Abstract
Background and objectives
AKI is a common and severe complication in patients with cirrhosis. AKI progression was previously shown to correlate with in-hospital mortality. Therefore, accurately predicting which patients are at highest risk for AKI progression may allow more rapid and targeted treatment. Urinary biomarkers of structural kidney injury associate with AKI progression and mortality in multiple settings of AKI but their prognostic performance in patients with liver cirrhosis is not well known.
Design, setting, participants, & measurements
A multicenter, prospective cohort study was conducted at four tertiary care United States medical centers between 2009 and 2011. The study comprised patients with cirrhosis and AKI defined by the AKI Network criteria evaluating structural (neutrophil gelatinase–associated lipocalin, IL-18, kidney injury molecule-1 [KIM-1], liver-type fatty acid–binding protein [L-FABP], and albuminuria) and functional (fractional excretion of sodium [FENa]) urinary biomarkers as predictors of AKI progression and in-hospital mortality.
Results
Of 188 patients in the study, 44 (23%) experienced AKI progression alone and 39 (21%) suffered both progression and death during their hospitalization. Neutrophil gelatinase–associated lipocalin, IL-18, KIM-1, L-FABP, and albuminuria were significantly higher in patients with AKI progression and death. These biomarkers were independently associated with this outcome after adjusting for key clinical variables including model of end stage liver disease score, IL-18 (relative risk [RR], 4.09; 95% confidence interval [95% CI], 1.56 to 10.70), KIM-1 (RR, 3.13; 95% CI, 1.20 to 8.17), L-FABP (RR, 3.43; 95% CI, 1.54 to 7.64), and albuminuria (RR, 2.07; 95% CI, 1.05–4.10) per log change. No biomarkers were independently associated with progression without mortality. FENa demonstrated no association with worsening of AKI. When added to a robust clinical model, only IL-18 independently improved risk stratification on a net reclassification index.
Conclusions
Multiple structural biomarkers of kidney injury, but not FENa, are independently associated with progression of AKI and mortality in patients with cirrhosis. Injury marker levels were similar between those without progression and those with progression alone.
Keywords: ARF, clinical nephrology, liver failure, outcomes, progression of renal failure
Introduction
AKI is common in patients with cirrhosis, complicating 20% of hospitalizations (1–3). The risk of death increases with peak severity of AKI (4–8) and progression of AKI to a higher stage defined by the AKI Network (AKIN) criteria. AKI progression is associated with mortality independent of the model of end stage liver disease (MELD) score (4). Intervening to prevent progression therefore may reduce mortality. Despite the overall grim prognosis for patients with cirrhosis and AKI, disease-specific treatments carrying the potential to improve outcomes, if correctly and judiciously applied, are available for patients with hepatorenal syndrome (HRS) (9–12). In addition, patients with progressive acute tubular necrosis (ATN) may be managed with dialysis, and those with severe, irreversible disease benefit from combined liver-kidney transplantation (13). For reasons of safety and equity, such aggressive therapies should ideally be offered only to those patients at greatest risk for progressive renal dysfunction and death. However, in practice, where clinically distinguishing the cause of AKI is frequently challenging, patients often receive a “kitchen sink” approach of multiple aggressive therapies irrespective of whether they are at high risk for AKI progression or death. If a patient is unlikely to progress or die, aggressive management can likely be held while time is taken to clarify the cause of AKI. However, if a patient is at high risk for adverse outcomes, early and aggressive action should be taken.
Unfortunately, predicting which patients will suffer progressive AKI, and identifying those progressors who will proceed to death, is clinically challenging. In patients with cirrhosis, an enlarged volume of fluid distribution, low protein intake, and decreased creatinine production secondary to muscle atrophy and liver dysfunction significantly dissociate creatinine levels from reflecting the true presence and severity of kidney dysfunction (14). Correspondingly, creatinine fluctuations early in the course of AKI are difficult to interpret, taking several days to resolve into a definitive trend demonstrating progression. As a result of this delay, potentially beneficial treatments may be deferred. An accurate, objective, and reproducible means of anticipating AKI progression or death at the time of AKI diagnosis is urgently needed to allocate treatments, stratify patients for inclusion in trials, and prioritize liver and kidney transplantations.
Research into structural AKI has been revolutionized by investigation of multiple urinary biomarkers of kidney tubular injury that independently predict AKI progression in multiple clinical settings (15–17). Among the most promising are neutrophil gelatinase–associated lipocalin (NGAL), IL-18, kidney injury molecule-1 (KIM-1), and liver-type fatty acid–binding protein (L-FABP). Although NGAL has been studied for early detection of AKI after liver transplantation (18,19) and for differential diagnosis of AKI in cirrhosis (20,21), few studies have evaluated these biomarkers for prognosis in patients with cirrhosis and AKI (20,21). With a unique mix of functional (HRS) and structural disease (ATN and GN), the association in cirrhosis between tubular injury biomarker levels and outcomes is unclear. In this setting, it is possible that biomarkers of tubular function, such as fractional excretion of sodium (FENa), and traditional markers of both glomerular and tubular injury, such as urine albumin, may also provide additional prognostic accuracy. Indeed, albuminuria is predictive of impending AKI in patients with cirrhosis (22). We conducted a multicenter prospective study evaluating urinary biomarkers of kidney injury and tubular function for prediction of AKI progression and progression with mortality in patients with cirrhosis.
Materials and Methods
Study Design
The details of the cohort and study design were previously described (4). This prospective, multicenter observational cohort study was conducted over 29 months between 2009 and 2011, at four tertiary care academic centers in the United States. Eligible patients were admitted with AKI (see independent variables below for definitions) or developed it during the course of hospitalization. Inclusion criteria included a known diagnosis of cirrhosis (see independent variables below for definitions), age≥18 years, and availability of a documented serum creatinine within 1 year before AKI. Major exclusion criteria included prior kidney or liver transplantation and advanced CKD or RRT at the time of enrollment. All consecutive eligible patients were enrolled within 5 days of meeting AKI criteria. Informed consent was obtained from all patients or their proxy decision-makers. The study was approved by the institutional review board at each participating institution.
Sample Collection and Biomarker Measurement
A fresh 10-ml urine sample was collected daily for 3 days. Samples were immediately refrigerated and centrifuged at 5000×g for 10 minutes at −4°C. Aliquots of 1 ml of supernatant were stored within 6 hours of collection at −80°C. No additives or protease inhibitors were utilized. All biomarkers were measured from frozen aliquots that did not undergo any additional freeze-thaw cycles. Laboratory measurements were performed by personnel blinded to patient information. ELISA methods, coefficients of variation, and detection ranges were as previously described for measurement of NGAL (23), IL-18 (24), KIM-1 (25), and L-FABP (25). Urine creatinine was measured by the modified Jaffe reaction.
Variables
Independent Variables.
Independent variables included cirrhosis, AKI, and baseline serum creatinine. Eligible patients carried a documented diagnosis of cirrhosis based on liver biopsy, when available, or on a combination of clinical, biochemical, imaging, and endoscopic findings.
AKI was defined as a rise in creatinine of 0.3 mg/dl or 50% from baseline as recommended by a working group composed of members of the International Ascites Club (IAC) and the Acute Dialysis Quality Initiative who based this cutoff on stage 1 of the AKIN criteria (26). Because documentation of urine output was incomplete, this aspect of the criteria was not utilized.
Baseline serum creatinine was defined as the most recent stable measurement before admission because the use of outpatient values results in less misclassification of AKI incidence, severity, and prognosis compared with utilizing hospital admission, hospital nadir, or imputed values (27). The median interval between creatinine utilized for baseline and hospital admission in this study was 26 days (interquartile range [IQR], 9–73).
Other Variables.
Baseline GFR was estimated via the Modification of Diet in Renal Disease 4 equation (28). CKD was defined as a GFR<60 ml/min per 1.73 m2. MELD and Child–Pugh scores were calculated on the day of first sample collection. HRS was diagnosed via the 2007 IAC criteria (29).
Outcomes.
Our primary outcomes consisted of progression to a higher AKIN stage and progression to a higher stage with subsequent death, which were compared separately with patients who did not progress. If patients who presented with stage 3 AKI but not requiring RRT subsequently required dialysis, this was considered progression. Patients who died without progression were excluded from the primary analysis because death may have been a competing risk for progression for these patients. Biomarker values for these excluded patients did not differ from those with progression and death (Supplemental Table 1).
Statistical Analyses
Categorical variables were expressed as proportions and compared using chi-squared and Fisher’s exact tests, as appropriate. Normally or near-normally distributed variables were reported as means with SDs and were compared by the t test. Non-normally distributed continuous variables were reported as medians with IQRs and were compared by the Wilcoxon rank-sum test. Normality was assessed using the Kolmogorov–Smirnov test. NGAL values were bounded at an upper limit of 1000 ng/ml with no lower bound. KIM-1 was bounded at an upper limit of 60 ng/ml and a lower limit of 0.056 ng/ml. L-FABP was bounded at an upper limit of 400 ng/ml and a lower limit of 0.57 ng/ml. IL-18 did not have an upper limit but the lower limit of detection for the assay was 25 pg/ml. All patients below this threshold were assigned a value of 15 pg/ml. Biomarker values from day 0 (the first day of sample collection) were used for all analyses.
Biomarkers were log-transformed and analyzed as continuous variables given their non-normal distribution. We determined crude and adjusted relative risks (RRs) for each biomarker for progression alone and progression with death using a Poisson logistic regression model with patients without progression of AKI as the reference group. Utilizing the clinical model we developed through our association of AKI progression with mortality (4), we adjusted for critical covariates including presence of CKD, demographics (race, age, and sex), MELD score, and serum sodium. RRs were calculated rather than odds ratios to avoid artificial inflation of point estimates due to high prevalence of outcomes. To assess biomarkers’ ability to discriminate risk, we calculated the area under the receiver operating curve for each biomarker for each outcome. To evaluate biomarkers for improvements in risk discrimination, we calculated a category-free net reclassification index (NRI) for each biomarker for the outcome of AKI progression and death. This was performed by utilizing binary logistic regression models (for no progression versus progression and death) constructed with the above-noted clinical variables and evaluating changes in model predictions with and without each biomarker. Finally, we determined the optimal cutoff for each biomarker for predicting AKI progression and death by maximizing the Youden index and calculated the RR for this outcome by the number of biomarkers above these cutoffs. This was achieved using a regression model with the above clinical variables and the number of biomarkers above their cutoff as an ordinal variable (with zero markers as the reference). Biomarkers were evaluated for collinearity using Pearson’s test (Supplemental Table 2) and were evaluated for consistency across days of sample collection using paired t tests. In supplemental analysis, biomarkers levels were compared across groups in those patients who did and did not meet IAC criteria for HRS. Goodness of fit was verified with the Hosmer–Lemeshow test. A two-sided P value <0.05 was considered significant for all analysis. Statistical analysis was performed using SAS (version 9.2; SAS Institute, Cary, NC) and R (version 2.10.1) software.
Results
Cohort Characteristics
A total of 219 patients with cirrhosis and AKI were prospectively enrolled. Thirty-one patients were subsequently excluded for the following reasons: prolonged interval between onset of AKI and time of first sample collection (n=15), lack of documented baseline creatinine level (n=4), recent treatment with nephrotoxins (n=3), diagnosis of acute hepatitis rather than cirrhosis (n=2), anuria (n=2), and other causes (n=5).
AKI Progression
Forty-four patients (23%) experienced AKI progression alone and 39 (21%) had AKI progression and subsequently died during their hospitalization. Ten patients (5%) died without progression and were excluded from the primary analysis. Baseline demographic, clinical, and laboratory data of the entire cohort and of those patients with and without AKI progression are shown in Tables 1 and 2. Neither baseline GFR nor the presence of CKD varied between the three groups. The Δ creatinine between baseline and admission did not differ between the three groups (0.8 mg/dl versus 0.8 versus 0.5; P=0.28). Baseline proteinuria was only present in 22 patients (12%) and was similar across groups. The majority of patients had decompensated cirrhosis as evidenced by the history of ascites (76%), hepatic encephalopathy (67%), variceal bleeding (23%), and spontaneous bacterial peritonitis (16%). Reasons for admission were similar across groups but rates of urinary tract infections and pneumonia differed during the course of hospitalization. The median Child–Pugh score was 10.5 and the median MELD score was 26.3. Both Child–Pugh (12 versus 10 versus 10; P<0.001) and MELD scores (34.3 versus 26.5 versus 22.1; P<0.001) were higher in those patients who progressed and died than in those with progression alone or those without progression.
Table 1.
Baseline and demographic characteristics
| Characteristic | Total (N=188) | No Progression (n=95) | Progression Alone (n=44) | Progression with Death (n=39) | Death Alone (n=10) | P Value |
|---|---|---|---|---|---|---|
| Age (yr) | 55±9.3 | 55.7±9.6 | 54.7±8.9 | 54.7±9 | 50.8±7 | 0.10 |
| Men | 133 (71) | 66 (69) | 32 (73) | 29 (74) | 6 (60) | 0.83 |
| BMI (kg/m2) | 30.1 (25.5–35.1) | 30 (24.5–35.3) | 29.6 (26–33.9) | 30.9 (27–34.8) | 26.7 (25.7–32.7) | 0.40 |
| Race/ethnicity | ||||||
| White | 134 (71) | 70 (74) | 37 (84) | 21 (54) | 6 (60) | 0.01 |
| Black | 27 (14) | 14 (15) | 3 (7) | 9 (23) | 1 (10) | 0.26 |
| Hispanic | 23 (12) | 10 (11) | 3 (7) | 8 (21) | 2 (20) | 0.06 |
| CKDa | 65 (35) | 31 (33) | 21 (48) | 12 (31) | 1 (10) | 0.48 |
| eGFR (ml/min per 1.73 m2) | 70 (55–98) | 70 (57–93) | 64 (43–88) | 81 (54–100) | 101 (78–115) | 0.14 |
| Proteinuriab | 22 (12) | 14 (15) | 3 (7) | 5 (13) | 0 (0) | 0.36 |
| Baseline SCr (mg/dl) | 1 (0.8–1.3) | 1 (0.8–1.3) | 1.1 (0.8–1.5) | 1 (0.8–1.3) | 0.7 (0.6–1) | 0.09 |
| Creatinine at meeting AKIN criteria (mg/dl) | 2.2 (1.6–2.9) | 2.1 (1.6–2.7) | 2.4 (1.6–3.3) | 2.1 (1.5–2.8) | 2.3 (1.6–4.1) | 0.50 |
| Peak creatinine | 2.7 (1.9–4.2) | 2.2 (1.7–3.1) | 3.4 (2.4–4.8) | 4 (3.1–5.5) | 2.5 (2.2–5.1) | <0.001 |
| Diabetes | 49 (26) | 29 (31) | 10 (23) | 9 (23) | 1 (10) | 0.56 |
| Active cancer | 21 (11) | 10 (11) | 5 (11) | 4 (10) | 2 (20) | 0.98 |
| Cause of cirrhosis | ||||||
| Alcohol | 55 (29) | 27 (28) | 12 (27) | 10 (26) | 6 (60) | 0.17 |
| Alcohol and HCV | 52 (28) | 26 (27) | 13 (30) | 12 (31) | 1 (10) | 0.57 |
| HCV | 32 (17) | 14 (15) | 10 (23) | 6 (15) | 2 (20) | 0.87 |
| NASH | 17 (9) | 10 (11) | 5 (11) | 2 (5) | 0 (0) | 0.62 |
| Cryptogenic | 12 (6) | 7 (7) | 3 (7) | 2 (5) | 0 (0) | 0.96 |
| Autoimmune | 11 (6) | 6 (6) | 0 (0) | 4 (10) | 1 (10) | 0.26 |
| Other | 10 (5) | 5 (5) | 1 (2) | 4 (10) | 0 (0) | 0.38 |
| Previous complications | ||||||
| Ascites | 142 (76) | 71 (75) | 35 (81) | 30 (77) | 6 (60) | 0.91 |
| Hepatic encephalopathy | 126 (67) | 61 (64) | 31 (70) | 28 (72) | 6 (60) | 0.77 |
| Variceal bleed | 44 (23) | 23 (24) | 12 (27) | 7 (18) | 2 (20) | 0.31 |
| SBP | 30 (16) | 13 (14) | 7 (16) | 9 (23) | 1 (10) | 0.53 |
Data are presented as the mean±SD, median (interquartile range), or n (%). P values were calculated using the universal F test across four groups. BMI, body mass index; SCr, serum creatinine; AKIN, AKI Network; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis; SBP, spontaneous bacterial peritonitis.
CKD is defined as an eGFR<60 ml/min per 1.73 m2 by the Modification of Diet in Renal Disease equation.
Microalbuminuria is defined as ≥30 mg/dl on dipstick testing or quantitative measurement before admission.
Table 2.
Hospital events and complications
| Events and Complications | Total (n=188) | No Progression (n=95) | Progression Alone (n=44) | Progression with Death (n=39) | Death Alone (n=10) | P Value |
|---|---|---|---|---|---|---|
| Reason for admission | ||||||
| Hepatic encephalopathy | 50 (27) | 30 (32) | 8 (18) | 10 (26) | 2 (20) | 0.33 |
| Refractory ascites/edema | 23 (12) | 11 (12) | 7 (16) | 5 (13) | 0 (0) | 0.53 |
| AKI | 22 (12) | 12 (13) | 7 (16) | 1 (3) | 2 (20) | 0.23 |
| Gastrointestinal bleeding | 14 (7) | 8 (8) | 2 (5) | 3 (8) | 1 (10) | 0.97 |
| Abdominal pain | 14 (7) | 5 (5) | 5 (11) | 2 (5) | 2 (20) | 0.67 |
| Jaundice | 10 (5) | 5 (5) | 1 (2) | 3 (8) | 1 (10) | 0.58 |
| Transplant work-up | 6 (3) | 5 (5) | 0 (0) | 0 (0) | 1 (10) | 0.43 |
| SBP | 6 (3) | 2 (2) | 1 (2) | 2 (5) | 1 (10) | 0.21 |
| Other | 39 (21) | 17 (18) | 12 (27) | 13 (33) | 0 (0) | 0.45 |
| Child–Pugh classa | <0.001b | |||||
| A | 4 (2) | 4 (4) | 0 (0) | 0 (0) | 0 (0) | |
| B | 60 (32) | 42 (45) | 14 (32) | 4 (10) | 0 (0) | |
| C | 123 (66) | 48 (51) | 30 (68) | 35 (90) | 10 (100) | |
| Hospital complications | ||||||
| UTI | 53 (28) | 14 (15) | 18 (41) | 16 (41) | 5 (50) | <0.001 |
| SBP | 37 (20) | 12 (13) | 11 (25) | 12 (31) | 2 (20) | 0.002 |
| Pneumonia | 35 (19) | 11 (12) | 4 (9) | 15 (38) | 5 (50) | <0.001 |
| Bacteremia | 33 (18) | 11 (12) | 8 (18) | 11 (28) | 3 (30) | 0.06 |
| ICU admission | 90 (48) | 24 (25) | 20 (45) | 38 (97) | 8 (80) | <0.001 |
| Mechanical ventilation | 62 (33) | 9 (9) | 13 (30) | 34 (87) | 6 (60) | <0.001 |
| Vasopressor therapy | 49 (26) | 6 (6) | 8 (18) | 29 (74) | 6 (60) | <0.001 |
| Dialysis | 44 (23) | 0 (0) | 16 (36) | 27 (69) | 1 (10) | <0.001 |
| Child–Pugh score | 10.5 (9–12) | 10 (8–11) | 10 (9–12) | 12 (11–13) | 12.5 (11–14) | <0.001 |
| MELD scorea | 26.3±9.5 | 22.1±8.4 | 26.5±6.9 | 34.3±9.3 | 34.3±6.1 | <0.001 |
| Serum sodium at enrollment (mmol/L) | 133 (130–138) | 134 (130–137) | 132 (128–137) | 133 (130–138) | 134 (124–143) | 0.31 |
| Hyponatremia at enrollmentc | 62 (33) | 29 (31) | 17 (39) | 13 (33) | 3 (30) | 0.30 |
| WBC (n=170) (×1000/µl) | 7.4 (5–10.9) | 6.1 (4.4–8.5) | 7.2 (5.2–10.1) | 12.4 (7.3–10.3) | 13.9 (10.4–14) | <0.001 |
| Hbg (n=170) (g/dl) | 8.7 (7.9–10.1) | 8.9 (8.2–10.1) | 8.8 (7.3–10.3) | 8.1 (7.5–9.1) | 8.3 (8–11.1) | 0.02 |
| AST (n=155) (U/L) | 64 (39–113) | 56 (39–89) | 50 (32–88) | 118 (49–276) | 116 (69–272) | <0.001 |
| ALT (n=155) (U/L) | 33 (18–51) | 29 (20–46) | 23 (14–47) | 39 (24–106) | 44 (30–79) | 0.001 |
| Midodrine | 80 (45) | 32 (34) | 25 (57) | 23 (59) | 6 (60) | <0.001 |
| Octreotide | 81 (46) | 32 (34) | 25 (57) | 24 (62) | 7 (70) | <0.001 |
| Length of stayd | 12 (6–19) | 9 (6–14) | 16 (10–35) | 15 (8–31) | 17 (5–26) | <0.001 |
Data are presented as the mean±SD, median (interquartile range), or n (%). P values were calculated using the universal F test across four groups. UTI, urinary tract infection; ICU, intensive care unit; MELD, model for end stage liver disease; WBC, white blood cell count; Hgb, hemoglobin; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Child–Pugh class and MELD score are at the time of enrollment.
Jonckheere–Terpstra trend test.
Serum sodium <130 mEq/L.
Days from admission until discharge or death.
Three urine samples were collected in 134 participants (71%), two samples in 42 participants (22%), and only one sample was collected in 12 participants (6%). The first sample was collected at a median 2 days (IQR, 1–3) after first meeting AKIN criteria. Median values for biomarkers are shown in Table 3. Sensitivity analysis using raw biomarker values and those corrected for urine creatinine showed minimal variation (data not shown). To facilitate cross-study comparison of results, NGAL, IL-18, KIM-1, and L-FABP are therefore presented as raw values. Log-transformed biomarkers demonstrated moderate correlations between each other (Supplemental Table 2).
Table 3.
Summary statistics for urine biomarkers by progression and mortality
| Marker | No Progression (n=95) | Progression without Death (n=44) | P Value (versus No Progression) | Progression with Death (n=39) | P Value (versus No Progression) | Overall P Valuea |
|---|---|---|---|---|---|---|
| Tubular injury marker | ||||||
| NGAL (ng/ml) | 76 (17–180) | 100 (49–544) | 0.02 | 366 (112–910) | <0.001 | <0.001 |
| IL-18 (pg/ml) | 15 (15–62) | 21 (15–67) | 0.38 | 90 (15–325) | <0.001 | <0.001 |
| KIM-1 (ng/ml) | 5 (1.8–11.7) | 5.8 (2.6–9.5) | 0.96 | 8.3 (4–17.1) | 0.004 | 0.01 |
| L-FABP (ng/ml) | 8 (3–19) | 12 (6–29) | 0.06 | 38 (13–73) | <0.001 | <0.001 |
| Tubular function marker | ||||||
| FENa (%) | 0.32 (0.1–0.89) | 0.15 (0.04–0.59) | 0.02 | 0.31 (0.1–0.91) | 0.97 | 0.04 |
| Glomerular injury marker | ||||||
| Albumin (mg/dl) | 21 (5–73) | 29 (9–164) | 0.23 | 84 (45–233) | <0.001 | <0.001 |
Data are presented as the median (interquartile range). NGAL, neutrophil gelatinase–associated lipocalin; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid–binding protein; FENa, fractional excretion of sodium.
P value from overall ANOVA model.
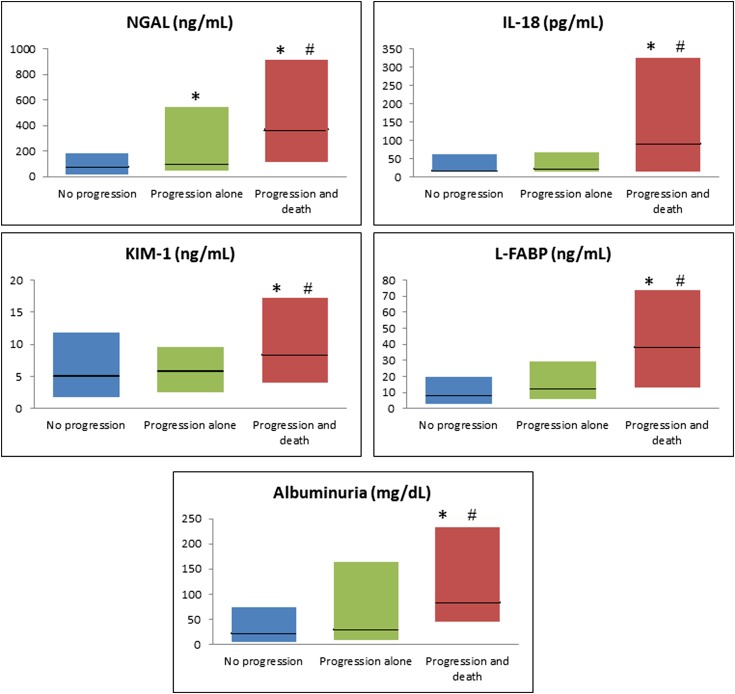
Median values for all biomarkers varied across the three groups. Tubular injury markers were highest in patients with progression and death. Although all tubular injury markers distinguished patients with progression and death from no progression, only NGAL distinguished progression alone from no progression. Microalbuminuria was higher in patients with progression and death than those with progression alone or no progression (84 mg/dl [IQR, 45–233] versus 29 [IQR, 9–164] versus 21 [IQR, 5–73]), respectively, and this distinction persisted when correcting for urinary creatinine. FENa was significantly lower in patients with progression alone but did not differ between those without progression and those with progression and death. In subgroup analysis, similar trends were seen in patients who did not meet IAC criteria for HRS but not in those who did (Supplemental Table 3). The medians and IQRs of injury markers are depicted in Figure 1. The biomarker levels over 3 days of sample collection are shown in Supplemental Figure 1. Median time from sample collection until death for those patients who died was 8 days (IQR, 5–19). There was no significant difference in any biomarkers between the patients who died before (n=26) versus after (n=23) 8 days.
Figure 1.
Biomarker levels for patients with no progression, progression alone, and progression with death. Biomarker values are presented for patients who did not have progression of AKI, those who had progression alone and those with progression and death. Data are presented as box plots with the horizontal black line representing the median and the shaded region representing the interquartile range. Blue bars depict patients without progression (n=95), green bars are patients with progression alone (n=44), and red bars are patients with progression and death (n=35). *Groups in which the biomarker level is statistically higher than in patients without progression; #Groups in which the biomarker level is significantly higher than patients with progression alone. KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid–binding protein; NGAL, neutrophil gelatinase–associated lipocalin.
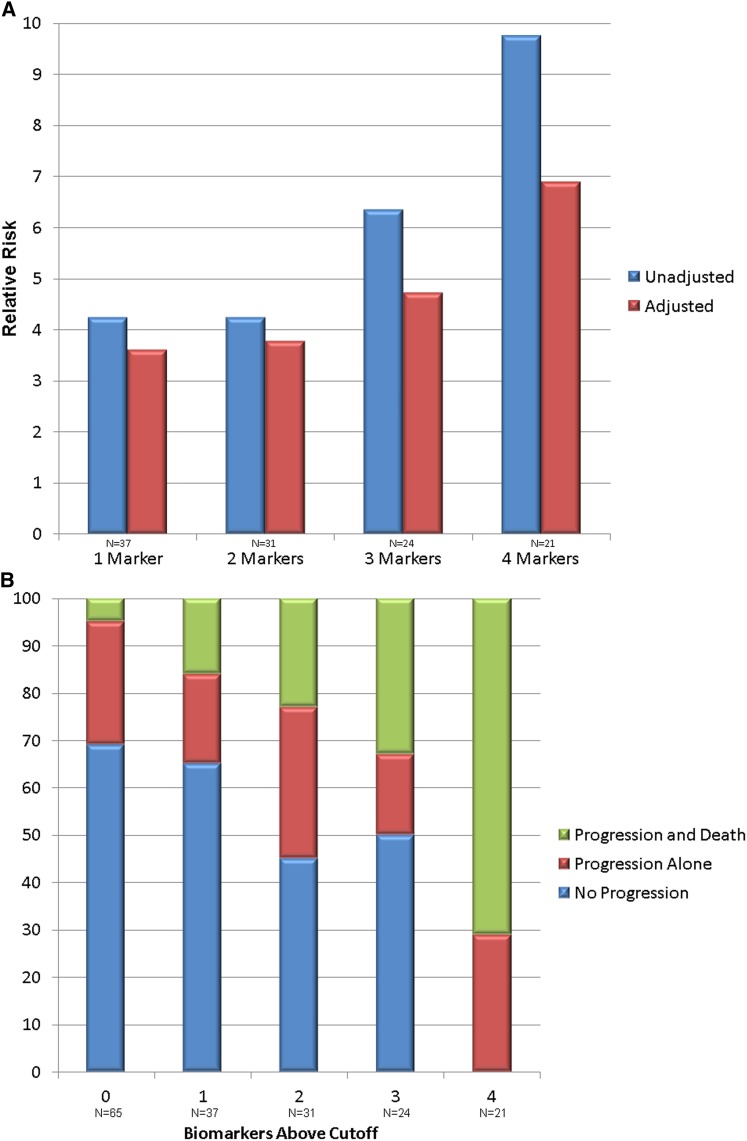
In multivariable analysis, RRs of 4.09 (95% confidence interval [95% CI], 1.56 to 10.70) for IL-18, 3.13 (95% CI, 1.20 to 8.17) for KIM-1, 3.43 (95% CI, 1.54 to 7.64) for L-FABP, and 2.07 (95% CI, 1.05 to 4.10) for albuminuria per log unit were independently associated with AKI progression and death relative to no progression (Table 4). NGAL exhibited a strong trend but did not reach statistical significance (RR, 2.30; 95% CI, 0.94 to 5.60), primarily due to significant collinearity with the MELD score. No biomarkers were independently associated with progression without death. FENa was not associated with the primary outcome on any analysis. Area under the receiver operating curves, optimal cutoffs, sensitivities, and specificities of each biomarker for AKI progression and death are shown in Table 5. The ability of biomarkers to improve risk discrimination as determined by the NRI is presented in Table 6. The four urinary biomarkers with the strongest risk discrimination (NGAL, IL-18, L-FABP, and albumin) were selected, and unadjusted and adjusted RRs for AKI progression and death by number of biomarkers above their optimal diagnostic cutoffs are shown in Figure 2A. Outcomes by number of biomarkers above the cutoff for AKI progression and death are shown in Figure 2B.
Table 4.
Association of biomarkers with the AKI progression and mortality
| Urine Biomarker (Log-Transformed)a | No Progression versus Progression without Death | No Progression versus Progression with Death | ||
|---|---|---|---|---|
| Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI)b | |
| Tubular injury marker | ||||
| NGAL | 2.24 (1.25 to 4.00) | 1.70 (0.82 to 3.54) | 5.18 (2.55 to 10.52) | 2.30 (0.94 to 5.60) |
| IL-18 | 1.33 (0.64 to 2.80) | 1.31 (0.55 to 3.14) | 4.92 (2.40 to 10.09) | 4.09 (1.56 to 10.70) |
| KIM-1 | 1.10 (0.66 to 1.81) | 0.95 (0.52 to 1.72) | 2.98 (1.42 to 6.24) | 3.13 (1.20 to 8.17) |
| L-FABP | 1.71 (0.96 to 3.06) | 1.86 (0.94 to 3.67) | 4.23 (2.20 to 8.15) | 3.43 (1.54 to 7.64) |
| Tubular function marker | ||||
| FENa | 0.59 (0.30 to 1.16) | 0.57 (0.25 to 1.31) | 1.24 (0.65 to 2.35) | 1.25 (0.52 to 2.97) |
| Glomerular injury marker | ||||
| Albumin | 1.26 (0.79 to 1.99) | 1.14 (0.65 to 1.98) | 2.48 (1.48 to 4.17) | 2.07 (1.05 to 4.10) |
RR, relative risk; 95% CI, 95% confidence interval.
Biomarkers are log10 transformed and RRs are per log-unit change.
Adjusted for CKD stage, demographics (race, age, and sex), MELD score, and serum sodium.
Table 5.
Biomarkers risk discrimination for AKI progression and death
| Urine Biomarker | AUC | Cutoff | Sensitivity | Specificity | Positive Likelihood Ratio | Negative Likelihood Ratio |
|---|---|---|---|---|---|---|
| Tubular injury marker | ||||||
| NGAL (ng/ml) | 0.77 (0.68–0.85) | 287 | 0.62 | 0.85 | 4.18 | 0.45 |
| IL-18 (pg/ml) | 0.71 (0.61–0.81) | 55 | 0.64 | 0.75 | 2.54 | 0.48 |
| KIM-1 (ng/ml) | 0.66 (0.56–0.76) | 3.3 | 0.90 | 0.38 | 1.45 | 0.27 |
| L-FABP (ng/ml) | 0.76 (0.66–0.85) | 21 | 0.67 | 0.81 | 3.52 | 0.41 |
| Tubular function marker | ||||||
| FENa (%) | 0.50 (0.39–0.61) | 0.10 | 0.92 | 0.20 | 1.16 | 0.40 |
| Glomerular injury marker | ||||||
| Albumin (mg/dl) | 0.73 (0.64–0.82) | 41 | 0.79 | 0.66 | 2.32 | 0.32 |
AUC, area under the receiver operating curve.
Table 6.
NRIs for biomarkers and AKI progression and death
| Urine Biomarker | Nonevent NRI | Event NRI | Overall NRI (95% CI) |
|---|---|---|---|
| NGAL | 0.09 | 0.14 | 0.23 (−0.12 to 0.58) |
| IL-18 | 0.32 | 0.19 | 0.51 (0.16 to 0.86) |
| KIM-1 | −0.06 | 0.19 | 0.12 (−0.23 to 0.45) |
| L-FABP | 0.28 | 0.03 | 0.31 (−0.04 to 0.66) |
| FENa | −0.01 | −0.03 | −0.04 (−0.41 to 0.33) |
| Albumin | 0.16 | 0.19 | 0.35 (−0.02 to 0.72) |
Clinical model includes CKD stage, demographics (race, age, and sex), MELD score, and serum sodium. NRI, net reclassification index.
Figure 2.
Association between the biomarker panel and AKI progression and death, as well as biomarker elevation and outcomes. (A) Association between the number of biomarkers above their optimal cutoff for AKI progression and death and the unadjusted and adjusted relative risk for this outcome. All values are relative to having no markers over their cutoffs (n=65). Markers used in the panel include NGAL, IL-18, L-FABP, and albumin. The adjusted model is adjusted for CKD stage, demographics (race, age, and sex), MELD score, and serum sodium. The 95% confidence intervals for adjusted relative risks are as follows: one marker, 1.21 to 10.67; two markers, 1.25 to 11.40; three markers, 1.62 to 13.72; and four markers, 2.32 to 20.46. Biomarker cutoffs are as follows: NGAL, 287 ng/ml; IL-18, 55 pg/ml; L-FABP, 21 ng/ml; and albumin, 41 mg/dl. (B) Association between biomarker elevation and outcomes. The percentage of patients without AKI progression or death, progression alone and progression and death by the number of biomarkers of structural injury above their optimal cutoff for prediction of progression and death. Biomarkers included in the panel include NGAL, IL-18, L-FABP, and albumin. MELD, model of end stage liver disease.
Discussion
In patients with the grave combination of cirrhosis and AKI, renal dysfunction is often progressive. We recently showed that AKI progression is associated with >3-fold odds of mortality independent of the MELD score (4). Progression of AKI strongly modifies the association between peak AKI severity and mortality. Patients who initially present with stage 1 AKI and progress to stage 2 have mortality of 29% versus 7% in those who present in stage 2 but do not progress (4). Similarly, those presenting in stage 1 and progressing to stage 3 have mortality of 50% versus 21% in those who present in stage 3 and do not progress. It is therefore critical to know which patients are destined to progress so as to guide prognosis and treatment decisions. Ideally, clinicians would identify patients at highest risk of both progression and death because they would warrant the earliest and most aggressive intervention. Unfortunately, the lack of objective tests to predict AKI progression delays initiation of treatment and hinders clinical trials. The efficacy of treatment for HRS declines with increasing creatinine at treatment initiation (30); it is likely that more accurate identification of patients at high risk for progression of their AKI would allow earlier commencing of therapy and improved outcomes.
Quantitating the degree of injury that the kidney has sustained may allow for more prescient prediction of AKI progression in patients with structural AKI. However, the standard metric of kidney function (serum creatinine) measures changes in filtration but does not directly reflect the degree, if any, of frank structural injury. Biomarkers reflecting tubular injury have been successfully associated with outcomes, including both worsening of AKI and mortality, in several settings including cardiac surgery (15), heart failure (31,32), intensive care unit (ICU) (16), and transplantation (17) settings. Additional data indicate that increased post-AKI albuminuria, generally a hallmark of glomerular injury but also associated with tubular injury, connotes worse prognosis (15).
In this study, there was a clear correlation between urinary injury biomarker levels and outcomes. NGAL, IL-18, KIM-1, L-FABP, and albuminuria were significantly higher in patients with AKI progression and death compared with patients with no progression, and IL-18, KIM-1, L-FABP, and albuminuria were independently associated with this outcome. Critically, this suggests that injury biomarkers may serve to identify patients at highest risk for the worst outcomes who may derive maximal benefit from early and aggressive interventions. Indeed, the likelihood of progression and death was progressively higher with an increasing number of elevated biomarkers. Assessed through the NRI, only IL-18 showed the ability to improve risk stratification for this outcome beyond our clinical model, although L-FABP and albumin demonstrated a strong trend toward such risk reclassification. However, compared with patients without progression, no biomarkers were independently associated with progression without death. In addition, biomarker values were similar in patients with death alone compared with those with progression and death. It is possible that biomarkers overall in the setting of cirrhosis may, with regard to prognosis, best serve as markers of severity of illness rather than predictors of AKI progression alone. As such, their elevation may precede deterioration of patients’ clinical status. Although patients with the worse outcomes had a higher frequency of ICU admissions and requirements for mechanical ventilation and vasopressor therapy, 44 patients (49%) who were admitted to the ICU had biomarkers drawn before ICU admission and 21 patients (43%) started on vasopressors had biomarkers drawn before pressor initiation. Alternatively, biomarkers of structural injury may associate with AKI progression in patients with tubular damage but not in those with a functional disease such as HRS. Because our cohort was analyzed as a whole, such a signal may have been lost.
Importantly, there was no difference in FENa between groups. The median FENa for all three groups was significantly below 1%, reflecting preserved sodium avidity in patients with cirrhosis even after tubular injury. Patients with cirrhosis with AKI suffer a mixture of structural (ATN) and functional (prerenal azotemia and HRS) causes of renal dysfunction. Despite this diverse physiology, the association between injury biomarkers and outcomes is similar to that seen where structural AKI predominates (15–18). There is evidence that some degree of tubular injury may be present even in those patients fulfilling criteria for HRS, albeit of a degree far milder than seen with ATN (21). It is striking then that, along with the negative findings regarding FENa, our results suggest the primacy of structural injury in determining outcomes when generally applied to a cohort composed of patients with cirrhosis and both “functional” and “structural” AKI.
This study has several important strengths. Unlike many studies of AKI and cirrhosis, it is not restricted to ICU patients, improving the generalizability of the findings. The size of this cohort is one of the largest in the literature for this difficult-to-study population. The evaluation of multiple biomarkers is critical in cirrhosis in which AKI is physiologically distinct from other settings such as surgery, sepsis, or the ICU. Finally, the prospective design allowed for robust and complete data collection on multiple critical covariates.
Our study is not without limitations. The cause of AKI was not considered and thus patients likely suffered from a mix of prerenal azotemia, ATN, and HRS. However, accurate adjudication of the cause of AKI must frequently be done retrospectively and thus would not be available to clinicians at the time of biomarker measurement. Potentially divergent associations by the cause of AKI between biomarkers and outcomes would, if anything, be expected to bias our results toward the null. The use of outpatient values for baseline creatinine results in the least misclassification of AKI incidence, severity, and prognosis, but this approach does mean that the exact timeframe for a rise in creatinine is unknown (27). Kidney injury biomarkers in hospitalized patients with cirrhosis without AKI are only minimally above normal ranges (J.M. Belcher, G. Garcia-Tsao, A.J. Sanyal, H. Thiessen-Philbrook, A.J. Peixoto, M.A. Perazella, N. Ansari, J. Lim, S.G. Coca, and C.R. Parikh, unpublished data) and the significantly elevated values even in those patients who do not experience progression or death therefore suggests that AKI is indeed ongoing at hospital admission.
In conclusion, this study confirms that multiple structural biomarkers of kidney injury, but not FENa, are independently associated with progression of AKI and mortality in patients with cirrhosis. Elevated injury markers were seen in patients who ultimately progressed and died, but levels were similar between those without progression and those with progression alone. Further research in a larger cohort is required to validate this finding and to determine whether biomarkers may identify patients with cirrhosis most likely to benefit from disease-specific AKI treatments.
Disclosures
None.
Supplementary Material
Acknowledgments
The biomarker assays for KIM-1 and L-FABP were donated by Seikisui Diagnostics. Neither the granting agency nor Seikisui Diagnostics participated in the protocol development, analysis, or interpretation of results.
The research reported in this article was funded by grants from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (1R21-DK078714 to C.R.P., supported by NIH P-30DK034989) and the George M. O’Brien Kidney Center at Yale (P30-DK079310). J.M.B. was supported by an institutional fellowship training grant from the NIH. C.R.P. is the coinventor on the IL-18 patent (no commercial value) licensed to the University of Colorado.
Drs. Isabel Butrymowicz and Harjit Bhogal are collaborators of the Translational Research Investigating Biomarker Endpoints in AKI Consortium.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09430913/-/DCSupplemental.
References
- 1.Arabi Y, Ahmed QA, Haddad S, Aljumah A, Al-Shimemeri A: Outcome predictors of cirrhosis patients admitted to the intensive care unit. Eur J Gastroenterol Hepatol 16: 333–339, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Cárdenas A, Ginès P, Uriz J, Bessa X, Salmerón JM, Mas A, Ortega R, Calahorra B, De Las Heras D, Bosch J, Arroyo V, Rodés J: Renal failure after upper gastrointestinal bleeding in cirrhosis: Incidence, clinical course, predictive factors, and short-term prognosis. Hepatology 34: 671–676, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Tandon P, Garcia-Tsao G: Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol 9: 260–265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR, TRIBE-AKI Consortium : Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology 57: 753–762, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenq CC, Tsai MH, Tian YC, Lin CY, Yang C, Liu NJ, Lien JM, Chen YC, Fang JT, Chen PC, Yang CW: RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med 33: 1921–1930, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Cholongitas E, Calvaruso V, Senzolo M, Patch D, Shaw S, O’Beirne J, Burroughs AK: RIFLE classification as predictive factor of mortality in patients with cirrhosis admitted to intensive care unit. J Gastroenterol Hepatol 24: 1639–1647, 2009 [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho JR, Villela-Nogueira CA, Luiz RR, Guzzo PL, da Silva Rosa JM, Rocha E, Moraes Coelho HS, de Mello Perez R: Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol 46: e21–e26, 2012 [DOI] [PubMed] [Google Scholar]
- 8.du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P: The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med 31: 1693–1699, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Dobre M, Demirjian S, Sehgal AR, Navaneethan SD: Terlipressin in hepatorenal syndrome: A systematic review and meta-analysis. Int Urol Nephrol 43: 175–184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appenrodt B, Zielinski J, Brensing KA, Heller J, Sauerbruch T, Schepke M: Degree of hepatic dysfunction and improvement of renal function predict survival in patients with HRS type I: A retrospective analysis. Eur J Gastroenterol Hepatol 21: 1428–1432, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Colle I, Durand F, Pessione F, Rassiat E, Bernuau J, Barrière E, Lebrec D, Valla DC, Moreau R: Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with Terlipressin: A retrospective analysis. J Gastroenterol Hepatol 17: 882–888, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH: Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl 16: 440–446, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS: Simultaneous liver-kidney transplantation summit: Current state and future directions. Am J Transplant 12: 2901–2908, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Francoz C, Prié D, Abdelrazek W, Moreau R, Mandot A, Belghiti J, Valla D, Durand F: Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: Impact on the model for end-stage liver disease score. Liver Transpl 16: 1169–1177, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR, TRIBE-AKI Consortium : Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23: 905–914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, Peterson JF, Parikh CR, May AK, Ware LB: Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol 5: 1497–1505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT: Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant 26: 1717–1723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portal AJ, McPhail MJ, Bruce M, Coltart I, Slack A, Sherwood R, Heaton ND, Shawcross D, Wendon JA, Heneghan MA: Neutrophil gelatinase—associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl 16: 1257–1266, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, Pereira G, Rodríguez E, Garcia E, Prado V, Poch E, Jiménez W, Fernández J, Arroyo V, Ginès P: Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 57: 267–273, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Verna EC, Brown RS, Farrand E, Pichardo EM, Forster CS, Sola-Del Valle DA, Adkins SH, Sise ME, Oliver JA, Radhakrishnan J, Barasch JM, Nickolas TL: Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 57: 2362–2370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slack AJ, McPhail MJ, Ostermann M, Bruce M, Sherwood R, Musto R, Dew T, Auzinger G, Bernal W, O’Grady J, Heneghan MA, Moore K, Wendon JA: Predicting the development of acute kidney injury in liver cirrhosis—an analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Aliment Pharmacol Ther 37: 989–997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Shibata M, Hirota M, Nozawa F, Okabe A, Kurimoto M, Ogawa M: Increased concentrations of plasma IL-18 in patients with hepatic dysfunction after hepatectomy. Cytokine 12: 1526–1530, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG, TRIBE-AKI Consortium : Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol 8: 1079–1088, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R, Ronco C, Genyk Y, Arroyo V: Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 60: 702–709, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF: Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 77: 536–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V: Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 56: 1310–1318, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P, Terlipressin Study Group : Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J Hepatol 55: 315–321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH: Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail 16: 49–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR: The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57: 1752–1761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.