The complement system is an essential part of innate immunity acting as a first-line defense against infection and provides an interface between innate and adaptive immunity (1,2). It consists of a network of soluble (fluid phase) and cell membrane proteins (solid phase).
The key step in the complement cascade is the cleavage of C3 to C3a and C3b leading to the formation of a C3 convertase via the classic, lectin, or alternative complement pathways (CP, LP, or AP, respectively). Whereas antigen-antibody complexes and carbohydrate moieties found primarily on the surface of microbial pathogens are needed to activate the CP or LP, respectively, the AP is capable of autoactivation by a mechanism called tick-over of C3. This mechanism occurs spontaneously at a low rate, generating a conformationally changed C3 capable of binding complement factor B (CFB), resulting in the cleavage of CFB by factor D, and generating Ba and Bb. This leads to the formation of the AP C3 convertase (C3bBb), whereas activation via the CP or LP results in formation of CP convertase (C4bC2a).
The C3b fragment, generated by any of the C3 convertases in the presence of factor B and D, associates with C3 convertase, generating even more C3 convertase and resulting in a potent amplification loop. The association of C3b with any of the C3 convertases results in formation of C5 convertase that cleaves C5 into C5a and C5b. C3a and C5a are anaphylatoxins and are among the most powerful effectors of complement activation capable of inducing chemotaxis, cell activation, and inflammatory signaling. C5b subsequently binds to C6, facilitating the binding of C7, C8, and C9, and culminating in the formation of the C5b-9 terminal membrane attack complex on cell surfaces leading to cell lysis.
Activation of the AP occurs in a sequential manner that is tightly regulated in order to restrain unwanted inflammation and self-damage. Several complement-regulatory and complement-inhibitory proteins operate at different levels of the cascade, particularly at the C3 and C5 convertase level, often in a redundant way. These include plasma proteins such as factor H (CFH) and factor I (CFI) and cell-bound and surface regulators such as membrane cofactor protein (CD46) (3,4).
CFI is responsible for the proteolytic inactivation of C3b to iC3b (inactive C3b) and ultimately the C3 breakdown products C3d and C3g, thus irreversibly preventing reassembly of the C3 convertase, whereas CFH accelerates the breakdown of C3 convertase by competing with CFB in binding to C3b and by accelerating the dissociation of the C3bBb convertase complex. On the other hand, surface regulators control C3 convertase via inactivation of C3b deposited on cell surfaces and basement membranes (5).
Genetic mutations in proteins that regulate the assembly and activity of C3 convertase or development of autoantibodies against either fluid phase or surface complement-regulating proteins can result in dysregulation of the AP. For example, mutations in C3 can render the protein resistant to cleavage by C3 convertase or to inactivation by CFH (6). Similarly, antibodies to CFH and CFB, can result in overactivation of AP (7,8). Antibodies to C3 convertase (C3 Nephritic factor; C3Nef) result in stabilization of the convertase and prolong its t1/2 by preventing it from inactivation and degradation by inhibitors, thereby resulting in persistent activation of AP (9,10). Certain genetic polymorphisms are also risk factors for AP dysregulation (11–13).
Whatever the mechanism, dysregulation of the AP results in activated complement products, including C3b and terminal complement factors, that are delivered indiscriminately to all surfaces, including glomeruli, and can be manifested as a thrombotic microangiopathy as in atypical hemolytic uremic (aHUS) syndrome or as a proliferative GN as in C3 glomerulopathy (14). C3 glomerulopathy is defined by the presence of dominant C3 in the absence of significant Ig deposition on immunofluorescence microscopy (15). The condition can be further subclassified as dense deposit disease (DDD) or C3GN based on electron microscopy findings.
Similar triggers, sites, and intensity in dysregulation of the AP have been described in C3GN and DDD. Mass spectrometry studies showed large amounts of C3 and C9 and smaller amounts of C8, C5, C7, and C6 present in both conditions, but they are otherwise indistinguishable (16). So what makes similar pathogenic processes result in C3GN in one patient and DDD in another?
Genetic mutations are not the answer. Mutations are found in a minority of the patients with DDD (16,17) and C3GN (16–18) but the individual frequency of each mutation is low. Moreover, what makes mutations in the same gene areas result in different patients’ phenotypes? For example, in a large series of patients with C3 glomerulopathy, mutations in CFH were described in areas previously identified in patients with aHUS (17). CFH mutation has been documented in a patient who first developed a C3 glomerulopathy and later aHUS (19). Conversely, two children with CFH deficiency and aHUS developed a C3 glomerulopathy after kidney transplantation (20). These data epitomize that the mutations by themselves are not sufficient to determine the phenotype. More likely, genetic mutations/polymorphisms are predisposing factors and additional triggers (e.g., environment, drugs, vaccinations, pregnancy, etc.) are required.
The presence of autoantibodies cannot explain the phenotype either. C3Nefs are found in >80% of patients with DDD and in 40%–50% of patients with C3GN (16,17). However, C3Nef is also found in healthy individuals (21) and in asymptomatic family members of patients with DDD (22), suggesting that the presence of C3Nef alone is not sufficient for development of the disease. Furthermore, a patient with anti-CFH autoantibodies developed a membranoproliferative pattern of injury in the native kidneys that recurred rapidly in the first transplant in the same pattern, but transformed into aHUS in the second transplant (23).
In this issue of CJASN, Zhang et al. sought to answer the puzzle by evaluating the complement biomarker profiles of a cohort of 34 patients with C3 glomerulopathy G (17 with DDD and 17 with C3GN) (24). Compared with normal controls, Zhang et al. found that patients with C3 glomerulopathy had significantly lower levels of C3 and CFB, significantly higher breakdown products (e.g., C3d, Bb, C5a), and increased soluble C5b-9 levels. Whereas C5 and properdin levels were significantly decreased in both DDD and C3GN (properdin levels even lower in C3GN), the breakdown product C5a was increased and C7 levels were significantly decreased only in C3GN. In addition, soluble C5b-9 was more likely to be elevated in C3GN than DDD. Taken together, the authors suggest that it is the degree of dysregulation at the level of C3 and C5 convertase that defines phenotype: greater dysregulation of C3 convertase=DDD, and greater dysregulation of the C5 convertase=C3GN. This is a start. However, there is great overlap in the results between both conditions to be useful in ascertaining individual diagnosis, as demonstrated in Figure 4 by Zhang et al., which depicts the complement biomarker assessment in the cohorts (24). For example, although Bb levels were significantly higher in patients with C3 glomerulopathy than in controls, differences were not statistically different between patients with C3GN versus DDD. Consistent with past observations, genetic analysis was not helpful in differentiating DDD from C3GN because there was no difference in the frequency of CFH and C3 risk alleles between the two groups.
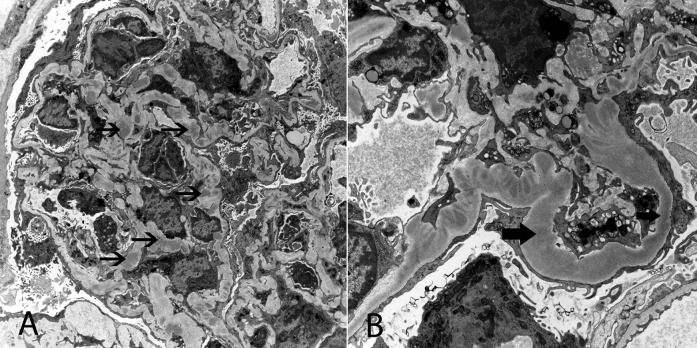
Some questions remain. If continuous activation of the AP is central to the disease and Zhang et al. found an increase in CFB split products (i.e., Ba and Bb), why they did not find similar results for C3a and C3c? Is it due to differences in t1/2 of the markers and their effect on in vitro testing? Why is properdin reduced in C3 glomerulopathy? Is it because of consumption/aggregation to C3 convertase? Considering that properdin is the only positive regulator of the CP acting to stabilize C3 convertase, why are the low levels of properdin not protective? The fact that soluble C5b-9 levels are more likely elevated in C3GN than DDD should lead to studies to determine whether this group may have a better response to C5 blockade with eculizumab. Widespread delivery of breakdown products (e.g., Bb) to mesangial and glomerular basement membrane areas certainly plays a role in the pathogenesis of C3 glomerulopathy, a process that may be worsened by blocking the complement cascade at the C5 levels. Most complement factors behave as an acute phase reactant. Considering that some patients with C3 glomerulopathy are known to have periods of exacerbation interposed by periods in which the urinary sediment appears relatively benign, it would have been helpful to know when the samples were collected during the clinical course of the disease (active versus quiescent). Do complement biomarker levels change with changes in GFR? Detail clinical information (e.g., BP, serum creatinine, proteinuria, hematuria, etc.) could have added to the phenotypic characterization of the patients, but unfortunately this information is not presented. Similarly, a full description of the renal pathology is not available. It is not uncommon to see a renal biopsy showing overlapping features of C3GN and DDD (Figure 1). Small patches of intramembranous dense deposits can be seen in C3GN. Conversely, mesangial and capillary wall deposits can be observed in DDD. This again raises the interesting issue of defining what distinguishes these two entities pathophysiologically.
Figure 1.
Electron microscopy spectrum in C3 glomerulopathy. Electron microscopy of a single glomerulus showing features of C3GN with mesangial and capillary wall electron dense deposits in some capillary loops (A), and features of DDD with dense intramembranous deposits in other capillary loops. (B) Thin black arrow points to discrete mesangial and capillary wall deposits typical of C3GN, and thick arrows points to dense intramembranous deposits typical of DDD. Original magnification, ×4700 in A; ×6800 in B.
Nevertheless, the authors should be congratulated for giving us a “beginning” toward the understanding of the pathologic basis in C3 glomerulopathy. Properly defining the etiologic basis of the pathologic process is essential in order to design rational forms of therapy and to be able to predict response to immune suppression, plasma infusion, complement-inhibitory drugs, or outcome after kidney transplantation.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Defining the Complement Biomarker Profile of C3 Glomerulopathy,” on pages 1876–1882.
References
- 1.Noris M, Remuzzi G: Overview of complement activation and regulation. Semin Nephrol 33: 479–492, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurman JM, Holers VM: The central role of the alternative complement pathway in human disease. J Immunol 176: 1305–1310, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Zipfel PF, Skerka C: Complement regulators and inhibitory proteins. Nat Rev Immunol 9: 729–740, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Ferreira VP, Pangburn MK, Cortés C: Complement control protein factor H: The good, the bad, and the inadequate. Mol Immunol 47: 2187–2197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipfel PF, Smith RJH, Skerka C: Factor I and factor H deficiency in renal diseases: similar defects in the fluid phase have a different outcome at the surface of the glomerular basement membrane. Nephrol Dial Transplant 24: 385–387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, Vazquez-Martul E, Torreira E, Montes T, Tortajada A, Pinto S, Lopez-Trascasa M, Morgan BP, Llorca O, Harris CL, Rodríguez de Córdoba S: Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest 120: 3702–3712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodship TH, Pappworth IY, Toth T, Denton M, Houlberg K, McCormick F, Warland D, Moore I, Hunze EM, Staniforth SJ, Hayes C, Cavalcante DP, Kavanagh D, Strain L, Herbert AP, Schmidt CQ, Barlow PN, Harris CL, Marchbank KJ: Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol Immunol 52: 200–206, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Strobel S, Zimmering M, Papp K, Prechl J, Józsi M: Anti-factor B autoantibody in dense deposit disease. Mol Immunol 47: 1476–1483, 2010 [DOI] [PubMed] [Google Scholar]
- 9.West CD: Nephritic factors predispose to chronic glomerulonephritis. Am J Kidney Dis 24: 956–963, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Williams DG: C3 nephritic factor and mesangiocapillary glomerulonephritis. Pediatr Nephrol 11: 96–98, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ: Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol 6: 1009–1017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Józsi M, Zipfel PF, Hageman GS, Smith RJ: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 43: 582–589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau KK, Smith RJ, Kolbeck PC, Butani L: Dense deposit disease and the factor H H402 allele. Clin Exp Nephrol 12: 228–232, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Fervenza FC: Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS). Semin Thromb Hemost 40: 416–421, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ: C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grünfeld JP, Niaudet P, Lesavre P, Frémeaux-Bacchi V: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Sethi S, Fervenza FC, Zhang Y, Zand L, Meyer NC, Borsa N, Nasr SH, Smith RJ: Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int 83: 293–299, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaziri-Sani F, Holmberg L, Sjöholm AG, Kristoffersson AC, Manea M, Frémeaux-Bacchi V, Fehrman-Ekholm I, Raafat R, Karpman D: Phenotypic expression of factor H mutations in patients with atypical hemolytic uremic syndrome. Kidney Int 69: 981–988, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Boyer O, Noel LH, Balzamo E, Guest G, Biebuyck N, Charbit M, Salomon R, Fremeaux-Bacchi V, Niaudet P: Complement factor H deficiency and posttransplantation. Clin Immunol Immunopathol 57: 10–18, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Spitzer RE, Stitzel AE, Tsokos GC: Production of IgG and IgM autoantibody to the alternative pathway C3 convertase in normal individuals and patients with membranoproliferative glomerulonephritis. Clin Immunol Immunopathol 57: 10–18, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Licht C, Heinen S, Józsi M, Löschmann I, Saunders RE, Perkins SJ, Waldherr R, Skerka C, Kirschfink M, Hoppe B, Zipfel PF: Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int 70: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lorcy N, Rioux-Leclercq N, Lombard ML, Le Pogamp P, Vigneau C: Three kidneys, two diseases, one antibody? Nephrol Dial Transplant 26: 3811–3813, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Nester CM, Martin B, Skjoedt MO, Meyer NC, Shao D, Borsa N, Palarasah Y, Smith RJH: Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol 9: 1876–1882, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]