Abstract
The thick ascending limb occupies a central anatomic and functional position in human renal physiology, with critical roles in the defense of the extracellular fluid volume, the urinary concentrating mechanism, calcium and magnesium homeostasis, bicarbonate and ammonium homeostasis, and urinary protein composition. The last decade has witnessed tremendous progress in the understanding of the molecular physiology and pathophysiology of this nephron segment. These advances are the subject of this review, with emphasis on particularly recent developments.
Keywords: Na transport, calcium receptor, water transport, acidosis, renal physiology
Introduction
The thick ascending limb (TAL) occupies a central anatomic and functional position in human renal physiology, with critical roles in the defense of the extracellular fluid volume, the urinary concentrating mechanism, calcium and magnesium homeostasis, bicarbonate and ammonium homeostasis, and urinary protein composition (1). The last decade has witnessed tremendous progress in the understanding of the molecular physiology and pathophysiology of this nephron segment. These advances are the subject of this review, with emphasis on particularly recent developments.
Anatomy and Morphology
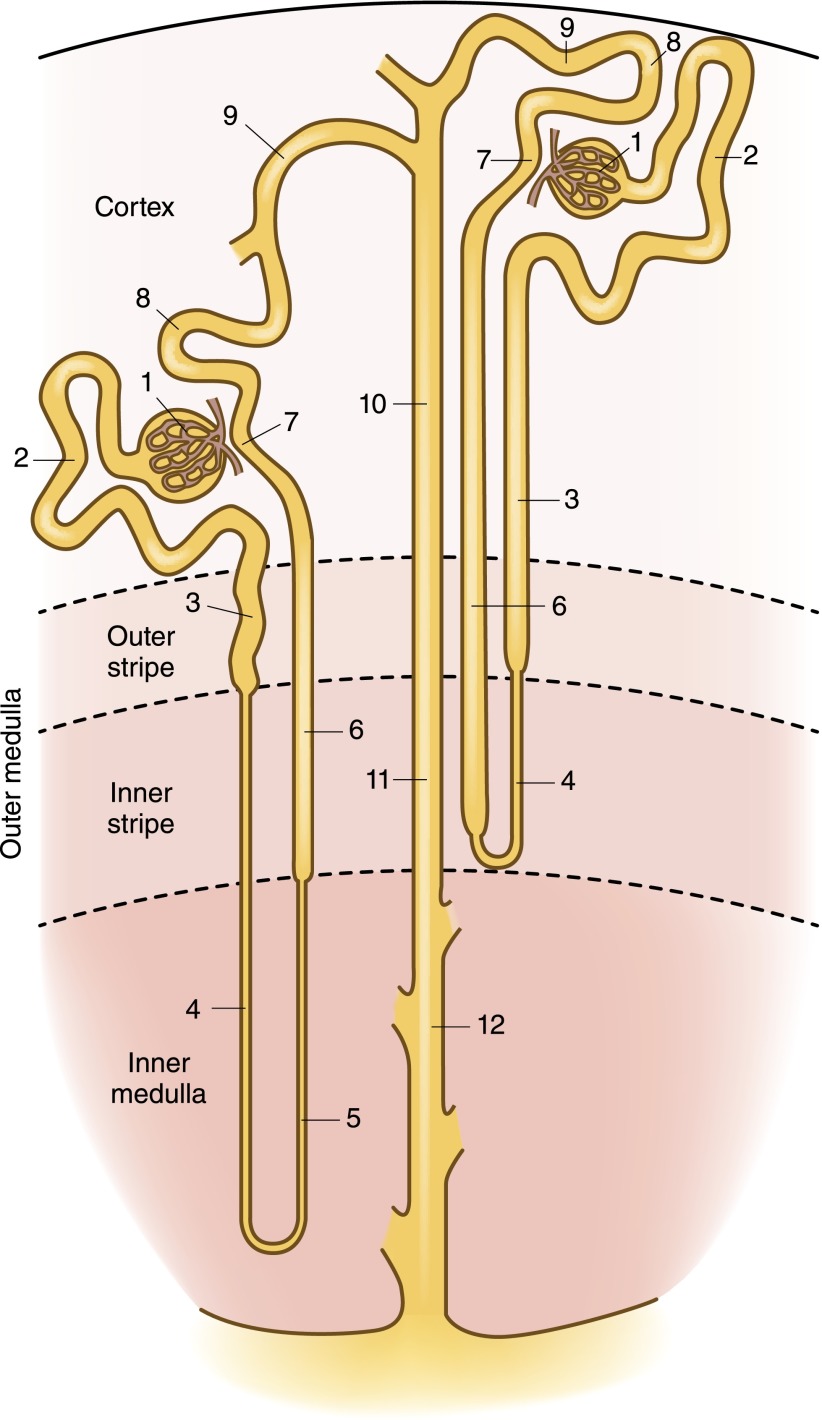
The loop of Henle encompasses the thin descending limb, the thin ascending limb, and the TAL. Short-looped nephrons that originate from superficial and midcortical nephrons have a short descending limb within the inner stripe of the outer medulla; close to the hairpin turn of the loop, these tubules merge into the TAL (see Figure 1). By contrast, long-looped nephrons originating from juxtamedullary glomeruli have a long ascending thin limb. The TALs of long-looped nephrons begin at the boundary between the inner and outer medulla, whereas the TALs of short-looped nephrons may be entirely cortical. The ratio of medullary to cortical TAL for a given nephron is a function of the depth of its origin such that superficial nephrons primarily contain cortical TALs, whereas juxtamedullary nephrons primarily contain medullary TALs.
Figure 1.
Organization of the nephron, showing both short-looped and long-looped nephrons. See text for details relevant to the thick ascending limb (TAL). Within the cortex, a medullary ray is delineated by a dashed line. Structures are noted as follows: 1, glomerulus; 2, proximal convoluted tubule; 3, proximal straight tubule; 4, descending thin limb; 5, ascending thin limb; 6, TAL; 7, macula densa; 8, distal convoluted tubule; 9, connecting tubule; 10, cortical collecting duct; 11, outer medullary collecting duct; 12, inner medullary collecting duct.
Aquaporin-1 expression is a marker of descending thin limbs and has been utilized to define the anatomy of the loops of Henle (2). The TAL begins abruptly after the thin ascending limb of long-looped nephrons and after an aquaporin-1–negative segment of short-limbed nephrons, immediately following the aquaporin-1–positive thin descending limb (2). The TAL then meets its parent glomerulus at the vascular pole; the plaque of renal tubular cells at this junction constitutes the macula densa, cells that share transport characteristics with adjacent TAL cells. The distal convoluted tubule (DCT) begins at a variable distance after the macula densa, with an abrupt transition between cortical TAL cells expressing the Na+-K+-2Cl− cotransporter (NKCC2; see Figure 2 and below) and DCT cells that express the thiazide-sensitive Na+-Cl− cotransporter (NCC).
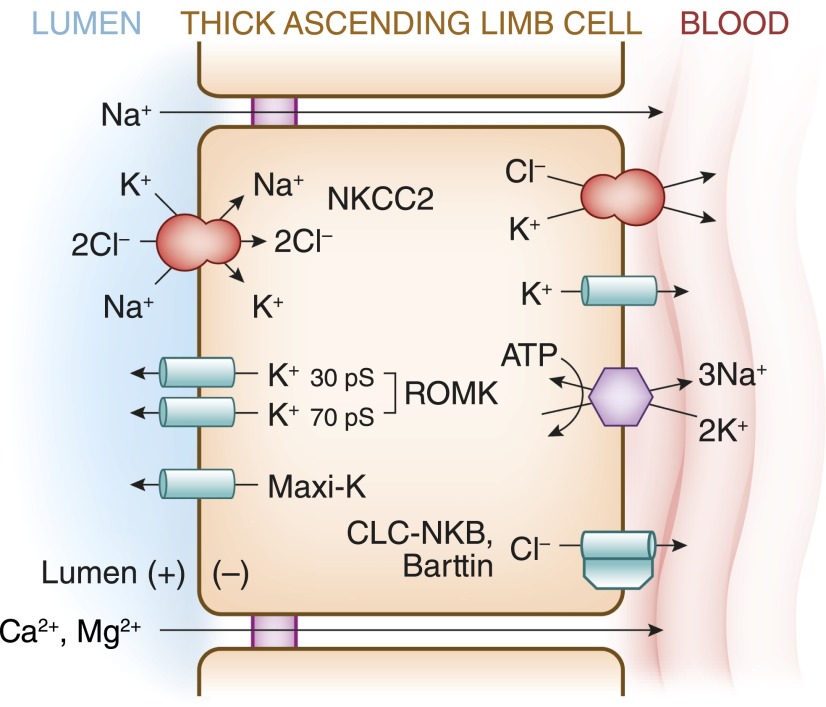
Figure 2.
Transepithelial Na+-Cl− transport pathways in the TAL. See text for details. Barttin, Cl− channel subunit; CLC-NKB, human Cl− channel; KCC4, K+-Cl− cotransporter-4; Maxi-K, calcium-activated maxi K+ channel (also known as the BK channel); NKCC2, Na+-K+-2Cl− cotransporter-2; ROMK, renal outer medullary K+ channel.
The TAL contains two morphologic subtypes: a rough-surfaced cell type (R cells) with prominent apical microvilli and a smooth-surfaced cell type (S cells) with an abundance of subapical vesicles (3,4) (see Figure 3). In the hamster TAL, cells can also be separated into those with high apical and low basolateral K+ conductance and a weak basolateral Cl− conductance (LBC cells), versus a second population with low apical and high basolateral K+ conductance, with high basolateral Cl− conductance (HBC cells) (3,5). The relative frequency of the morphologic and functional subtypes in the cortical and medullary TAL suggests that HBC cells correspond to S cells and LBC cells to R cells (3). Molecular characterization of this heterogeneity is still rudimentary; however, R and S cells clearly differ in the expression pattern of EGF (6) and NKCC2 (4). The functional correlates of this heterogeneity are discussed in the following sections.
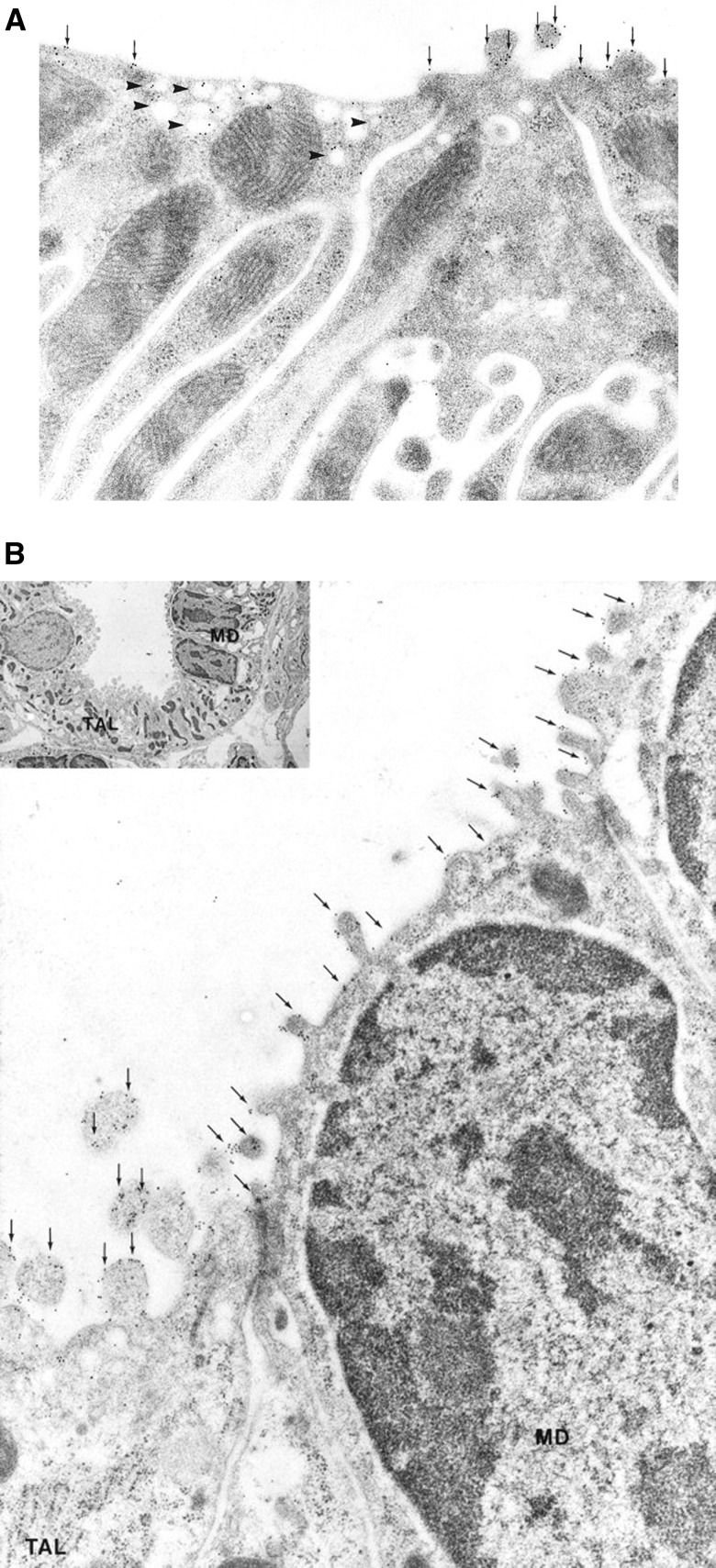
Figure 3.
Ultrastructural localization of NKCC2 protein in the TAL and macula densa (MD). (A) Immunoelectron microscopy of NKCC2 in the TAL. NKCC2 labeling is associated with apical plasma membrane (arrows) and small intracellular vesicles (arrowheads) of TAL cells. Both smooth-surfaced cells (left) and rough-surfaced cells (right) are labeled, with greater labeling of intracellular vesicles in smooth-surfaced cells. (B) Immunogold localization of NKCC2 in ions of MD. Abundant NKCC2 labeling is associated with apical plasma membrane (arrows) of MD cells and TAL cells. Inset: overview showing MD cells and TAL cells. Regions indicated by MD are shown at higher power in main panel from adjacent section. Original magnification, ×3500 in B; ×44,000 in B inset. Reprinted from reference 4, with permission.
Apical Transport of Na+, K+, and Cl−
The TAL reabsorbs approximately 30% of filtered Na+-Cl−, with a steady drop in the luminal Na+-Cl− concentration from approximately 140 mM in the inner stripe of the outer medulla to 30–60 mM at the macula densa (7). In addition to maintenance of the extracellular fluid volume and defense of arterial perfusion, Na+-Cl− absorption by the TAL plays a pivotal role in the urinary concentrating mechanism. Specifically, active Na+-Cl− absorption by the water-impermeable TAL dilutes the luminal fluid and drives the countercurrent multiplication that generates the axial osmolality gradient in the outer medulla, required for the vasopressin-dependent absorption of water by the collecting duct.
The cells of the medullary TAL, cortical TAL, and macula densa share the same basic transport mechanisms (see Figure 2). Na+, K+, and Cl− are cotransported across the apical membrane by NKCC2, an electroneutral Na+-K+-2Cl− cotransporter that is exquisitely sensitive to furosemide, a “loop” diuretic known for 4 decades to inhibit transepithelial Cl− transport by the TAL (8). This transporter generally requires the simultaneous presence of all three ions such that the transport of Na+ and Cl− across the epithelium is mutually codependent and dependent on the luminal presence of K+ (9). Functional expression of NKCC2 in Xenopus laevis oocytes yields Cl−- and Na+-dependent uptake of 86Rb+ (a radioactive substitute for K+) and Cl−- and K+-dependent uptake of 22Na+ (9–11), sensitive to micromolar concentrations of furosemide, bumetanide, and other loop diuretics (9).
NKCC2 is expressed along the entire TAL, in both R and S cells (4) (see Figure 3). NKCC2 expression in subapical vesicles is particularly prominent in smooth cells (4), consistent with the evolving understanding of vesicular trafficking in the regulation of NKCC2 (7). NKCC2 is also expressed in macula densa cells (4) (Figure 1), which are known to demonstrate apical Na+-K+-2Cl− cotransport activity (12). Luminal loop diuretics applied at the macula densa block both tubuloglomerular feedback (13) and the suppression of renin release by luminal Cl− (14), indicating that NKCC2 in the macula densa functions as the tubular sensor for both processes. The ability of loop diuretics to block tubuloglomerular feedback has been linked to their greater renal functional tolerance versus thiazides in advanced CKD (15). In contrast with the inhibitory effect of loop diuretics, volume depletion induced by thiazides augments the tubuloglomerular feedback response (16), causing a sharp drop in GFR in patients with CKD (15).
Alternative splicing of exon 4 of solute carrier family 12, member 1 (SLC12A1, the gene encoding NKCC2) yields NKCC2 proteins that differ in primary sequence within transmembrane domain 2 and the adjacent intracellular loop. There are thus three different variants of exon 4, denoted A, B, and F; the variable inclusion of these cassette exons yields distinct NKCC2-A, NKCC2-B, and NKCC2-F isoforms (9,11). Kinetic transporter characterization reveals that these isoforms differ dramatically in ion affinities (9,11). In particular, NKCC2-F has a very low affinity for Cl− (Km of 113 mM) and NKCC2-B has a very high affinity (Km of 8.9 mM); NKCC2-A has an intermediate Cl− affinity (Km of 44.7 mM) (11). These isoforms differ in axial distribution along the tubule, with the F cassette expressed in the inner stripe of the outer medulla, the A cassette in the outer stripe, and the B cassette in cortical TAL (17). There is thus an axial distribution of the anion affinity of NKCC2 along the TAL, from a low-affinity, high-capacity transporter (NKCC2-F) in the inner stripe of the outer medulla to a high-affinity, low-capacity transporter (NKCC2-B) in the cortical TAL. This arrangement fits with the need for a progressive increase in the Cl− affinity of the transporter as the luminal Cl− concentration drops along the length of the TAL.
Microperfused TALs develop a lumen-positive potential difference when the tubular solutions contain Na+-Cl− (18). This lumen-positive potential difference plays a critical role in physiology of the TAL, driving the paracellular transport of Na+, Ca2+, and Mg2+ (see Figure 2). The conductivity of the apical membrane of TAL cells is predominantly K+ selective; luminal recycling of K+ via Na+-K+-2Cl− cotransport and apical K+ channels, along with basolateral depolarization due to Cl− exit through Cl− channels, generates the lumen-positive transepithelial potential difference (19,20).
Apical K+ channels are critical for transepithelial Na+-Cl− transport by the TAL. In microperfusion studies, the combined removal of K+ from luminal perfusate and pharmacological blockade of apical K+ channels results in a marked decrease in Na+-Cl− reabsorption (21). Apical K+ channels are thus required for sustained functioning of NKCC2; the low luminal concentration of K+ in this nephron segment would otherwise become limiting for transepithelial Na+-Cl− transport. The net transport of K+ across perfused TAL epithelium is <10% that of Na+ and Cl− (22); approximately 90% of the K+ transported by NKCC2 is recycled across the apical membrane via K+ channels, resulting in minimal net K+ absorption by the TAL (20).
Three subtypes of apical K+ channels have been identified in the TAL, with differing unitary conductance characteristics: a 30-picosiemen (pS) channel, a 70-pS channel, and a high-conductance, calcium-activated maxi K+ channel (also known as the big K+ or BK channel) (23–25) (see Figure 2). The 70-pS channel mediates approximately 80% of the apical K+ conductance of TAL cells (26). The low-conductance 30-pS channel shares several biophysical and regulatory characteristics with the cloned renal outer medullary K+ channel (ROMK; otherwise known as KIR1.1 or KCNJ1), the cardinal inward-rectifying K+ channel that was initially cloned from rat renal outer medulla (27). ROMK protein has been identified at the apical membrane of medullary TAL, cortical TAL, and macula densa (28). The 30-pS channel is completely absent from the apical membrane of mice with homozygous deletion of the gene encoding ROMK (29), providing genetic evidence that ROMK mediates this 30-pS conductance. Notably, not all cells in the TAL are labeled with ROMK antibody (28), suggesting that ROMK might be absent in the HBC cells with high basolateral Cl− conductance and low apical/high basolateral K+ conductance (also see above) (3,5). HBC cells are thought to correspond to the smooth-surfaced morphologic subtype of TAL cells (S cells) (3); however, the relative expression of ROMK protein by immunoelectron microscopy in R and S cells has not yet been published. Regardless, the heterogeneity of ROMK expression indicates that apical K+ recycling is not present in all epithelial cells within the TAL.
ROMK plays a critical role in Na+-Cl− absorption by the TAL, given that loss-of-function mutations in the gene encoding this channel are associated with Bartter’s syndrome (30) (see Table 1). This genetic phenotype was initially discordant with the data suggesting that the higher-conductance 70-pS K+ channel is the dominant channel at the apical membrane of TAL cells (26). This paradox was resolved by the observation that the 70-pS channel is also absent from the TAL of ROMK knockout mice, indicating that ROMK proteins form a subunit of the 70-pS channel (31). ROMK activity in the TAL is clearly modulated by associations with other proteins such that coassociation with other subunits to generate the 70-pS channel is perfectly compatible with the known physiology of this protein. ROMK thus associates with scaffolding proteins Na+/H+ exchanger regulatory factor (NHERF)-1 and NHERF-2, via the C-terminal PDZ binding motif of ROMK; NHERF-2 is coexpressed with ROMK in the TAL (32). The association of ROMK with NHERFs serves to bring ROMK into closer proximity to the cystic fibrosis transmembrane regulator protein (CFTR) (32). This ROMK-CFTR interaction is in turn required for the native ATP and glybenclamide sensitivity of apical K+ channels in the TAL (33). Impaired CFTR-dependent regulation of ROMK in the TAL may potentially explain the propensity for hypochloremic alkalosis and “pseudo-Bartter’s syndrome” in patients with cystic fibrosis (33,34).
Table 1.
Genetic classification of Bartter’s syndrome
| Subtype | Protein | Function | Phenotype | Comments/Variants |
|---|---|---|---|---|
| I | NKCC2 | Na-K-2Cl cotransporter | Antenatal BS | Variant presentations (e.g., acidosis, normokalemia) |
| II | ROMK | K+ channel | Antenatal BS | Transient neonatal hyperkalemia |
| III | CLC-NKB | Cl− channel | Classic BS | No nephrocalcinosis |
| Gitelman’s syndrome in some patients | ||||
| Deafness with CLC-NKA loss | ||||
| IV | Barttin | Cl− channel subunit | Antenatal BS with deafness | No nephrocalcinosis |
| Hypomagnesemia | ||||
| Renal failure | ||||
| V | CaSR | Calcium receptor | BS-like | Hypocalcemia, autosomal dominant |
See the text for details. NKCC2, Na+-K+-2Cl− cotransporter-2; BS, Bartter’s syndrome; ROMK, renal outer medullary K+ channel; CLC-NKB, human Cl− channel; Barttin, Cl− channel subunit; CaSR, calcium-sensing receptor.
Basolateral Transport
The basolateral Na+/K+-ATPase is the primary exit pathway for Na+ at the basolateral membrane of TAL cells. The Na+ gradient generated by this Na+/K+-ATPase activity also drives the apical entry of Na+, K+, and Cl− via NKCC2, the furosemide-sensitive Na+-K+-2Cl− cotransporter (20). Inhibition of Na+/K+-ATPase with ouabain thus collapses the lumen-positive potential difference and abolishes transepithelial Na+-Cl− transport in the TAL (35). The basolateral exit of Cl− from TAL cells is primarily but not exclusively (36) electrogenic, mediated primarily by Cl− channels (19,20). Intracellular Cl− activity during transepithelial Na+-Cl− transport is above its electrochemical equilibrium (37), with an intracellular-negative voltage of −40 to −70 mV that drives basolateral Cl− exit (19,20). Reductions in basolateral Cl− depolarize the basolateral membrane, whereas increases in intracellular Cl− induced by luminal furosemide have a hyperpolarizing effect (37).
At least two CLC chloride channels, CLC-K1 and CLC-K2 (denoted CLC-NKA and CLC-NKB in humans), are coexpressed in the TAL (38). Several lines of evidence indicate that the dominant Cl− channel in the TAL is encoded by CLC-K2/CLC-NKB. First, CLC-K1 is expressed at both apical and basolateral membranes of the thin ascending limb and the phenotype of CLC-K1 knockout mice is more consistent with primary dysfunction of thin ascending limbs (39), rather than the TAL. CLC-K2 protein, in turn, is heavily expressed at the basolateral membrane of the TAL, with additional expression in the DCT, connecting tubule, and α-intercalated cells (40). Second, loss-of-function mutations in CLC-NKB are associated with Bartter’s syndrome (41), genetic evidence for a dominant role of this channel in Na+-Cl− transport by the human TAL. Finally, an in vivo study using whole-cell recording techniques suggests that CLC-K2 is the dominant Cl− channel in TAL (42).
A key advance in the physiology of the TAL was the characterization of the “Barttin” subunit of CLC-K channels, which is coexpressed with CLC-K1 and CLC-K2 in several nephron segments, including the TAL (38). The human CLC-NKA and CLC-NKB paralogs are not functional in the absence of Barttin coexpression (43); hence, the full functional characterization of these channels depended on the discovery of Barttin. CLC-NKB coexpressed with Barttin is highly selective for Cl−, with a permeability series of Cl− >> Br− = NO3− > I− (38,43). Strikingly, despite the considerable homology between the CLC-NKA/NKB proteins, these channels differ considerably in pharmacologic sensitivity to various Cl− channel blockers (44). This pharmacologic divergence suggests that the possibility that novel inhibitors specific for CLC-NKB could eventually be developed; such inhibitors would be expected to function as novel loop diuretics that would not require tubular excretion for natriuretic effects, acting instead at the basolateral membrane.
Electroneutral K+-Cl− cotransport (see Figure 2) mediates K+-dependent Cl− exit at the TAL basolateral membrane (37). The K+-Cl− cotransporter KCC4 is expressed at the basolateral membrane of medullary and cortical TAL, in addition to macula densa. To account for the effects on transmembrane potential difference of basolateral barium and/or increased K+, it was previously suggested that the basolateral membrane of the TAL contains a barium-sensitive K+-Cl− transporter (37,45); this is consistent with the barium sensitivity of KCC4 (46). Increases in basolateral K+ cause Cl−-dependent cell swelling in the Amphiuma early distal tubule, an analog of the mammalian TAL. In Amphiuma LBC cells with low basolateral conductance, analogous to mammalian LBC cells (3,5), this cell swelling was not accompanied by changes in basolateral membrane voltage or resistance (47), consistent with electroneutral K+-Cl− transport. By extension, KCC4 may play an important role in the basolateral chloride transport of LBC cells.
Epithelial cells within the TAL are influenced by changes in interstitial osmolality, swelling under hypotonic conditions, and shrinking under hypertonic conditions. Notably, however, the apical membrane of the TAL is completely water impermeable and TAL segments have an extremely low transepithelial water permeability. The basolateral membrane, however, expresses abundant Aquaporin-1 water channel protein, allowing for water flux across the basolateral membrane and changes in cell volume in response to changes in interstitial osmolality (48).
Paracellular Transport
The transport stoichiometry of NKCC2 (1Na+/1K+/2Cl−) is such that additional transport mechanisms are necessary to balance the transport of Na+ with the exit of double the amount of Cl− at the basolateral membrane; this additional Na+ is transported across the epithelium via the paracellular pathway (49,50) (see Figure 2). The ratio of net Cl− transepithelial absorption to net Na+ absorption through the paracellular pathway is thus 2.4±0.3 in microperfused mouse medullary TAL segments (50), close to the ratio of 2.0 expected if 50% of Na+ transport occurs via the paracellular pathway. The combination of a cation-permeable paracellular pathway and an “active transport” lumen-positive potential difference (19), generated indirectly by the basolateral Na+/K+-ATPase (35), results in a doubling of active Na+-Cl− transport for a given level of oxygen consumption (49).
Tight junctions in the TAL are cation selective, with relative permeability of Na+ to that of Cl− (PNa/PCl) of 2–5 (19,50). The reported transepithelial resistance in the TAL is between 10 and 50 Ω cm2; although this resistance is higher than that of the proximal tubule, the TAL is not considered a “tight” epithelium. Notably, however, water permeability of the TAL is extremely low, <1% that of the proximal tubule (19). These “hybrid” characteristics—relatively low resistance and very low water permeability—allow the TAL to generate and sustain Na+-Cl− gradients of up to 120 mM (19).
The tight junctions of epithelia function as charge- and size-selective “paracellular channels,” physiologic characteristics that are conferred by integral membrane proteins that cluster together at the tight junction; changes in the expression of these proteins can have marked effects on permeability, without affecting the number of junctional strands (51). In particular, the charge and size selectivity of tight junctions is conferred in large part by the claudins, a large (>20) gene family of tetraspan transmembrane proteins. Mouse TAL cells coexpress claudin-3, claudin-10, claudin-11, claudin-14, claudin-16, and claudin-19 (52–54). Notably, the expression of claudin-19 in TAL cells is heterogeneous (53), analogous perhaps to the heterogeneity of ROMK expression (see above).
The TAL reabsorbs approximately 50%–60% of filtered magnesium and approximately 20% of filtered calcium, exclusively via the paracellular pathway. Mutations in human claudin-16 (paracellin-1) and claudin-19 (52) are associated with hereditary hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), genetic evidence that these claudins are critical for the cation selectivity of TAL tight junctions. Heterologous expression of claudin-16 (paracellin-1) in the anion-selective LLC-PK1 cell line increases Na+ permeability, without affecting Cl− permeability (55). Claudin-19 in turn reduces PCl in LLC-PK1 cells, without affecting cation permeability (56). The claudin-16 and claudin-19 proteins physically interact (56,57) and coexpression of claudin-16 and claudin-19 synergistically increases the PNa/PCl ratio in LLC-PK1 cells (56). Knockdown of claudin-16 in transgenic mice increases Na+ absorption in the downstream collecting duct, with development of hypovolemic hyponatremia after treatment with amiloride (58). Claudin-19 knockdown mice exhibit an increase in fractional excretion of Na+ and a doubling in serum aldosterone (57). Both strains exhibit hypermagnesuria and hypercalciuria, with hypomagnesemia, replicating the human FHHNC phenotype. In summary, claudin-16 and claudin-19 are critical for the cation selectivity of tight junctions in the TAL, contributing significantly to the transepithelial absorption of Na+, Ca2+, and Mg2+ in this nephron segment.
Other claudins expressed in the TAL either modulate the function of claudin-16/claudin-19 heterodimers or have independent effects on paracellular transport. Claudin-14 interacts with claudin-16, disrupting cation selectivity of the paracellular barrier in cells that also coexpress claudin-19 (59). Claudin-14 expression in the TAL is calcium dependent, via the calcium-sensing receptor (CaSR), providing a novel axis for calcium-dependent regulation of paracellular calcium transport (see below) (59–61). Claudin-10 in turn appears to specifically modulate paracellular Na+ permeability, with impaired paracellular Na+ transport but enhanced paracellular Ca2+ and Mg2+ in claudin-10 knockout mice (54).
Transport of NH4+ and HCO3−
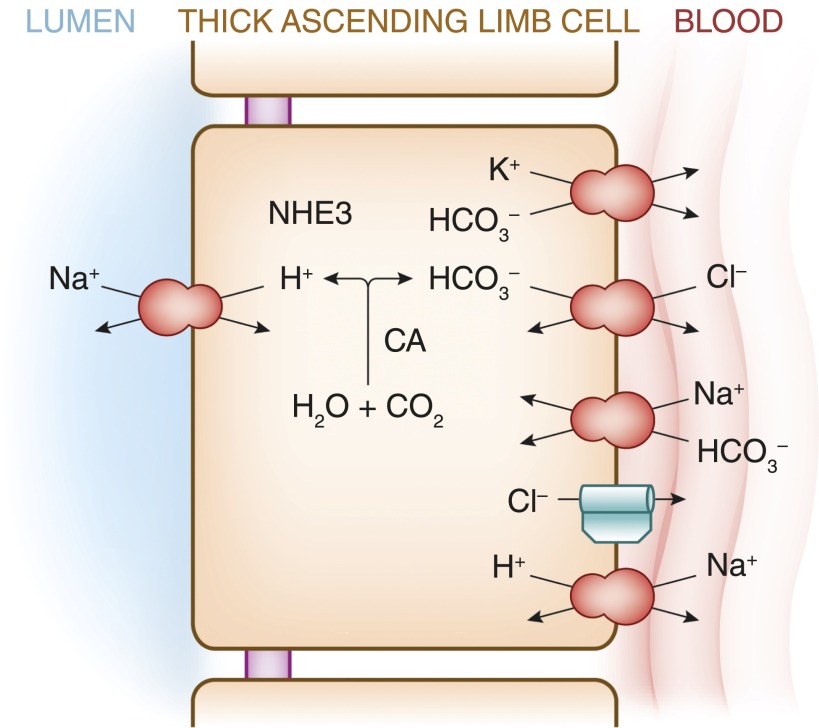
The TAL also plays an important role in acid-base physiology, functioning in both renal bicarbonate reabsorption and ammonium (NH4+) excretion. Approximately 15% of filtered bicarbonate is reabsorbed by the TAL. Apical, carbonic anhydrase-dependent (62) bicarbonate reabsorption is accomplished by Na+/H+ exchange, primarily mediated by the Na+/H+ exchanger NHE3 (63) (see Figure 4). The apical exit of an H+ ion is accompanied by basolateral exit of HCO3− (i.e., bicarbonate absorption). There are several basolateral exit mechanisms for bicarbonate in the TAL (64), including Cl−/HCO3− exchange, K+-HCO3− cotransport (likely mediated by the K+-Cl− cotransporter KCC4), and Na+/H+ exchange. A basolateral Na+-HCO3− cotransporter (NBCn1) is heavily expressed in the TAL but is thought to primarily function in bicarbonate entry at the basolateral membrane, rather than exit (65). Bicarbonate reabsorption by the TAL is regulated by acid-base status, and is upregulated in acidosis and downregulated in metabolic alkalosis (66).
Figure 4.
Bicarbonate transport pathways within the TAL. See text for details. NHE3, Na+/H+ exchanger-3; CA, carbonic anhydrase.
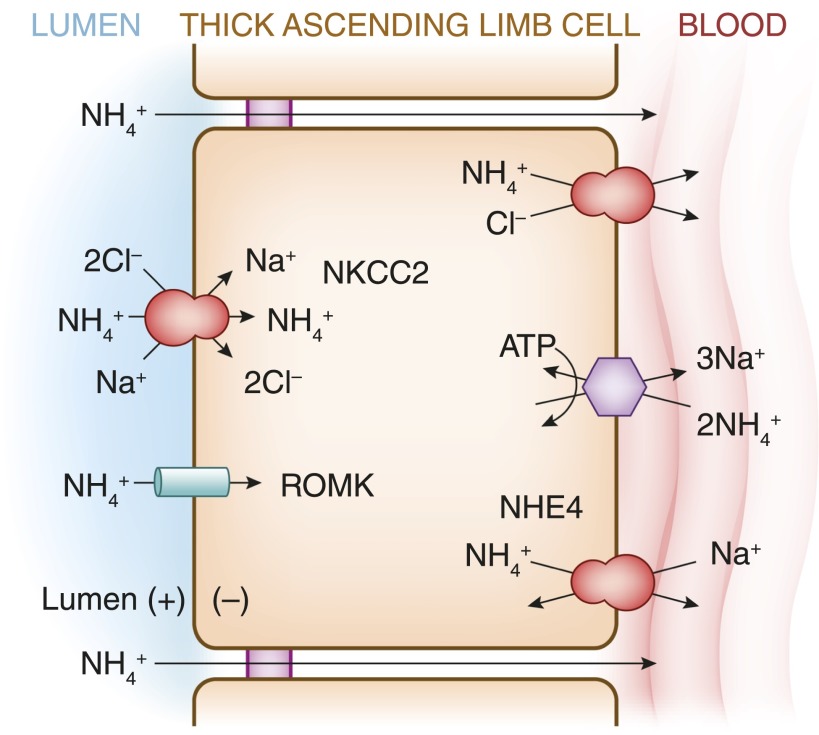
Ammonium is generated by the proximal tubule in response to metabolic acidosis, reabsorbed by the TAL (67), and is concentrated by countercurrent multiplication within the medullary interstitium (67,68), from whence it is transported down its concentration gradient via apical NH3 carriers in the collecting duct (69). The NH4+ ion has the same ionic radius as K+ and can be transported by NKCC2 (70) and other K+ transporters (see Figure 5). NH4+ transport via apical K+ channels and paracellular transport play lesser roles under physiologic conditions (67). NH4+ exits the TAL predominantly via the basolateral Na+/H+ exchanger NHE4, functioning in Na+/NH4+ exchange mode (71). The capacity of the TAL to reabsorb NH4+ and, as a result, the corticomedullary NH4+ gradient is increased during acidosis (67,68), due in part to an induction of NKCC2 (70) and NHE4 (71). Dysfunction or inhibition of the TAL, as in Bartter’s syndrome or loop diuretic administration for example, typically causes metabolic alkalosis, somewhat obscuring the role of the TAL in acid and NH4+ homeostasis. Notably, however, pediatric patients with Bartter’s syndrome due to NKCC2 deficiency can initially present with metabolic acidosis (72), perhaps because of a defect in medullary NH4+ accumulation.
Figure 5.
Ammonium transport pathways within the TAL. See text for details. NH4+, ammonium; NHE4, Na+/H+ exchanger-4.
Increasing the luminal K+ concentration in perfused TAL markedly inhibits active NH4+ absorption, likely because of competition between K+ and NH4+ for transport via NKCC2 (67). Hyperkalemia thus induces acidosis in rats by reducing NH4+ accumulation by the TAL, collapsing the NH4+ gradient between the vasa recta (surrogate for interstitial fluid) and collecting duct (73). Clinically, patients with hyperkalemic acidosis due to hyporeninemic hypoaldosteronism can demonstrate an increase in urinary NH4+ excretion in response to normalization of plasma K+ with cation-exchange resins (74), indicating a significant role for hyperkalemia in generation of the acidosis. This physiology may gain broader relevance if and when novel potassium binders (75) become clinically available for chronic management of hyperkalemia.
Regulation of Ion Transport in the TAL
Activating Influences
Transepithelial Na+-Cl− transport by the TAL is regulated by multiple competing neurohumoral influences. In particular, increases in intracellular cAMP tonically stimulate ion transport in the TAL; the list of stimulatory hormones and mediators that increase cAMP in this nephron segment includes vasopressin, parathyroid hormone (PTH), glucagon, calcitonin, and β-adrenergic activation. These overlapping cAMP-dependent stimuli are thought to result in maximal baseline stimulation of transepithelial Na+-Cl− transport (76). This baseline activation is in turn modulated by a number of negative influences, most prominently prostaglandin E2 (PGE2) and extracellular Ca2+. Other hormones and autocoids working through cGMP-dependent signaling, including nitric oxide, also have potent negative effects on Na+-Cl− transport within the TAL (7). By contrast, angiotensin II has a stimulatory effect on Na+-Cl− transport within the TAL (77,78).
Vasopressin, acting through V2 receptors (79), is the most extensively studied positive modulator of transepithelial Na+-Cl− transport in the TAL. Vasopressin activates apical Na+-K+-2Cl− cotransport within minutes in perfused mouse TAL segments, and also exerts longer-term influence on NKCC2 expression and function. The acute activation of apical Na+-K+-2Cl− cotransport is achieved at least in part by the stimulated exocytosis of NKCC2 proteins, from subapical vesicles to the plasma membrane (7). Activation of NKCC2 is also associated with the phosphorylation of a cluster of N-terminal threonines in the transporter protein; treatment of rats with the V2 agonist desmopressin (dDAVP; Sanofi-Aventis) induces phosphorylation of these residues in vivo, as measured with a phospho-specific antibody (80). These threonine residues are substrates for the homologous STE20/SPS1-related proline/alanine-rich kinase (SPAK) and oxidative stress–responsive kinase 1 (OSR1) kinases, initially identified by Gagnon et al. as key regulatory kinases for NKCC1 and other cation-chloride cotransporters (81). SPAK and OSR1, in turn, are activated by upstream WNK (with no lysine [K]) kinases.
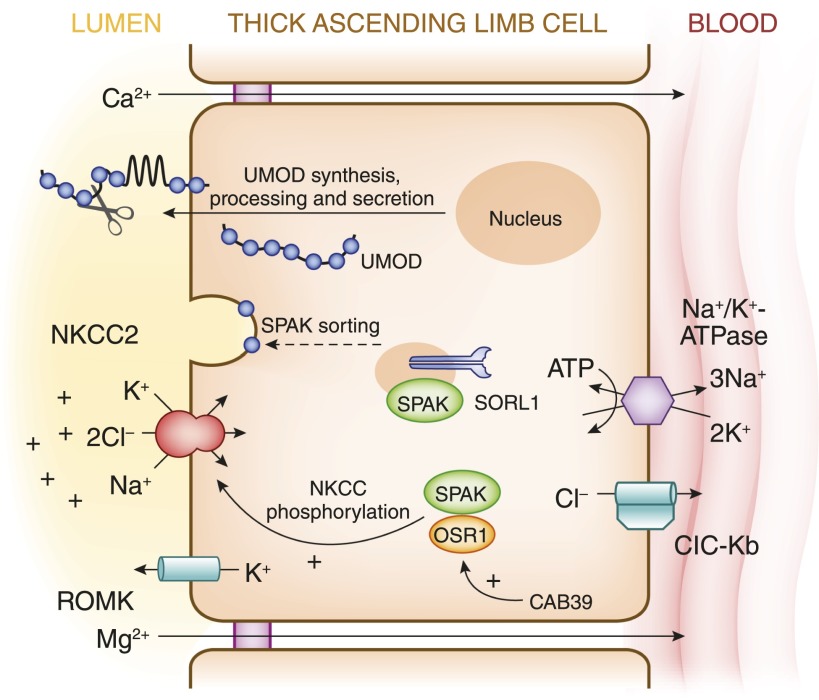
The N-terminal phosphorylation of NKCC2 by OSR1 kinase appears to be critical for activity of the transporter in the native TAL. The N terminus of NKCC2 contains a predicted binding site for SPAK and OSR1 (82), proximal to the sites of regulatory phosphorylation; the analogous binding site is required for activation of the NKCC1 cotransporter (83). SPAK and OSR1 also require the sorting protein-related receptor with A-type repeats 1 (SORL1) (see also Figure 6) for proper trafficking within TAL cells such that targeted deletion of SORL1 results marked reduction in N-terminal NKCC2 phosphorylation (84). Of the two kinases, OSR1 is evidently more critical for NKCC2 function in the TAL, given the loss of function of the TAL with reduced N-terminal NKCC2 phosphoprotein in mice with targeted TAL-specific deletion of OSR1 (85).
Figure 6.
Model for the interaction of ROMK, NKCC2, SORL1, SPAK/OSR1, and CAB39 with uromodulin in the regulation of ion transport in the TAL. CAB39, calcium-binding protein 39; OSR1, oxidative stress–responsive kinase 1; SORL1, sorting protein-related receptor with A-type repeats 1; SPAK, STE20/SPS1-related proline/alanine-rich kinase; Modified from reference 104, with permission.
The role of the upstream WNK kinases is illustrated by the phenotype of a “knock-in” mouse strain in which mutant SPAK or OSR1 cannot be activated by upstream WNK kinases (86); these mice have a marked reduction in N-terminal phosphorylation of both NKCC2 and the thiazide-sensitive NCC, with associated salt-sensitive hypotension. The upstream WNK kinases appear to regulate SPAK/OSR1 and NKCC2 in chloride-dependent fashion, phosphorylating and activating SPAK/OSR1 and the transporter in response to a reduction in intracellular chloride concentration (87). However, the adaptor protein calcium-binding protein 39 can dimerize and activate SPAK or OSR1 kinase monomers, bypassing the upstream phosphorylation by WNK kinases (88) (see also Figure 6).
Vasopressin has also been shown to alter the stoichiometry of furosemide-sensitive apical Cl− transport in the TAL, from a K+-independent Na+-Cl− mode to the classic Na+-K+-2Cl− cotransport stoichiometry (49). Underscoring the metabolic advantages of paracellular Na+ transport, which is critically dependent on the apical entry of K+ via Na+-K+-2Cl− cotransport (see above), vasopressin accomplishes a doubling of transepithelial Na+-Cl− transport without affecting transcellular Na+-Cl− transport. This doubling in transepithelial absorption occurs without an increase in O2 consumption (49), highlighting the energy efficiency of ion transport by the TAL. The mechanism of this switch remains unknown. However, vasopressin induces cAMP-dependent phosphorylation of several serines and threonines in NKCC2 (89), potentially modulating K+ dependence of the transporter.
In addition to its acute effects on NKCC2, vasopressin increases transepithelial Na+-Cl− transport by activating apical K+ channels and basolateral Cl− channels in the TAL (76,90). Vasopressin also has considerable long-term effects on transepithelial Na+-Cl− transport by the TAL. Sustained increases in circulating vasopressin result in marked hypertrophy of medullary TAL cells, accompanied by a doubling in baseline active Na+-Cl− transport (90). Water restriction or treatment with dDAVP also results in an increase in abundance of the NKCC2 protein in rat TAL cells. Consistent with a direct effect of vasopressin-dependent signaling, expression of NKCC2 is reduced in mice with a heterozygous deletion of the Gs stimulatory G protein, through which the V2 receptor activates cAMP generation (90).
Inhibitory Influences
The tonic stimulation of transepithelial Na+-Cl− transport by cAMP-generating hormones is modulated by a number of negative neurohumoral influences. In particular, extracellular Ca2+ and PGE2 exert potent inhibitory effects, through a plethora of synergistic mechanisms. Both extracellular Ca2+ and PGE2 activate the Gi inhibitory G protein in TAL cells, opposing the stimulatory, Gs-dependent effects of vasopressin on intracellular levels of cAMP (91). Extracellular Ca2+ exerts its effect through the CaSR, which is heavily expressed at the basolateral membrane of TAL cells (91,92); PGE2 primarily signals through EP3 PG receptors (76). The increases in intracellular Ca2+ due to the activation of the CaSR and other receptors directly inhibits cAMP generation by a Ca2+-inhibitable adenylate cyclase that is expressed in the TAL, accompanied by an increase in phosphodiesterase-dependent degradation of cAMP (91,93). Abrogation of the tonic negative effect of PGE2 with indomethacin results in a considerable increase in abundance of the NKCC2 protein (90), whereas targeted deletion of the CaSR in mouse TAL activates NKCC2 via increased N-terminal phosphorylation (60).
Activation of the CaSR and other receptors in the TAL also results in the downstream generation of AA metabolites with potent negative effects on Na+-Cl− transport. Extracellular Ca2+ thus activates phospholipase A2 in TAL cells, leading to the liberation of AA. This AA is in turn metabolized by cytochrome P450 ω-hydroxylase to 20-hydroxyeicosatetraenoic acid, or by cyclooxygenase-2 to PGE2; cytochrome P450 ω-hydroxylation generally predominates in response to activation of the CaSR in TAL (91). 20-Hydroxyeicosatetraenoic acid inhibits apical Na+-K+-2Cl− cotransport, apical K+ channels, basolateral Cl− channels, and the basolateral Na+/K+-ATPase (76,91,94).
The relative importance of the CaSR in the regulation of Na+-Cl− transport by the TAL is dramatically illustrated by the phenotype of rare patients with gain-of-function mutations in this receptor. In addition to suppressed PTH and hypocalcemia, the usual phenotype caused by gain-of-function mutations in the CaSR (autosomal dominant hypoaparathyroidism), these patients manifest a hypokalemic alkalosis, polyuria, and increases in circulating renin and aldosterone (95,96). Therefore, the persistent inhibition of Na+-Cl− transport in the TAL by these overactive mutants of the CaSR causes a rare subtype of Bartter’s syndrome, type V in the genetic classification of this disease (91) (see Table 1).
Activation of the CaSR also modulates the claudin repertoire of TAL cells, leading to PTH-independent hypercalciuria (59–61,92). This involves a novel feedback mechanism wherein activation of the CaSR downregulates two microRNAs (miR-9 and miR-374) that otherwise bind to the 3′-untranslated region of the claudin-14 mRNA and destabilize the transcript (59). This CaSR-dependent downregulation of these microRNAs leads to increased expression of claudin-14 protein, inhibition of claudin-16/claudin-19 heterodimers, reduced paracellular calcium permeability in the TAL, and hypercalciuria (59–61). This interaction provides a potential signaling pathway to explain the association between common variants in the human claudin-14 gene and hypercalciuric nephrolithiasis (97).
Uromodulin
TAL cells are unique in expressing the membrane-bound, glycosyl-phosphatidylinositol–anchored protein uromodulin (Tamm–Horsfall glycoprotein) (see Figure 7), which is not expressed by macula densa cells or the downstream DCT. Uromodulin can be released by proteolytic cleavage at the apical membrane and is secreted as the most abundant protein in normal human urine (20–100 mg/d) (1).
Figure 7.
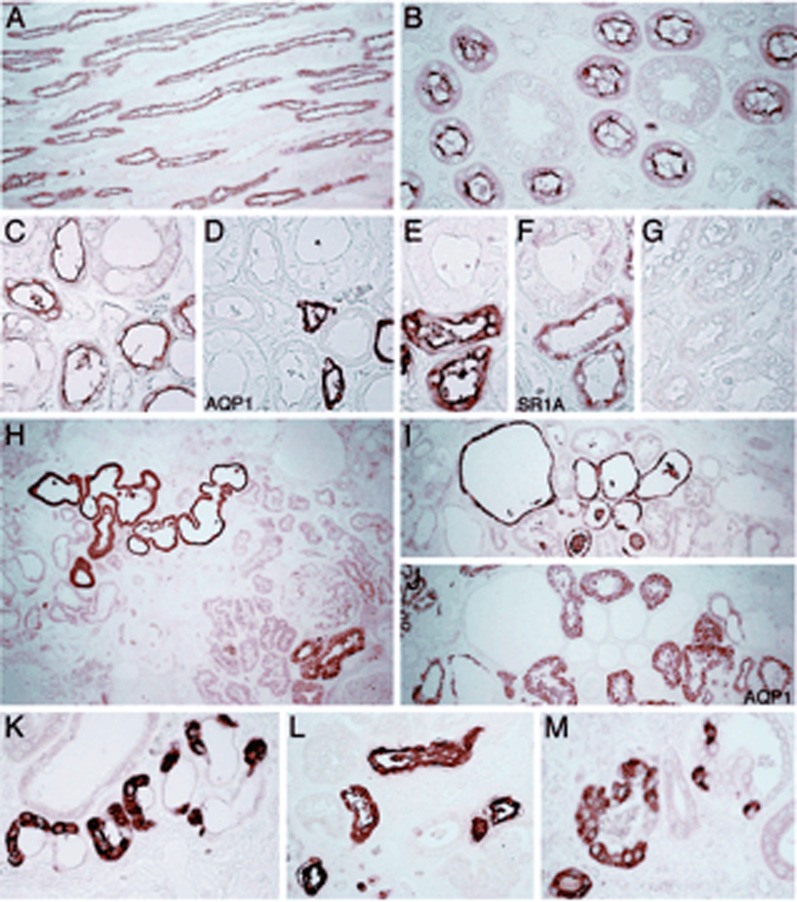
Expression patterns and distribution of uromodulin in normal and diseased kidney. The segmental distribution and staining pattern of uromodulin was compared in normal kidneys (A–G), and in three kidneys with UAKD due to UMOD mutations (H–M). In the normal human kidney, uromodulin is distributed primarily in the TAL segments (A), with a distinct apical membrane reactivity (B). The segmental distribution to the TAL was demonstrated by lack of cross-reactivity with AQP1 (C and D) and codistribution with SR1A on serial sections (E and F). No specific staining was detected when using nonimmune IgG (G). (H–M) The expression and staining pattern for uromodulin was significantly modified in the three kidneys harboring UMOD mutations. Intense staining for uromodulin was detected in a subset of tubule profiles (H and I) that are sometimes enlarged or cystic. The tubule profiles stained for uromodulin are negative for AQP1 (I and J). (K–M) At higher magnification, the staining for uromodulin is intense, diffusely intracellular, and also heterogeneous within tubular cells. AQP1, aquaporin 1; SR1A, serotonin receptor 1A; UAKD, uromodulin-associated kidney disease. Modified from reference 102, with permission.
Uromodulin has a host of emerging roles in the physiology and biology of the TAL. A high-salt diet increases uromodulin expression (1), suggesting a role in ion transport. In this regard, uromodulin facilitates membrane trafficking and function of the NKCC2 protein (98), with similar effects on apical ROMK protein (99). Uromodulin also protects against nephrolithiasis, with the development of calcium oxalate stones in uromodulin knockout mice and evident protective alleles in humans (1). Potential mechanisms for this effect include reduced aggregation of nascent crystals (1) and activation of downstream transient receptor potential cation channel subfamily V member 5 epithelial calcium channels in the DCT by secreted uromodulin (100), with the development of hypercalciuria under uromodulin-deficient conditions. Other possible roles for uromodulin include a defensive role against urinary tract infection and possible roles in innate immunity (1). In disease, interactions between monoclonal free light chains and uromodulin are thought to be critical for cast formation and AKI in cast nephropathy associated with multiple myeloma (101).
Autosomal dominant mutations in the UMOD gene encoding uromodulin are associated with medullary cystic disease type 2 and familial juvenile hyperuricemic nephropathy. Now collectively referred to as uromodulin-associated kidney disease (UAKD), this syndrome includes progressive tubulointerstitial damage and CKD, variably penetrant hyperuricemia and gout, and variably penetrant renal cysts that are typically confined to the corticomedullary junction (1). The causative mutations tend to affect conserved cysteine residues win the N-terminal half of the protein, leading to protein misfolding and retention within the endoplasmic reticulum (1,102) (see Figure 7).
Genome-wide association studies recently linked more common genetic variants in the UMOD promoter with the risk of CKD and hypertension (1). These susceptibility variants have a high frequency (approximately 0.8) and confer an approximately 20% higher risk for CKD and a 15% risk for hypertension (103). These polymorphisms are associated with more abundant renal uromodulin transcript and higher urinary uromodulin excretion (103,104), due to activating effects on the UMOD promoter (103). Overexpression of uromodulin in transgenic mice leads to distal tubular injury, with segmental dilation and increased tubular cast area relative to wild-type mice. Similar lesions were increased in frequency in older humans homozygous for susceptibility variants in UMOD, compared with those homozygous for protective variants (103). Uromodulin-transgenic mice also manifested salt-sensitive hypertension, owing to activation of the SPAK kinase and activating N-terminal phosphorylation of NKCC2. Again, human hypertensive individuals homozygous for susceptibility variants in UMOD appear to have an analogous phenotype, with exaggerated natriuresis in response to furosemide compared with those who are homozygous for protective variants (103). These findings are compatible with the stimulatory effects of uromodulin on the NKCC2 (98) and ROMK (99) transport proteins. Uromodulin excretion appears to parallel transport activity of the TAL and with common polymorphisms in the KCNJ1 gene encoding ROMK and two genes involved in regulating SPAK/OSR1 kinase activity (SORL1 and CAB39) (104). These latter genetic data link uromodulin function with the various signaling pathways that control Na+-Cl− transport within the TAL (see Figure 6).
Pathophysiology of the TAL
It is no surprise that the TAL plays a significant role in the pathophysiology of disease, given its pivotal role in so many aspects of renal physiology. An understanding of TAL physiology leads to greater bedside appreciation of the mechanisms associated with its involvement in human pathophysiology. For example, as discussed above, hyperkalemia leads to an increase in tubular K+ concentration within the TAL, competition between tubular K+ and NH4+ for apical transport via NKCC2 (67), reduced transepithelial NH4+ transport, blunted countercurrent multiplication of interstitial NH4+ concentration, reduced urinary NH4+ excretion, and metabolic acidosis (73,74). Other associations between TAL dysfunction and human disease are more complex, such as the calcium-dependent regulation of claudin-10 and its genetic role in hypercalciuric nephrolithiasis (59–61) (see the discussion of paracellular transport).
Inhibition of TAL function with loop diuretics can also have predictable beneficial effects in specific renal syndromes. For example, by collapsing the lumen-positive potential difference and thus reducing paracellular calcium transport, loop diuretics—combined with adequate saline administration—can enhance calcium excretion in hypercalcemia (105). Loop diuretics combined with oral salt supplementation are also an effective chronic therapy for the syndrome of inappropriate antidiuretic hormone (106), blunting the countercurrent mechanism, increasing water excretion, and correcting the associated hyponatremia. An acquired dysfunction of NKCC2 after ureteral obstruction can in turn lead to the salt wasting and impaired urinary concentrating ability that is characteristic of postobstructive renal function (107).
Hereditary loss of function or dysfunction of TAL is discussed in the various sections above. Loss-of-function mutations in TAL Na+-Cl− transport are associated with Bartter’s syndrome, familial hypokalemic metabolic alkalosis. Patients with “classic” Bartter’s syndrome typically suffer from polyuria and impaired urinary concentrating ability. They may have an increase in urinary Ca2+ excretion and approximately 20% are hypomagnesemic (108); other features include marked elevation of plasma angiotensin II, plasma aldosterone, and plasma renin. By contrast, patients with Gitelman’s syndrome, caused by recessive loss of function mutations of NCC (the thiazide-sensitive Na+-Cl− cotransporter in the DCT), are markedly hypocalciuric and universally hypomagnesemic. Patients with “antenatal” Bartter’s syndrome present earlier in life with a severe systemic disorder characterized by marked electrolyte wasting, polyhydramnios, and significant hypercalciuria with nephrocalcinosis. Genetic classification of Bartter’s syndrome is outlined in Table 1. Although there is significant phenotypic overlap and phenotypic variability, the various phenotypes are predictable in many respects from the underlying physiology of the genes involved. Other genetic causes of TAL dysfunction include UAKD and FHHNC; although the associated defects in TAL are less severe than in Bartter’s syndrome, these disorders can also encompass polyuria and impaired concentrating ability, due to dysfunction in the countercurrent mechanism. Relative hypovolemia and the associated neurohumoral response can lead to hyperuricemia with or without gout, as has been reported in Bartter’s syndrome (109), UAKD (1), and FHHC (110).
Finally, a considerable body of evidence links hypertension with increased NKCC2 activity in both human hypertension and animal models of hypertension (7). Much of this association may be due to gain-of-function variants in the UMOD gene encoding uromodulin (1,103). These provocative findings highlight the role of the TAL in hypertension and may lead to a reappraisal of the therapeutic approach to hypertensive individuals with common, at-risk UMOD genotypes. Furthermore, the association of UMOD variation with an increased risk of CKD identifies uromodulin as a potential therapeutic target in both hypertension and CKD.
Disclosures
D.B.M. has been a consultant to ZS Pharma and receives authorship and royalty fees from UpToDate.
Acknowledgments
This review is dedicated to the memory of Steven C. Hebert, who made multiple seminal contributions to the physiology and pathophysiology of the TAL.
D.B.M. is supported by the National Institutes of Health (DK070756) and the US Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O: The rediscovery of uromodulin (Tamm-Horsfall protein): From tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S, Pallone T, Smith BL, Christensen EI, Agre P, Maunsbach AB: Aquaporin-1 water channels in short and long loop descending thin limbs and in descending vasa recta in rat kidney. Am J Physiol 268: F1023–F1037, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Tsuruoka S, Koseki C, Muto S, Tabei K, Imai M: Axial heterogeneity of potassium transport across hamster thick ascending limb of Henle’s loop. Am J Physiol 267: F121–F129, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA: Ultrastructural localization of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol 275: F885–F893, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Yoshitomi K, Kondo Y, Imai M: Evidence for conductive Cl- pathways across the cell membranes of the thin ascending limb of Henle’s loop. J Clin Invest 82: 866–871, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung JY, Song JH, Li C, Yang CW, Kang TC, Won MH, Jeong YG, Han KH, Choi KB, Lee SH, Kim J: Expression of epidermal growth factor in the developing rat kidney. Am J Physiol Renal Physiol 288: F227–F235, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ares GR, Caceres PS, Ortiz PA: Molecular regulation of NKCC2 in the thick ascending limb. Am J Physiol Renal Physiol 301: F1143–F1159, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burg M, Stoner L, Cardinal J, Green N: Furosemide effect on isolated perfused tubules. Am J Physiol 225: 119–124, 1973 [DOI] [PubMed] [Google Scholar]
- 9.Hebert SC, Mount DB, Gamba G: Molecular physiology of cation-coupled Cl- cotransport: The SLC12 family. Pflugers Arch 447: 580–593, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Plata C, Mount DB, Rubio V, Hebert SC, Gamba G: Isoforms of the Na-K-2Cl cotransporter in murine TAL II. Functional characterization and activation by cAMP. Am J Physiol 276: F359–F366, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Giménez I, Isenring P, Forbush B: Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem 277: 8767–8770, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Lapointe JY, Laamarti A, Bell PD: Ionic transport in macula densa cells. Kidney Int Suppl 67: S58–S64, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Carretero OA: An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int 38: 1206–1210, 1990 [DOI] [PubMed] [Google Scholar]
- 14.He XR, Greenberg SG, Briggs JP, Schnermann J: Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension 26: 137–142, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Wilcox CS: New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol 13: 798–805, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Okusa MD, Persson AE, Wright FS: Chlorothiazide effect on feedback-mediated control of glomerular filtration rate. Am J Physiol 257: F137–F144, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Igarashi P, Vanden Heuvel GB, Payne JA, Forbush B, 3rd: Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am J Physiol 269: F405–F418, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Burg MB, Green N: Function of the thick ascending limb of Henle’s loop. Am J Physiol 224: 659–668, 1973 [DOI] [PubMed] [Google Scholar]
- 19.Greger R: Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev 65: 760–797, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Hebert SC, Andreoli TE: Control of NaCl transport in the thick ascending limb. Am J Physiol 246: F745–F756, 1984 [DOI] [PubMed] [Google Scholar]
- 21.Greger R, Schlatter E: Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle’s loop of rabbit kidney. Pflugers Arch 392: 92–94, 1981 [DOI] [PubMed] [Google Scholar]
- 22.Stokes JB: Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest 70: 219–229, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi J, Guggino WB: Membrane stretch: A physiological stimulator of Ca2+-activated K+ channels in thick ascending limb. Am J Physiol 257: F347–F352, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Bleich M, Schlatter E, Greger R: The luminal K+ channel of the thick ascending limb of Henle’s loop. Pflugers Arch 415: 449–460, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Wang WH: Two types of K+ channel in thick ascending limb of rat kidney. Am J Physiol 267: F599–F605, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Lu M: Effect of arachidonic acid on activity of the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol 106: 727–743, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC: Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC: Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol 273: F739–F748, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Lu M, Wang T, Yan Q, Yang X, Dong K, Knepper MA, Wang W, Giebisch G, Shull GE, Hebert SC: Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter’s) knockout mice. J Biol Chem 277: 37881–37887, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP: Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Wang T, Yan Q, Wang W, Giebisch G, Hebert SC: ROMK is required for expression of the 70-pS K channel in the thick ascending limb. Am J Physiol Renal Physiol 286: F490–F495, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Yoo D, Flagg TP, Olsen O, Raghuram V, Foskett JK, Welling PA: Assembly and trafficking of a multiprotein ROMK (Kir 1.1) channel complex by PDZ interactions. J Biol Chem 279: 6863–6873, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Leng Q, Egan ME, Caplan MJ, Boulpaep EL, Giebisch GH, Hebert SC: CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest 116: 797–807, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahida R, Mohammed H, Guy L: Pseudo-Bartter’s syndrome revealing cystic fibrosis in an infant caused by 3849 + 1G>A and 4382delA compound heterozygosity. Acta Paediatr 100: e234–e235, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Hebert SC, Culpepper RM, Andreoli TE: NaCl transport in mouse medullary thick ascending limbs. II. ADH enhancement of transcellular NaCl cotransport; origin of transepithelial voltage. Am J Physiol 241: F432–F442, 1981 [DOI] [PubMed] [Google Scholar]
- 36.Greger R, Schlatter E: Properties of the basolateral membrane of the cortical thick ascending limb of Henle’s loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch 396: 325–334, 1983 [DOI] [PubMed] [Google Scholar]
- 37.Greger R, Oberleithner H, Schlatter E, Cassola AC, Weidtke C: Chloride activity in cells of isolated perfused cortical thick ascending limbs of rabbit kidney. Pflugers Arch 399: 29–34, 1983 [DOI] [PubMed] [Google Scholar]
- 38.Waldegger S, Jeck N, Barth P, Peters M, Vitzthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW: Barttin increases surface expression and changes current properties of ClC-K channels. Pflugers Arch 444: 411–418, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Morimoto T, Kondo Y, Iinuma K, Uchida S, Sasaki S, Marumo F, Imai M: Analysis of NaCl transport in thin ascending limb of Henle’s loop in CLC-K1 null mice. Am J Physiol Renal Physiol 282: F451–F457, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi K, Uchida S, Mizutani S, Sasaki S, Marumo F: Intrarenal and cellular localization of CLC-K2 protein in the mouse kidney. J Am Soc Nephrol 12: 1327–1334, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP: Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet 17: 171–178, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Palmer LG, Frindt G: Cl- channels of the distal nephron. Am J Physiol Renal Physiol 291: F1157–F1168, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Estévez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, Jentsch TJ: Barttin is a Cl- channel beta-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414: 558–561, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Picollo A, Liantonio A, Didonna MP, Elia L, Camerino DC, Pusch M: Molecular determinants of differential pore blocking of kidney CLC-K chloride channels. EMBO Rep 5: 584–589, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Stefano A, Greger R, Desfleurs E, de Rouffignac C, Wittner M: A Ba(2+)-insensitive K+ conductance in the basolateral membrane of rabbit cortical thick ascending limb cells. Cell Physiol Biochem 8: 89–105, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Mercado A, Song L, Vazquez N, Mount DB, Gamba G: Functional comparison of the K+-Cl- cotransporters KCC1 and KCC4. J Biol Chem 275: 30326–30334, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Guggino WB: Functional heterogeneity in the early distal tubule of the Amphiuma kidney: Evidence for two modes of Cl- and K+ transport across the basolateral cell membrane. Am J Physiol 250: F430–F440, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Cabral PD, Herrera M: Membrane-associated aquaporin-1 facilitates osmotically driven water flux across the basolateral membrane of the thick ascending limb. Am J Physiol Renal Physiol 303: F621–F629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun A, Grossman EB, Lombardi M, Hebert SC: Vasopressin alters the mechanism of apical Cl- entry from Na+:Cl- to Na+:K+:2Cl- cotransport in mouse medullary thick ascending limb. J Membr Biol 120: 83–94, 1991 [DOI] [PubMed] [Google Scholar]
- 50.Hebert SC, Andreoli TE: Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl- absorption. J Gen Physiol 87: 567–590, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furuse M, Furuse K, Sasaki H, Tsukita S: Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153: 263–272, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S: Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angelow S, El-Husseini R, Kanzawa SA, Yu AS: Renal localization and function of the tight junction protein, claudin-19. Am J Physiol Renal Physiol 293: F166–F177, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Müller D: Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A 109: 14241–14246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou J, Paul DL, Goodenough DA: Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118: 5109–5118, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Himmerkus N, Shan Q, Goerke B, Hou J, Goodenough DA, Bleich M: Salt and acid-base metabolism in claudin-16 knockdown mice: Impact for the pathophysiology of FHHNC patients. Am J Physiol Renal Physiol 295: F1641–F1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J: Claudin-14 regulates renal Ca⁺⁺ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toka HR, Al-Romaih K, Koshy JM, DiBartolo S, 3rd, Kos CH, Quinn SJ, Curhan GC, Mount DB, Brown EM, Pollak MR: Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 23: 1879–1890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT: Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am J Physiol Renal Physiol 304: F761–F769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Good DW: Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. Am J Physiol 248: F821–F829, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Wang T, Hropot M, Aronson PS, Giebisch G: Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol 281: F1117–F1122, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Bourgeois S, Massé S, Paillard M, Houillier P: Basolateral membrane Cl(-)-, Na(+)-, and K(+)-coupled base transport mechanisms in rat MTALH. Am J Physiol Renal Physiol 282: F655–F668, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Odgaard E, Jakobsen JK, Frische S, Praetorius J, Nielsen S, Aalkjaer C, Leipziger J: Basolateral Na+-dependent HCO3- transporter NBCn1-mediated HCO3- influx in rat medullary thick ascending limb. J Physiol 555: 205–218, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capasso G, Unwin R, Ciani F, De Santo NG, De Tommaso G, Russo F, Giebisch G: Bicarbonate transport along the loop of Henle. II. Effects of acid-base, dietary, and neurohumoral determinants. J Clin Invest 94: 830–838, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Good DW: Ammonium transport by the thick ascending limb of Henle’s loop. Annu Rev Physiol 56: 623–647, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Packer RK, Desai SS, Hornbuckle K, Knepper MA: Role of countercurrent multiplication in renal ammonium handling: Regulation of medullary ammonium accumulation. J Am Soc Nephrol 2: 77–83, 1991 [DOI] [PubMed] [Google Scholar]
- 69.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM: A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M: Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+-K+(NH4+)-2Cl- cotransporter of the rat medullary thick ascending limb. J Biol Chem 273: 33681–33691, 1998 [DOI] [PubMed] [Google Scholar]
- 71.Bourgeois S, Meer LV, Wootla B, Bloch-Faure M, Chambrey R, Shull GE, Gawenis LR, Houillier P: NHE4 is critical for the renal handling of ammonia in rodents. J Clin Invest 120: 1895–1904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bettinelli A, Ciarmatori S, Cesareo L, Tedeschi S, Ruffa G, Appiani AC, Rosini A, Grumieri G, Mercuri B, Sacco M, Leozappa G, Binda S, Cecconi M, Navone C, Curcio C, Syren ML, Casari G: Phenotypic variability in Bartter syndrome type I. Pediatr Nephrol 14: 940–945, 2000 [DOI] [PubMed] [Google Scholar]
- 73.DuBose TD, Jr, Good DW: Chronic hyperkalemia impairs ammonium transport and accumulation in the inner medulla of the rat. J Clin Invest 90: 1443–1449, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szylman P, Better OS, Chaimowitz C, Rosler A: Role of hyperkalemia in the metabolic acidosis of isolated hypoaldosteronism. N Engl J Med 294: 361–365, 1976 [DOI] [PubMed] [Google Scholar]
- 75.Buysse JM, Huang IZ, Pitt B: PEARL-HF: Prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol 8: 17–28, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Féraille E, Doucet A: Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: Hormonal control. Physiol Rev 81: 345–418, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Silva GB, Garvin JL: Angiotensin II-dependent hypertension increases Na transport-related oxygen consumption by the thick ascending limb. Hypertension 52: 1091–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu P, Wang M, Luan H, Li L, Wang L, Wang WH, Gu R: Angiotensin II stimulates basolateral 10-pS Cl channels in the thick ascending limb. Hypertension 61: 1211–1217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mutig K, Paliege A, Kahl T, Jöns T, Müller-Esterl W, Bachmann S: Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Giménez I, Forbush B: Short-term stimulation of the renal Na-K-Cl cotransporter (NKCC2) by vasopressin involves phosphorylation and membrane translocation of the protein. J Biol Chem 278: 26946–26951, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Gagnon KB, England R, Delpire E: Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol 290: C134–C142, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Delpire E, Gagnon KB: Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol Genomics 28: 223–231, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Gagnon KB, England R, Delpire E: A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem 20: 131–142, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Reiche J, Theilig F, Rafiqi FH, Carlo AS, Militz D, Mutig K, Todiras M, Christensen EI, Ellison DH, Bader M, Nykjaer A, Bachmann S, Alessi D, Willnow TE: SORLA/SORL1 functionally interacts with SPAK to control renal activation of Na(+)-K(+)-Cl(-) cotransporter 2. Mol Cell Biol 30: 3027–3037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS: Impaired phosphorylation of Na(+)-K(+)-2Cl(-) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci U S A 108: 17538–17543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanović S, Jovanović A, O’Shaughnessy KM, Alessi DR: Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juárez P, Muñoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G: Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci U S A 105: 8458–8463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ponce-Coria J, Gagnon KB, Delpire E: Calcium-binding protein 39 facilitates molecular interaction between Ste20p proline alanine-rich kinase and oxidative stress response 1 monomers. Am J Physiol Cell Physiol 303: C1198–C1205, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA: Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci U S A 107: 15653–15658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Knepper MA, Kim GH, Fernández-Llama P, Ecelbarger CA: Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol 10: 628–634, 1999 [DOI] [PubMed] [Google Scholar]
- 91.Hebert SC: Calcium and salinity sensing by the thick ascending limb: A journey from mammals to fish and back again. Kidney Int Suppl 91 (Suppl): S28–S33, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P: PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122: 3355–3367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Jesus Ferreira MC, Héliès-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardès D: Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J Biol Chem 273: 15192–15202, 1998 [DOI] [PubMed] [Google Scholar]
- 94.Gu RM, Yang L, Zhang Y, Wang L, Kong S, Zhang C, Zhai Y, Wang M, Wu P, Liu L, Gu F, Zhang J, Wang WH: CYP-omega-hydroxylation-dependent metabolites of arachidonic acid inhibit the basolateral 10 pS chloride channel in the rat thick ascending limb. Kidney Int 76: 849–856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T: Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet 360: 692–694, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C: Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13: 2259–2266, 2002 [DOI] [PubMed] [Google Scholar]
- 97.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d’Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K: Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41: 926–930, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, Raffi H, Rampoldi L, Uchida S, Hille C, Dosche C, Kumar S, Castañeda-Bueno M, Gamba G, Bachmann S: Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. J Biol Chem 286: 30200–30210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Renigunta A, Renigunta V, Saritas T, Decher N, Mutig K, Waldegger S: Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function. J Biol Chem 286: 2224–2235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf MT, Wu XR, Huang CL: Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int 84: 130–137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ying WZ, Allen CE, Curtis LM, Aaron KJ, Sanders PW: Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Invest 122: 1777–1785, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dahan K, Devuyst O, Smaers M, Vertommen D, Loute G, Poux JM, Viron B, Jacquot C, Gagnadoux MF, Chauveau D, Büchler M, Cochat P, Cosyns JP, Mougenot B, Rider MH, Antignac C, Verellen-Dumoulin C, Pirson Y: A cluster of mutations in the UMOD gene causes familial juvenile hyperuricemic nephropathy with abnormal expression of uromodulin. J Am Soc Nephrol 14: 2883–2893, 2003 [DOI] [PubMed] [Google Scholar]
- 103.Trudu M, Janas S, Lanzani C, Debaix H, Schaeffer C, Ikehata M, Citterio L, Demaretz S, Trevisani F, Ristagno G, Glaudemans B, Laghmani K, Dell’Antonio G, Loffing J, Rastaldi MP, Manunta P, Devuyst O, Rampoldi L; Swiss Kidney Project on Genes in Hypertension (SKIPOGH) team: Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression. Nat Med 19: 1655–1660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olden M, Corre T, Hayward C, Toniolo D, Ulivi S, Gasparini P, Pistis G, Hwang SJ, Bergmann S, Campbell H, Cocca M, Gandin I, Girotto G, Glaudemans B, Hastie ND, Loffing J, Polasek O, Rampoldi L, Rudan I, Sala C, Traglia M, Vollenweider P, Vuckovic D, Youhanna S, Weber J, Wright AF, Kutalik Z, Bochud M, Fox CS, Devuyst O: Common variants in UMOD associate with urinary uromodulin levels: A meta-analysis. J Am Soc Nephrol 25: 1869–1882, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suki WN, Yium JJ, Von Minden M, Saller-Hebert C, Eknoyan G, Martinez-Maldonado M: Acute treatment of hypercalcemia with furosemide. N Engl J Med 283: 836–840, 1970 [DOI] [PubMed] [Google Scholar]
- 106.Decaux G, Waterlot Y, Genette F, Hallemans R, Demanet JC: Inappropriate secretion of antidiuretic hormone treated with frusemide. Br Med J (Clin Res Ed) 285: 89–90, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hwang SJ, Haas M, Harris HW, Jr, Silva P, Yalla S, Sullivan MR, Otuechere G, Kashgarian M, Zeidel ML: Transport defects of rabbit medullary thick ascending limb cells in obstructive nephropathy. J Clin Invest 91: 21–28, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guay-Woodford LM: Bartter syndrome: Unraveling the pathophysiologic enigma. Am J Med 105: 151–161, 1998 [DOI] [PubMed] [Google Scholar]
- 109.Meyer WJ, 3rd, Gill JR, Jr, Bartter FC: Gout as a complication of Bartter’s syndrome. A possible role for alkalosis in the decreased clearance of uric acid. Ann Intern Med 83: 56–59, 1975 [DOI] [PubMed] [Google Scholar]
- 110.Tasic V, Dervisov D, Koceva S, Weber S, Konrad M: Hypomagnesemia with hypercalciuria and nephrocalcinosis: Case report and a family study. Pediatr Nephrol 20: 1003–1006, 2005 [DOI] [PubMed] [Google Scholar]