Abstract
Background and objectives
Loss of renal function in patients with primary membranous nephropathy cannot be reliably predicted by laboratory or clinical markers at the time of diagnosis. M-type phospholipase A2 receptor autoantibodies have been shown to be associated with changes in proteinuria. Their eventual effect on renal function, however, is unclear.
Design, setting, participants, & measurements
In this prospective, open, multicenter study, the potential role of M-type phospholipase A2 receptor autoantibodies levels on the increase of serum creatinine in 118 consecutive patients with membranous nephropathy and positivity for serum M-type phospholipase A2 receptor autoantibodies was analyzed. Patients were included in the study between April of 2010 and December of 2012 and observed until December of 2013. The clinical end point was defined as an increase of serum creatinine by ≥25% and serum creatinine reaching ≥1.3 mg/dl.
Results
Patients were divided into tertiles according to their M-type phospholipase A2 receptor autoantibody levels at the time of inclusion in the study: tertile 1 levels=20–86 units/ml (low), tertile 2 levels=87–201 units/ml (medium), and tertile 3 levels ≥202 units/ml (high). The median follow-up time of all patients in the study was 27 months (interquartile range=18–33 months). The clinical end point was reached in 69% of patients with high M-type phospholipase A2 receptor autoantibodies levels (tertile 3) but only 25% of patients with low M-type phospholipase A2 receptor autoantibodies levels. The average time to reach the study end point was 17.7 months in patients with high M-type phospholipase A2 receptor autoantibodies levels and 30.9 months in patients with low M-type phospholipase A2 receptor autoantibodies levels. A multivariate Cox regression analysis showed that high M-type phospholipase A2 receptor autoantibodies levels—in addition to men and older age—are an independent predictor for progressive loss of renal function.
Conclusions
High M-type phospholipase A2 receptor autoantibodies levels were associated with more rapid loss of renal function in this cohort of patients with primary membranous nephropathy and therefore, could be helpful for treatment decisions.
Keywords: membranous nephropathy, nephrotic syndrome, renal function decline, risk factors
Introduction
The prediction of clinical outcome of patients with primary membranous nephropathy (MN) and nephrotic proteinuria is still a dilemma. The patients may develop a spontaneous remission of proteinuria with very good prognosis or a progressive loss of renal function and ESRD (1). A number of studies has shown that immunosuppressive therapies lead to remission of proteinuria and maintenance of renal function (2–5). However, it is still a challenge to decide for which patient and at what time in the course of the disease an immunosuppressive therapy should be started. Using three cohorts of patients with primary MN, Cattran et al. (6) validated a model, which allows prediction of progression of renal failure. In this model, a persistent high level of proteinuria (>8 g/24 h), the slope of creatinine clearance over 6 months, and an elevated serum creatinine at the time of diagnosis are predictors for loss of renal function. The discovery that M-type phospholipase A2 receptor autoantibodies (PLA2R-Abs) are detectable in about 70% of patients with primary MN allows us to assess their potential role as a pathogenetic mechanism on the clinical outcome of patients with primary MN (7). Several studies have shown a close association of serum PLA2R-Ab levels and changes in proteinuria (8–10). Two retrospective analyses have suggested that high PLA2R-Ab levels at the time of diagnosis may be predictors of unfavorable clinical outcome (11,12). To test whether PLA2R-Ab levels could be a risk factor for loss of renal function, we performed a prospective analysis in patients with MN who were positive for PLA2R-Ab in the serum.
Materials and Methods
Patients and Study Design
Inclusion criteria for participating in this prospective, multicenter, open clinical study were histologic diagnosis of primary MN, a positive PLA2R-Ab test within 6 months of renal biopsy, and no immunosuppressive therapy before inclusion in the study. Patients were included in the study between April of 2010 and December of 2012. Patients with a secondary form of MN were excluded from the study. Screening for secondary MN included serologic tests for lupus erythematodes and hepatitis, a detailed medical history, and screening for malignancies depending on the age and additional risk factors of the patient. During the study follow-up, the treating physicians decided on the therapeutic strategy without any recommendations. The most common factors for starting an immunosuppressive treatment were (1) severe clinical symptoms (nephrotic syndrome and edema) that were not properly controlled and (2) decline of renal function. The decision of which immunosuppressive therapy to use was made by the treating physicians on the basis of their experience.
PLA2R-Ab levels, 24-hour protein excretion, and serum creatinine were measured every 3 months. The study end point was defined as an increase of serum creatinine by ≥25% compared with the serum creatinine level at study inclusion and reaching a serum creatinine level of ≥1.3 mg/dl. This decrease in renal function is considered relevant in patients with MN; in these patients, the overall decline in renal function over time is moderate, because they may have spontaneous remission, stable disease, or progressive decrease of renal function.
In line with previous publications (11,12), patients were divided in three tertiles depending on the total IgG PLA2R-Ab level at the start of the study (low, medium, and high PLA2R-Ab levels).
The study was approved by the local ethics committee of the Chamber of Physicians in Hamburg and conducted in accordance with the ethical principles stated by the Declaration of Helsinki. Informed consent was obtained from all participating patients.
PLA2R-Ab Measurement
Serum levels of PLA2R-Ab were first measured by an indirect immunofluorescence test (8,13). After development of an ELISA (14) by EUROIMMUN AG, we continued measurements of total IgG and IgG4 subclass PLA2R-Ab by ELISA. The ELISA was chosen because of better quantification of the PLA2R-Ab levels. Because we were involved in the validation of this ELISA (14) and were experienced in performing this test, all measurements were performed in our laboratory. According to the manufacturer, the ELISA results were considered positive at a level >20 units/ml for IgG PLA2R-Ab and >0.259 units/ml for IgG4 PLA2R-Ab.
Histologic Studies
Interstitial fibrosis, glomerular scarring, and hypertensive nephrosclerosis were analyzed with standard periodic acid–Schiff staining. Interstitial fibrosis was classified as minor when ≤15% of the tubulointerstitial space was involved, moderate when >15% but ≤50% of the tubulointerstitial space was involved, and extended when >50% of the tubulointerstitial space was involved. Glomerular lesions were assessed by electron microscopy according to the classification of Ehrenreich and Churg (15).
Statistical Analyses
Data are given as mean values±SDs or median values and interquartile ranges (IQRs) when appropriate. Statistical significance was defined as a P value <0.05 (α=0.05). Patients were divided in three groups corresponding to the tertiles of the total IgG PLA2R-Ab level at the start of the study (low, medium, and high). Survival analysis was done on the clinical end point defined as an increase of serum creatinine by ≥25% and a serum creatinine of ≥1.3 mg/dl by computing Kaplan–Meier curves for these tertiles. The Kaplan–Meier curves were compared pairwise by log rank tests to test for significant differences. The resulting P values were Bonferroni adjusted (P′=P×3; 3=number of comparisons). A multivariate Cox regression analysis was performed on the clinical end point using clinical variables measured at the beginning of the study (age, sex, total IgG PLA2R-Ab levels, IgG4 PLA2R-Ab levels, proteinuria, serum albumin, serum creatinine, time between renal biopsy and measurement of PLA2R-Ab levels, glomerular lesions according to the work by Ehrenreich and Churg [15], and BP) as well as immunosuppressive treatment during follow-up. These explanatory variables were chosen because of their potential influence on the development of renal function (and proteinuria). The explanatory variables serum creatinine, proteinuria, time between renal biopsy, and measurement of PLA2R-Ab levels and total IgG PLA2R-Ab levels were ln transformed before the analysis. The strength and significance of the effects of the explanatory variables were indicated by hazard ratios, their associated confidence intervals, and P values in a forest plot. All statistical analyses were done using SPSS, version 21.
Results
Clinical Baseline Characteristics
One hundred sixty-eight consecutive patients with biopsy-proven primary MN were tested for PLA2R-Ab in the serum. One hundred eighteen patients were positive for PLA2R-Ab and included in this study (Table 1).
Table 1.
Clinical baseline characteristics of the patients at the time of inclusion in the study
| Clinical Baseline Characteristics | All Patients | Tertile 1 (Low) | Tertile 2 (Medium) | Tertile 3 (High) | P Value Low Versus High |
|---|---|---|---|---|---|
| Number of patients | 118 | 40 | 39 | 39 | |
| PLA2R-Ab level total IgG (units/ml) | 300±443 | 55±17 | 141±32 | 711±583 | <0.001 |
| PLA2R-Ab level IgG4 (units/ml) | 42.3±65.0 | 7.8±5.1 | 20.9±13.1 | 99.1±88.2 | <0.001 |
| Males (%) | 86 (73%) | 29 (73%) | 31 (79%) | 26 (67%) | 0.86 |
| Age (yr) | 53.1±16.3 | 48.2±15.8 | 52.9±15.3 | 58.2±16.5 | <0.01 |
| Time from renal biopsy to first serum measurement (mo)a | 0.75 (0.25–1.0) | 0.5 (0.0–0.75) | 0.75 (0.25–1.0) | 0.75 (0.25–1.5) | 0.06 |
| Proteinuria (g/24 h)a | 7.5 (5.0–10.7) | 6.4 (4.8–8.7) | 7.3 (5.8–10.4) | 9.1 (4.3–12.2) | 0.07 |
| Serum albumin (g/L) | 25.7±5.5 | 26.6±5.2 | 26.3±6.6 | 24.3±4.4 | 0.04 |
| Serum creatinine (mg/dl)a | 1.0 (0.8–1.3) | 1.0 (0.7–1.2) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) | 0.12 |
| Patients on immunosuppression during follow-up (%) | 93 (79) | 26 (65) | 31 (79) | 36 (92) | 0.31 |
| Systolic BP (mmHg) | 136.1±18.9 | 129.6±18.1 | 138.1±20.5 | 140.7±16.6 | <0.01 |
Patients were divided in tertiles depending on the total IgG PLA2R-Ab level at the start of the study: tertile 1, low PLA2R-Ab levels; tertile 2, medium PLA2R-Ab levels; tertile 3, high PLA2R-Ab levels. P values are given for the comparison of the patients in tertiles 1 (low PLA2R-Ab levels) and 3 (high PLA2R-Ab levels). PLA2R-Ab, phospholipase A2 receptor autoantibody.
Data are shown as medians and interquartile ranges.
The time between a renal biopsy (diagnosis of primary MN) and the study inclusion (first measurement of PLA2R-Ab levels) was 0.75 months (IQR=0.25–1.0 months). The time from disease onset (start of symptoms) to the time of diagnosis (time of renal biopsy) was 2 months (IQR=0–4.5 months) for 55 of 118 patients. This time is 1 month (IQR=0–4 months) for 21 patients in tertile 1, 3 months (IQR=1.5–3.5 months) for 16 patients in tertile 2, and 2 months (IQR=1–6 months) for 18 patients in tertile 3 (all differences are not statistically significant). At the time of inclusion in the study, almost all patients (111 of 118; 94%) were treated with an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker. Most patients received diuretics (100 of 118; 85%) and lipid-lowering drugs (72 of 118; 61%). Forty-nine patients were treated with anticoagulants. The use of anticoagulants was decided on by the treating physicians. Treatment was prophylactic in 26 patients and therapeutic in 23 patients. A thrombotic event occurred in 14 patients before start of the study or during the follow-up.
Patients were divided in three groups (tertiles) depending on the total IgG PLA2R-Ab level at the start of the study. PLA2R-Ab levels at inclusion in the study were 20–86 units/ml in tertile 1 (low PLA2R-Ab levels), 87–201 units/ml in tertile 2 (medium PLA2R-Ab levels), and 202–2852 units/ml in tertile 3 (high PLA2R-Ab levels). The majority of patients was men. Patients in tertile 3 were older than those in tertile 1 (P<0.05). Proteinuria was higher in tertile 3 than in tertile 1, but this difference did not reach statistical significance. Patients in the different tertiles had similar serum creatinine levels at the start of the study. In tertile 3, there were more patients receiving immunosuppressive treatment during the study follow-up than in tertile 1. This difference did not reach statistical significance. Patients in tertile 3 had significantly higher BP levels than patients in tertile 1. The median follow-up time of all patients in the study was 27 months (IQR=18–33 months).
Analysis of Risk Factors for Progression of Renal Failure
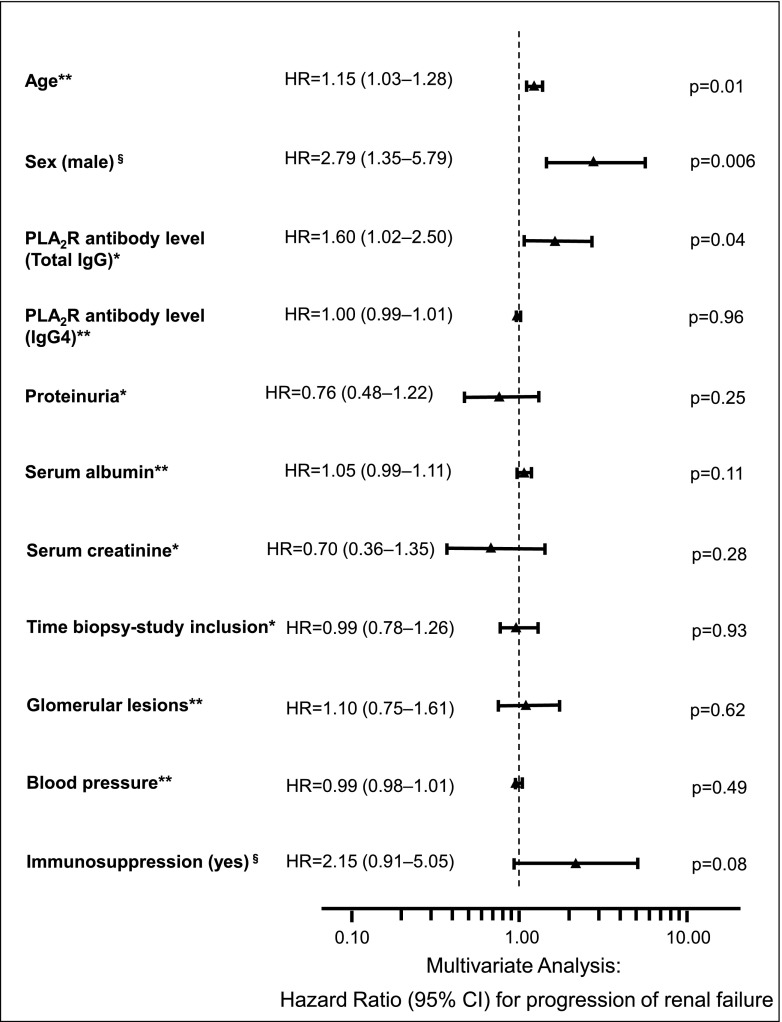
We performed a multivariate Cox regression analysis to identify risk factors associated with progression of renal failure (Figure 1). All available clinical factors (age, sex, total IgG PLA2R-Ab levels, IgG4 PLA2R-Ab levels, proteinuria, serum albumin, serum creatinine, time between renal biopsy and measurement of PLA2R-Ab levels, glomerular lesions according to the work by Ehrenreich and Churg [15], BP, and use of immunosuppressive treatment) were included in this analysis. The hazard ratios and their confidence intervals in Figure 1 indicate that, on average and adjusting for the effects of all other variables in the analysis, the risk of reaching the clinical end point (defined as an increase of serum creatinine by ≥25% and reaching a serum creatinine level of ≥1.3 mg/dl) is higher in older patients and increased by 15% every 5 years. Likewise, this risk increases by 60% per unit increase in the natural logarithm of the total IgG PLA2R-Ab level and is about 2.8 times higher in men compared with women. After adjusting for the effects of all other variables, IgG4 subclass PLA2R-Ab levels were not associated with outcome.
Figure 1.
HRs with 95% confidence intervals and P values as estimated for explanatory variables by multivariate Cox regression analysis. The explanatory variables serum creatinine, proteinuria, time between renal biopsy, and measurement of M-type phospholipase A2 receptor autoantibody (PLA2R-Ab) levels and total IgG PLA2R-Ab levels were ln transformed before analysis. Effects of age, sex, and PLA2R-Ab levels are significant at α=0.05. The corresponding HRs indicate higher risks of reaching the study end point with older age, with higher PLA2R-Ab levels, and for men compared with women. 95% CI, 95% confidence interval; HR, hazard ratio. *The HRs for serum creatinine, proteinuria, time between renal biopsy, and measurement of PLA2R-Ab levels and total IgG PLA2R-Ab levels show the increase of risk per unit increase in the natural logarithm of the variable. **The HRs for serum albumin, glomerular lesions, BP, and IgG4 PLA2R-Ab levels show the increase of risk per unit increase of the variable. The HR for age shows the increase of risk per 5 years. §The variables sex and immunosuppression are dichotomous. The HRs show the risk in men compared with women and patients who received immunosuppression compared with those who did not.
Glomerular Lesions and Tubulointerstitial Fibrosis
All 118 biopsies of the participating patients were analyzed for the degree of tubulointerstitial fibrosis, glomerular lesions, hypertensive damage, and glomerular scarring (Table 2).
Table 2.
Renal biopsy findings
| Renal Biopsy Findings | Number of Patients (%) | P Value | |||||
|---|---|---|---|---|---|---|---|
| All Patients | Tertile 1 (Low) | Tertile 2 (Medium) | Tertile 3 (High) | Tertile 1 Versus 2 | Tertile 1 Versus 3 | Tertile 2 Versus 3 | |
| Interstitial fibrosis, % | |||||||
| Minor | 82 (69) | 32 (80) | 28 (72) | 22 (56) | 0.86 | 0.38 | 0.59 |
| Moderate | 23 (19) | 2 (5) | 9 (23) | 12 (31) | 0.06 | 0.02 | 0.63 |
| Extended | 13 (11) | 6 (15) | 2 (5) | 5 (13) | 0.27 | >0.99 | 0.44 |
| Glomerular lesions (electron microscopy), % | |||||||
| I | 9 (8) | 4 (10) | 2 (5) | 3 (8) | 0.68 | >0.99 | >0.99 |
| II | 73 (62) | 21 (53) | 28 (72) | 24 (62) | 0.47 | 0.71 | 0.72 |
| III | 22 (19) | 8 (20) | 6 (15) | 8 (21) | 0.77 | >0.99 | 0.77 |
| IV | 14 (12) | 7 (18) | 3 (8) | 4 (10) | 0.32 | 0.53 | >0.99 |
| Nephrosclerosis, % | |||||||
| No | 67 (57) | 29 (73) | 23 (59) | 15 (38) | 0.60 | 0.13 | 0.33 |
| Yes | 51 (43) | 11 (27) | 16 (41) | 24 (62) | 0.50 | 0.07 | 0.34 |
| Minor | 23 (19) | 4 (10) | 9 (23) | 10 (26) | 0.24 | 0.16 | >0.99 |
| Moderate | 24 (20) | 6 (15) | 5 (13) | 13 (33) | >0.99 | 0.20 | 0.12 |
| Severe | 4 (3) | 1 (3) | 2 (5) | 1 (3) | >0.99 | >0.99 | >0.99 |
| Scarred glomeruli per biopsy specimen, % | 10±17 | 9±15 | 10±17 | 13±18 | 0.72 | 0.24 | 0.42 |
Interstitial fibrosis, glomerular lesions assessed by electron microscopy according to the classification by Ehrenreich and Churg (15), hypertensive glomerulosclerosis, and the percentage of scarred glomeruli per renal biopsy specimen are presented.
Interstitial fibrosis was found in 100 of 118 biopsy specimens. There were no significant differences in the numbers of patients with extensive interstitial fibrosis between the different tertiles. There were significantly more patients with moderate interstitial fibrosis in tertile 3 than in tertile 1 (P=0.02). The number of patients with minor interstitial fibrosis was smaller in tertile 1 than in tertile 3. This difference did not reach statistical significance.
There were no statistically significant differences in the numbers of patients with stages I–IV glomerular lesions between the different tertiles. More patients with hypertensive nephrosclerosis were found in tertile 3 compared with tertile 1. This difference was not statistically significant, and most of these patients had minor or moderate lesions. The percentage of scarred glomeruli per biopsy specimen was not significantly different between the tertiles. The average number of glomeruli per biopsy specimen was 14.9±9.8, and 13 (11%) biopsy specimens had <5 glomeruli.
Progression of Renal Failure in the Different PLA2R-Ab–Level Tertiles
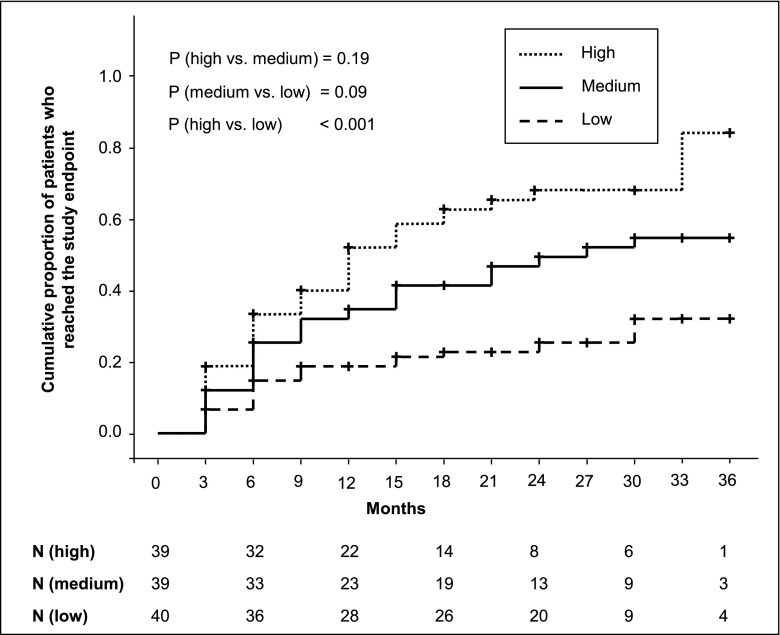
Patients were divided in tertiles depending on the total IgG PLA2R-Ab level at the start of the study (Table 3). The number of patients reaching a serum creatinine increase by ≥25% and a serum creatinine level of ≥1.3 mg/dl was significantly higher in tertile 3 (27 of 39 patients; 69%) than in tertile 1 (10 of 40 patients; 25%; Fisher exact test P=0.03). Patients in tertile 3 reached the end point significantly faster than patients in tertile 1 (Figure 2). Patients not reaching the study end point were followed for 24 months (IQR=18–30 months). This follow-up time was not different between the three tertiles (tertile 1: 24 months, IQR=15.75–27 months; tertile 2: 24 months, IQR=21–30 months; tertile 3: 27 months, IQR=20.25–30 months).
Table 3.
Number of patients reaching the study end point and time when the study end point was reached
| M-Type Phospholipase A2 Receptor Autoantibody Level at Study Inclusion | Number of Patients Reaching the Study End Point (%) | Mean Time (mo) to Reach Study End Point | |
|---|---|---|---|
| Time | 95% Confidence Interval | ||
| Tertile 1 (20–86 units/ml) | 10 (25) | 30.9 | 27.1 to 34.7 |
| Tertile 2 (87–201 units/ml) | 22 (56) | 26.9 | 22.0 to 31.8 |
| Tertile 3 (≥202 units/ml) | 27 (69) | 17.7 | 14.1 to 21.2 |
| Overall | 59 (50) | 26.9 | 24.0 to 29.8 |
Figure 2.
Survival analysis by M-type PLA2R-Ab–level tertile. Patients in tertile 3 (high PLA2R-Ab levels) reached the study end point, defined as an increase of serum creatinine by ≥25% compared with the serum creatinine level at study inclusion and reaching a serum creatinine level of ≥1.3 mg/dl, significantly faster than patients in tertile 1 (low PLA2R-Ab levels). The cumulative rate of patients who reached the study end point during follow-up was larger in patients in tertile 3 than in patients in tertile 1. P values are from log rank tests and Bonferroni adjusted. N, number of patients at risk at the different time points.
Proteinuria and PLA2R-Ab Levels at the End of the Follow-Up
Proteinuria and PLA2R-Ab levels at the time when the patients reached the study end point or the end of the follow-up (for patients who did not reach the end point) are shown in Table 4. Proteinuria of patients in tertile 3 was 3.7 g/24 h (IQR=0.7–9.6 g/24 h) and significantly higher than proteinuria in patients in tertile 1 (low PLA2R-Ab levels), which was 1.4 g/24 h (IQR=0.3–4.0 g/24 h; P=0.03). PLA2R-Ab levels were also significantly higher in patients in tertile 3 compared with patients in tertile 1 (48 units/ml, IQR=3–166 units/ml versus 5 units/ml, IQR=2–36 units/ml; P=0.03).
Table 4.
Proteinuria and M-type phospholipase A2 receptor autoantibody levels at the time of reaching the study end point or the end of follow-up
| At End of Follow-Up | All Patients | Tertile 1 (Low) | Tertile 2 (Medium) | Tertile 3 (High) | P Value | ||
|---|---|---|---|---|---|---|---|
| Tertile 1 Versus 2 | Tertile 1 Versus 3 | Tertile 2 Versus 3 | |||||
| Proteinuria (g/24 h) | 2.1 (0.6–6.0) | 1.4 (0.3–4.0) | 2.5 (0.8–5.4) | 3.7 (0.7–9.6) | 0.14 | 0.03 | 0.24 |
| PLA2R-Ab level (units/ml) | 12 (2–76) | 5 (2–36) | 18 (2–66) | 48 (3–166) | 0.38 | 0.03 | 0.12 |
Proteinuria and PLA2R-Ab levels are measured at the time when the patients reached the study end point or the end of the follow-up (for patients who did not reach the study end point). Data on proteinuria were available for 35 patients in tertile 1, 31 patients in tertile 2, and 30 patients in tertile 3. Data on PLA2R-Ab levels were available for 36 patients in tertile 1, 37 patients in tertile 2, and 34 patients in tertile 3. Data are presented as medians and interquartile ranges.
Immunosuppressive Treatment Used in the Study
During follow-up, 93 of 118 (79%) patients were treated with immunosuppressive therapy. The mean time from study inclusion to initiation of immunosuppressive treatment is 3.7±5.5 months.
Cyclosporin A (CsA) was used in 61 patients and applied two times daily for a mean time of 10.5±7.5 months (12.6±7.7 months in patients of tertile 1, 10.1±7.3 months in patients of tertile 2, and 9.1±7.6 months in patients of tertile 3; none of the differences were statistically significant). In 51 of these patients, CsA was given combined with glucocorticoids.
Cyclophosphamide (Cyc) was given in 40 patients. In 38 of these patients, Cyc was given combined with glucocorticoids. In 25 patients, Cyc was applied orally, and 15 patients received intravenous Cyc.
Rituximab (RTX) was used in 17 patients. Thirteen patients received RTX combined with glucocorticoids. The number of RTX infusions that the patients received was one in four patients, two in seven patients, three in one patient, four in four patients, and five in one patient.
A summary of the immunosuppressants applied is presented in Tables 5 and 6. In tertile 1, 26 patients received immunosuppressive therapy. The medication was switched in 11 of these patients. In tertile 2, immunosuppression was switched in nine of 31 patients. In tertile 3, immunosuppression was switched in 13 of 36 patients. There was no difference in the rate or dosage of the different drugs used between the three groups (Tables 5 and 6).
Table 5.
Immunosuppressive treatment used in the study: number of patients receiving immunosuppression
| PLA2R-Ab Level | Patients without IS | Patients with IS | Number of Patients (%) Receiving Immunosuppression | ||||
|---|---|---|---|---|---|---|---|
| CsA | Cyc | RTX | Glc | Others | |||
| Tertile 1 (low) | 14 | 26 | 19 (73) | 11 (42) | 5 (19) | 20 (77) | 5 (19): 2 Tac, 3 Aza |
| Tertile 2 (medium) | 8 | 31 | 19 (61) | 12 (39) | 5 (16) | 24 (77) | 5 (16): 2 Chloramb, 1 Tac, 1 MMF, 1 Aza |
| Tertile 3 (high) | 3 | 36 | 23 (64) | 17 (47) | 7 (19) | 33 (92) | 4 (11): 3 Tac, 1 MMF |
There were no differences in the immunosuppressants used in patients from the different tertiles. IS, immunosuppressive therapy; CsA, cyclosporin A; Cyc, cyclophosphamide; RTX, rituximab; Glc, glucocorticoids; Tac, tacrolimus; Aza, azathioprine; Chloramb, chlorambucil; MMF, mycophenolate moletil.
Table 6.
Immunosuppressive treatment used in the study: dosage of the used immunosuppressants
| PLA2R-Ab Level | CsA (mg/d) | Cyc (Cumulative; g) | RTX (Cumulative; mg) |
|---|---|---|---|
| Tertile 1 (low) | 207±88 | 13.9±13.0 | 1590±919 |
| Tertile 2 (medium) | 232±77 | 18.8±14.9 | 1506±633 |
| Tertile 3 (high) | 210±69 | 9.4±11.8 | 2313±659 |
CsA is given as the mean dosage per day used in patients of the tertile during the follow-up. Cyc and RTX are given as cumulative dosage per patient during the follow-up.
Discussion
In this study, we prospectively followed 118 patients with biopsy-proven primary MN who were positive for PLA2R-Ab. Our data show that patients with the highest PLA2R-Ab levels reach more frequently and faster a serum creatinine increase by ≥25% and a serum creatinine level of 1.3 mg/dl. Furthermore, the multivariate Cox regression analysis shows that PLA2R-Ab levels together with men and older age are predictors for the increase in serum creatinine. Being a man has been discussed to be a predictor for an unfavorable outcome in patients with MN (16), and in our cohort, older patients had a 15% higher risk to reach the study end point per 5 years of age.
How could high PLA2R-Ab levels affect an increase of serum creatinine in patients with primary MN? Impaired renal function in patients with primary MN has been associated with the degree of interstitial fibrosis in biopsy samples, whereas a multivariate analysis did not show an association of glomerular and interstitial lesions with remission of proteinuria or progression of renal function loss (17,18). We have analyzed both glomerular damage and the degree of tubulointerstitial fibrosis in three patient cohorts. Although most patients had signs of interstitial fibrosis, there was no correlation between the degree of interstitial fibrosis and patients reaching the study end point. Patients with high PLA2R-Ab levels, however, had significantly more moderate interstitial fibrosis compared with the patients in tertile 1. This was not reflected in the serum creatinine levels at the time of inclusion in the study, and therefore, we cannot conclude the role that interstitial fibrosis, in fact, plays in our findings.
In the model by Cattran et al. (6), persistent high levels of proteinuria are predictors for loss of renal function. Although not statistically significant, in patients with the highest PLA2R-Ab levels, proteinuria at inclusion in the study was higher compared with proteinuria in patients from tertiles 1 and 2. To better define whether proteinuria could be a potential mediator of the more pronounced increase of the serum creatinine in patients with high PLA2R-Ab levels, we assessed the PLA2R-Ab levels and proteinuria at either the time of reaching the study end point or the end of follow-up. Patients having the highest PLA2R-Ab levels had higher levels of proteinuria at the end of the observation. Because high PLA2R-Ab levels are associated with a longer time to remission of proteinuria (8), high PLA2R-Ab levels may mediate progression of renal function because of persistence of nephrotic-range proteinuria.
Although immunosuppressive treatments reduce proteinuria and improve long-term renal outcome in some patients with primary MN, there are differences between the medications used for treatment. In a recent controlled prospective study, it was shown that the progressive decline of renal function in patients with primary MN was more pronounced in patients who were treated with CsA compared with patients receiving alkylating agents (19). The effect of the treatment on proteinuria was not different between both protocols. The known nephrotoxic effects of CsA might have played a role in this difference (20). Because 79% of our patients were treated with immunosuppressants, we analyzed whether there were differences in the drugs and the doses applied. We did not find differences in the numbers of patients who were treated with CsA or the cumulative doses. Thus, it is very unlikely that the used immunosuppressive treatment accounts for the greater loss of renal function seen in patients with high PLA2R-Ab levels.
One of the central questions in the management of patients with primary MN is whether immunosuppressive treatment can be guided by PLA2R-Ab levels. In our view, a number of questions have to be answered before reaching this conclusion. First, at what level should the PLA2R-Ab decrease to consider treatment a success? Second, do the antibody levels have to completely disappear from the blood? Third, is the absolute or relative decrease of PLA2R-Ab level (or both) more important? The potential role of PLA2R-Ab levels on renal outcome was addressed in two recent retrospective analyses (11,12), which suggested that high PLA2R-Ab levels might be predictors for worse outcome. However, these studies do not allow us to draw a reliable conclusion on whether PLA2R-Ab levels are, indeed, associated with loss of renal function. For the patients presented in these studies, no eventual risk factors for the development of renal failure, such as histologic findings, treatment protocols, or time-dependent development of disease, were presented.
Patients of tertile 3 in our cohort had significantly higher BPs compared with patients in tertile 1. Additionally, hypertensive damage was more common in the renal biopsies of patients with high PLA2R-Ab levels compared with patients in the tertile with low PLA2R-Ab levels. High BP is a risk factor for loss of renal function in chronic renal disease. High BP is also associated with progressive loss of renal function in patients with primary MN and thus, has to be considered as potential mediator of impairment of renal function (21).
Our study has some limitations. Renal function was measured by serum creatinine and not measured GFR. Because the study was not a randomized, controlled study and the treatment decisions were made by the caring physicians and not per the protocol, our data do not allow for comparison of the different immunosuppressive therapies and their roles in the outcome of renal function.
Independent of the ultimate mechanisms of how PLA2R-Ab levels effect progressive loss of renal function in primary MN, our data show that, in addition to the already described predictors, PLA2R-Ab levels can be included as an early parameter for the assessment of risk for progressive loss of renal function.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Eugen Kinzler, Daniela Bergleiter, and Catharina Verkooyen for their technical assistance.
This work is supported by a fellowship from the Z-Project of the Klinische Forschergruppe 228 (to E.H.), a grant from the Else-Kroener-Fresenius Stiftung (to R.A.K.S.), and Deutsche Forschungsgemeinschaft Grants STA193/9-1 (to R.A.K.S.) and STA193/9-2 (to R.A.K.S.).
The following colleagues participated in the recruitment of the patients for this study: F. Aedtner, L. Arndt, P. Arnold, A. Assenmacher, V. Bajeski, J. Beckermann, M. L. Beckmann, T. Benzing, M. Bieringer, K. Biolik, F. Bozkurt, J. Bramstedt, J. Braun, M. Busch, E. Büssemaker, W. Clasen, E. G. Dannemann, A. Daul, F. Dellanna, O. Dorsch, J. Floege, L. Fricke, B. Friedrich, J. Galle, T. Gerhardt, P. Gerke, J. Gerth, A. Goldmann, S. Grosser, U. Haberstroh, A. Hamadeh, M. Heckel, T. Hermle, B. Hohenstein, W. Holl, M. Hollenbeck, W. Jabs, J. Jacobsen, O. Jung, F. Keller, M. Ketteler, F. Kiziler, R. Kleinecke, A. Klemm, M. Kohnle, B. Kortus-Götze, M. Kube, D. Lange, F. Lange-Hüsken, C. Laube, O. Laue, T. Leingärtner, J. Lepenies, B. Linke, O. Loke, G. Loley, V. Lufft, C. Marx, K. Messtorff, W. Meyer, M. Oppermann, F. Özcan, H. J. Pavenstädt, A. Plöger, F. Reichenberger, R. Reinking, P. Roch, D. Schaumann, P. Schilken, H. Schlee, D. Schmiedel, R. Schneidenbach, J. Schnierda, A. Schnitzler, M. Schulze, H. M. Seuffert, J. Seyfried, J. Siegmund, E. Siwek-Orman, M. Spiess, A. Stahn, R. Storkenmaier, S. Teichler, F. P. Tillmann, K. Toussaint, C. Tripps, S. Weiner, H. Wiedemann, G. Wirtz, T. Wollweber, H. G. Wullstein, and A. Zander. The affiliation for each colleague is provided in the Supplemental Material.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03850414/-/DCSupplemental.
References
- 1.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, Minari M, Scalia A, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, Joshi K, Sakhuja V: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, Rambaldi A, Marasà M, Remuzzi G: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North America Nephrotic Syndrome Study Group : Cyclosporine in patients with steroid-resistant membranous nephropathy: A randomized trial. Kidney Int 59: 1484–1490, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Beck LH, Jr., Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA: Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25: 1357–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck LH, Jr., Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofstra JM, Beck LH, Jr., Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A₂ receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, Hofstra JM, Hoxha E, Stahl RA, Lambeau G, Stöcker W, Schlumberger W: Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421: 213–218, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Ehrenreich T, Churg J: Pathology of membranous nephropathy. Pathol Annu 3: 145–186, 1968 [Google Scholar]
- 16.Cattran DC, Reich HN, Beanlands HJ, Miller JA, Scholey JW, Troyanov S, Genes, Gender and Glomerulonephritis Group : The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant 23: 2247–2253, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bohle A, Wehrmann M, Bogenschütz O, Batz C, Vogl W, Schmitt H, Müller CA, Müller GA: The long-term prognosis of the primary glomerulonephritides. A morphological and clinical analysis of 1747 cases. Pathol Res Pract 188: 908–924, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Sprangers B, Bomback AS, Cohen SD, Radhakrishnan J, Valeri A, Markowitz GS, D’Agati V, Appel GB: Idiopathic membranous nephropathy: Clinical and histologic prognostic features and treatment patterns over time at a tertiary referral center. Am J Nephrol 36: 78–89, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, Gaskin GJ, Jayne DR, O’Donoghue D, Boulton-Jones M, Mathieson PW: Immunosuppression for progressive membranous nephropathy: A UK randomised controlled trial. Lancet 381: 744–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naesens M, Kuypers DR, Sarwal M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Glassock RJ: Diagnosis and natural course of membranous nephropathy. Semin Nephrol 23: 324–332, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.