cellular variant (six of 109), 5.5% perihilar variant (six of 109), and 10.1% collapsing variant (11 of 109). The suPAR levels in these groups of patients were not significantly different (P=0.53). Because patients with the tip variant accounted for almost half of all patients with FSGS, we subdivided the 109 patients into the tip variant group (n=54) and other variants group (n=55). suPAR levels also did not statistically significantly differ between the two groups (median, 3011 [IQR, 2236–4096] pg/ml versus 2998 [IQR, 2157–4476] pg/ml; P=0.84). Most patients with FSGS had no or mild chronic tubulointerstitial lesions, and <10% of the patients manifested moderate to severe lesions. We observed no significant differences in suPAR levels between the groups of patients with different severities of chronic tubulointerstitial lesions (absent: median, 2658 [IQR, 2202–3250] pg/ml; mild: median, 2985 [IQR, 2235–4358] pg/ml; moderate: median, 2924 [IQR, 1945–3751] pg/ml; P=0.78). Acute tubulointerstitial lesions were found in most of the patients with FSGS (89.9%); however, suPAR levels were still similar in the groups with and without acute tubulointerstitial lesions (median, 2985 [IQR, 2235–4358] pg/ml versus 2807 [IQR, 2053–3445] pg/ml; P=0.45).
Correlation between suPAR Levels and Steroid Response in Patients with FSGS
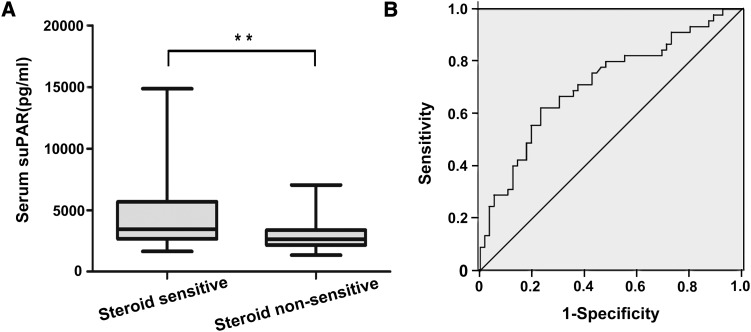
To determine whether suPAR levels were associated with steroid response in patients with FSGS, we performed a multiple logistic regression analysis. In the backward stepwise logistic regression model for the analysis of correlates of steroid response, including age, sex, eGFR, 24-hour urine protein, serum albumin, serum uric acid, lg suPAR, and histologic variants as covariates, suPAR was independently associated with steroid response (odds ratio, 85.02; 95% CI, 6.39 to 1130.44; P=0.001) (Table 3) in patients with FSGS. Consistently, the patients who were sensitive to steroids had significantly higher suPAR levels than the patients who were not sensitive to steroids (including steroid-dependent and steroid-resistant patients) (P=0.001) (Figure 3A). In both the tip variant group and the other variants group, suPAR levels were always higher in the patients who were sensitive to steroids than in the patients who were not sensitive (tip variant: P=0.03; other variants: P=0.001) (Supplemental Table 3).
Table 3.
Logistic regression analysis of correlates of steroid response in patients with FSGS
| Variable | Steroid Response | |||
|---|---|---|---|---|
| Univariable Model | Multivariable Model | |||
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sex | 0.56 (0.22 to 1.44) | 0.23 | ||
| Age | 1.02 (0.99 to 1.05) | 0.21 | ||
| eGFR | 0.99 (0.98 to 1.00) | 0.29 | ||
| 24-hr urine protein | 1.00 (0.88 to 1.15) | 0.96 | ||
| Serum albumin | 1.06 (0.95 to 1.18) | 0.34 | ||
| Serum uric acid | 0.84 (0.83 to 0.85) | 0.37 | ||
| Lg suPAR | 55.96 (5.41 to 578.63) | 0.001 | 85.02 (6.39 to 1130.44) | 0.001 |
| Histologic variants | 0.14 | |||
| Tip | Reference | |||
| NOS | 0.57 (0.23 to 1.44) | 0.24 | ||
| Cellular | 0.15 (0.02 to 1.38) | 0.09 | ||
| Perihilar | 0.38 (0.06 to 2.24) | 0.28 | ||
| Collapsing | 0.19 (0.04 to 0.98) | 0.05 | ||
NOS, not otherwise specified; OR, odds ratio.
Figure 3.
The relationship between suPAR levels and steroid response in patients with FSGS. (A) **P=0.001 for steroid-sensitive versus non–steroid-sensitive patients. The box plots indicate the median and interquartile ranges (25th and 75th percentiles), and the whiskers indicate the 1st and 99th percentiles. (B) suPAR cutoff value of 3400 pg/ml produced an area under the curve of 0.71 (P<0.001), a sensitivity of 0.62, and a specificity of 0.77.
Predictive Value of Baseline suPAR Levels in Steroid Response and Therapeutic Efficacy in Patients with FSGS
Using ROC curve analysis, we sought to determine whether the baseline suPAR level could act as a predictor of steroid response in patients with FSGS. The cutoff value of 3400 pg/ml was chosen in the ROC analysis so that we could evaluate the ability of suPAR to predict steroid responsiveness (it was the cutoff that maximized the Youden index). The ROC AUC was 0.71 (95% CI, 0.61 to 0.81; P<0.001), and the sensitivity and specificity were 0.62 and 0.77, respectively (Figure 3B).
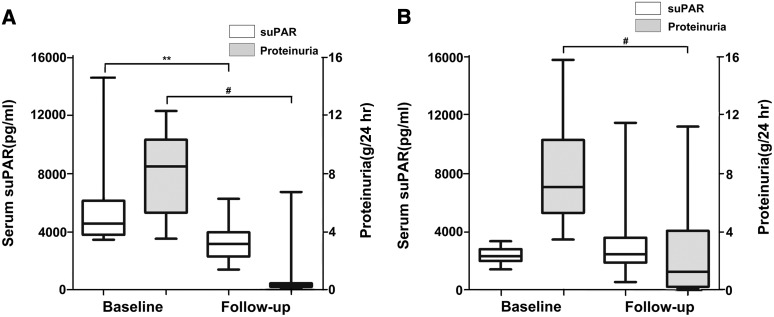
According to the cutoff value of 3400 pg/ml, patients with FSGS were further divided into two groups: a group with suPAR level ≥3400 pg/ml (n=37), defined as the high-suPAR group, and a group with suPAR level <3400 pg/ml (n=47), defined as the low-suPAR group. During follow-up, in the high-suPAR group, serum suPAR levels were significantly decreased (median, 4553 [IQR, 3771–6120] pg/ml versus 3149 [IQR, 2278–3953] pg/ml; P=0.002) (Figure 4A), consistent with proteinuria reduction (7.99±2.66 versus 1.02±1.69 g/24 hours; P<0.001). However, suPAR levels did not change in the low-suPAR group (median, 2359 [IQR, 2023–2842] pg/ml versus 2490 [IQR, 1916–3623] pg/ml; P=0.09) (Figure 4B) even though they exhibited reduced proteinuria (7.90±3.24 versus 2.54±2.97 g/24 hours; P<0.001). In addition, a higher proportion of patients with suPAR level ≥3400 pg/ml achieved complete remission (75.7% [28 of 37]) compared with patients with suPAR level <3400 pg/ml (27.7% [13 of 47]; P=0.001).
Figure 4.
suPAR changes from baseline to follow-up in patients with FSGS. The suPAR levels and proteinuria changes in (A) the high-suPAR group (suPAR level ≥3400 pg/ml; *P=0.002 for baseline suPAR versus follow-up suPAR; #P<0.001 for baseline proteinuria versus follow-up proteinuria) and (B) the low-suPAR group (suPAR level <3400 pg/ml; #P<0.001 for baseline proteinuria versus follow-up proteinuria). The box plots indicate the median and interquartile ranges (25th and 75th percentiles), and the whiskers indicate the 1st and 99th percentiles.
Discussion
With suPAR standard assay, we found that serum suPAR levels were much higher in patients with primary FSGS than in those with MCD or MN who also presented heavy proteinuria. suPAR levels were elevated in 48.6% of patients with FSGS but in <5% of those with MCD and MN. In addition, the study revealed that FSGS was an independent factor correlated with suPAR levels. Furthermore, suPAR levels were associated with steroid response in patients with FSGS.
A low eGFR is associated with serum suPAR elevation in glomerular diseases, including FSGS (15). To eliminate the contribution of severe renal dysfunction to suPAR elevation and to safely treat patients with FSGS with a full dose of prednisone, we selected patients whose eGFR was >40 ml/min per 1.73 m2; in contrast, other studies did not use an eGFR cutoff value during enrollment of patients with FSGS. Our study used the CKD-EPI formula for eGFR calculation, as did the study by Meijers et al. However, other studies used different formulas (16,32,33). Meijers et al. found an inverse correlation between eGFR and suPAR levels in patients without FSGS; however, they did not show the data in patients with FSGS (15). Huang et al. stated that plasma suPAR levels were positively correlated with the severity of chronic tubulointerstitial injury in patients with FSGS (14), but there were no correlations between suPAR and chronic tubulointerstitial lesions in our study. FSGS histologic variants had no association with suPAR levels. Differences in case inclusion criterion, disease activity, race, and other factors may all contribute to the variations in suPAR levels in the patients with primary FSGS.
Steroids are the first-line therapy for FSGS. All 109 patients were initially treated with prednisone at a daily dose of 1 mg/kg per day. Meijers et al. reported that suPAR levels did not differentiate patients with FSGS who were steroid sensitive from patients who were steroid resistant (15). In contrast, our study revealed a prominent correlation between suPAR levels and steroid responsiveness. The patients with FSGS who were steroid sensitive consistently exhibited higher suPAR levels than the patients who were not steroid sensitive (including steroid-dependent and –resistant patients). Studies showed that the tip variant tended to exhibit good response to steroid treatment (34,35), so histologic variants may affect the relationship between suPAR levels and steroid response. On the basis of logistic regression analysis for correlates of steroid response, including histologic variants, as covariates, we still found that suPAR was independently associated with steroid response. Therefore, histologic variants did not affect the correlation of suPAR levels with steroid response.
Furthermore, at the 6-month follow-up, the patients with FSGS who had a baseline suPAR level ≥3400 pg/ml presented a higher rate of suPAR decrease, as well as complete remission, than patients with suPAR level <3400 pg/ml. Therefore, in our FSGS cohort, a suPAR level >3400 pg/ml indicated a good response to steroids and a good prognosis, even though we did not obtain a very high AUC. A study by Wada et al. showed that patients with FSGS without renal impairment who took steroids/immunosuppressants had significantly lower levels of suPAR (16). Huang et al. also found decreased plasma suPAR levels in patients with FSGS with complete remission (14). However, they had no consistent therapy regimen and follow-up, and sample sizes were small. The results suggested that a serum suPAR level assay may help evaluate steroid response and prognosis in patients with FSGS.
The current rationale for the steroid treatment of primary FSGS is that the permeability factor is derived from immune system dysregulation (1). Several pieces of evidence have suggested that primary FSGS may be caused by certain circulating factors that are secreted from monocytes, neutrophils, and T cells and that act on podocytes (1,2,6,11,36,37). A recent study by Ding et al. highlighted the immune-mediated pathogenicity of primary FSGS, especially in patients who are sensitive to steroids (38). In our study, the initial steroid responsiveness was related to higher suPAR levels (≥3400 pg/ml), which suggested that serum suPAR elevation may possibly underlie the pathogenesis of some patients with FSGS. suPAR level assays may help determine a therapeutic regimen for patients with FSGS. However, FSGS is a profoundly heterogeneous disease entity. Therefore, it is unlikely that a biomarker could reflect the pathogenesis of all patients with FSGS and thereby serve as a general FSGS marker (39,40).
A limitation of our study was that only patients with an eGFR≥40 ml/min per 1.73 m2 were included. Accordingly, most of the enrolled patients presented mild or even no chronic tubulointerstitial injury on histopathologic examination. Therefore, we could not determine the effects of the different degrees of eGFR and chronic tubulointerstitial injury on suPAR levels. Another study limitation was that we could not measure the suPAR levels at each visit during follow-up because of limits on blood sample collection. A continuous suPAR level assay would help to further evaluate the association between suPAR levels and steroid treatment response, especially steroid relapse. In a further study, we will attempt to collect more blood samples to obtain dynamic serum suPAR levels and explore their clinical implications.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (no. 2012CB517606), a grant from the National Natural Science Foundation of China (no. 30900686), and the Natural Science Foundation of Jiangsu Province (BK2012780). Serum samples were from the Renal Disease Biobank of National Clinical Research Center of Kidney Diseases, Jinling Hospital.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02370314/-/DCSupplemental.
See related editorial, “The Hype Cycle for Soluble Urokinase Receptor in FSGS: Passing the Trough of Disillusionment?,” on pages 1835–1836.
References
- 1.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Audard V, Fan Q, Pawlak A, Lang P, Sahali D: Immunopathogenesis of idiopathic nephrotic syndrome. Contrib Nephrol 169: 94–106, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Sharma R, McCarthy ET, Savin VJ: The focal segmental glomerulosclerosis permeability factor: Biochemical characteristics and biological effects. Exp Biol Med (Maywood) 229: 85–98, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F: Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 334: 878–883, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Gbadegesin R, Lavin P, Foreman J, Winn M: Pathogenesis and therapy of focal segmental glomerulosclerosis: An update. Pediatr Nephrol 26: 1001–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy ET, Sharma M, Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC: Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: Evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol 10: 529–537, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Tain YL, Chen TY, Yang KD: Implications of serum TNF-beta and IL-13 in the treatment response of childhood nephrotic syndrome. Cytokine 21: 155–159, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Mauer SM, Hellerstein S, Cohn RA, Sibley RK, Vernier RL: Recurrence of steroid-responsive nephrotic syndrome after renal transplantation. J Pediatr 95: 261–264, 1979 [DOI] [PubMed] [Google Scholar]
- 10.Rea R, Smith C, Sandhu K, Kwan J, Tomson C: Successful transplant of a kidney with focal segmental glomerulosclerosis. Nephrol Dial Transplant 16: 416–417, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A, Anarat A, Emre S, Ghiggeri GM, Ozaltin F, Haffner D, Gipson DS, Kaskel F, Fischer DC, Schaefer F, Reiser J, PodoNet and FSGS CT Study Consortia : Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 23: 2051–2059, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, Chu R, Chen Y, Zhao MH: Plasma soluble urokinase receptor levels are increased but do not distinguish primary from secondary focal segmental glomerulosclerosis. Kidney Int 84: 366–372, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Meijers B, Maas RJ, Sprangers B, Claes K, Poesen R, Bammens B, Naesens M, Deegens JK, Dietrich R, Storr M, Wetzels JF, Evenepoel P, Kuypers D: The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int 85: 636–640, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Wada T, Nangaku M, Maruyama S, Imai E, Shoji K, Kato S, Endo T, Muso E, Kamata K, Yokoyama H, Fujimoto K, Obata Y, Nishino T, Kato H, Uchida S, Sasatomi Y, Saito T, Matsuo S: A multicenter cross-sectional study of circulating soluble urokinase receptor in Japanese patients with glomerular disease. Kidney Int 85: 641–648, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Braun N, Schmutzler F, Lange C, Perna A, Remuzzi G, Risler T, Willis NS: Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev 16: CD003233, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gipson DS, Trachtman H, Kaskel FJ, Radeva MK, Gassman J, Greene TH, Moxey-Mims MM, Hogg RJ, Watkins SL, Fine RN, Middleton JP, Vehaskari VM, Hogan SL, Vento S, Flynn PA, Powell LM, McMahan JL, Siegel N, Friedman AL: Clinical trials treating focal segmental glomerulosclerosis should measure patient quality of life. Kidney Int 79: 678–685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banfi G, Moriggi M, Sabadini E, Fellin G, D’Amico G, Ponticelli C: The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin Nephrol 36: 53–59, 1991 [PubMed] [Google Scholar]
- 20.Stirling CM, Mathieson P, Boulton-Jones JM, Feehally J, Jayne D, Murray HM, Adu D: Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. QJM 98: 443–449, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int 82: 840–856, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Koukoulaki M, Goumenos DS: The accumulated experience with the use of mycophenolate mofetil in primary glomerulonephritis. Expert Opin Investig Drugs 19: 673–687, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Dimkovic N, Jovanovic D, Kovacevic Z, Rabrenovic V, Nesic V, Savin M, Mitic B, Ratkovic M, Curic S, Mitic I, Pljesa S, Perunicic-Pekovic G, Marinkovic J, Popovic J, Vujic D: Mycophenolate mofetil in high-risk patients with primary glomerulonephritis: results of a 1-year prospective study. Nephron Clin Pract 111: c189–c196, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry G, Toronto Glomerulonephritis Registry Group : Focal and segmental glomerulosclerosis: Definition and relevance of a partial remission. J Am Soc Nephrol 16: 1061–1068, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Tang X, Xu F, Chen DM, Zeng CH, Liu ZH: The clinical course and long-term outcome of primary focal segmental glomerulosclerosis in Chinese adults. Clin Nephrol 80: 130–139, 2013 [DOI] [PubMed] [Google Scholar]
- 27.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D’Agati V, Appel G: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkwood BR, Sterne JAC: Essential Medical Statistics, 2nd Ed., Oxford, UK, Blackwell Science Publications, 2003 [Google Scholar]
- 32.Bock ME, Price HE, Gallon L, Langman CB: Serum soluble urokinase-type plasminogen activator receptor levels and idiopathic FSGS in children: A single-center report. Clin J Am Soc Nephrol 8: 1304–1311, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franco Palacios CR, Lieske JC, Wadei HM, Rule AD, Fervenza FC, Voskoboev N, Garovic VD, Zand L, Stegall MD, Cosio FG, Amer H: Urine but not serum soluble urokinase receptor (suPAR) may identify cases of recurrent FSGS in kidney transplant candidates. Transplantation 96: 394–399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Malhotra KP, Prasad N, Jain M: Morphological features and prognostic significance of tip variant of focal segmental glomerulosclerosis: Study of an Indian cohort. Indian J Pathol Microbiol 53: 248–252, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Shalhoub RJ: Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 2: 556–560, 1974 [DOI] [PubMed] [Google Scholar]
- 37.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M: A glomerular permeability factor produced by human T cell hybridomas. Kidney Int 40: 453–460, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Ding WY, Koziell A, McCarthy HJ, Bierzynska A, Bhagavatula MK, Dudley JA, Inward CD, Coward RJ, Tizard J, Reid C, Antignac C, Boyer O, Saleem MA: Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J Am Soc Nephrol 25: 1342–1348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyrier A: Focal and segmental glomerulosclerosis: Multiple pathways are involved. Semin Nephrol 31: 326–332, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Sever S, Trachtman H, Wei C, Reiser J: Is there clinical value in measuring suPAR levels in FSGS? Clin J Am Soc Nephrol 8: 1273–1275, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.