Abstract
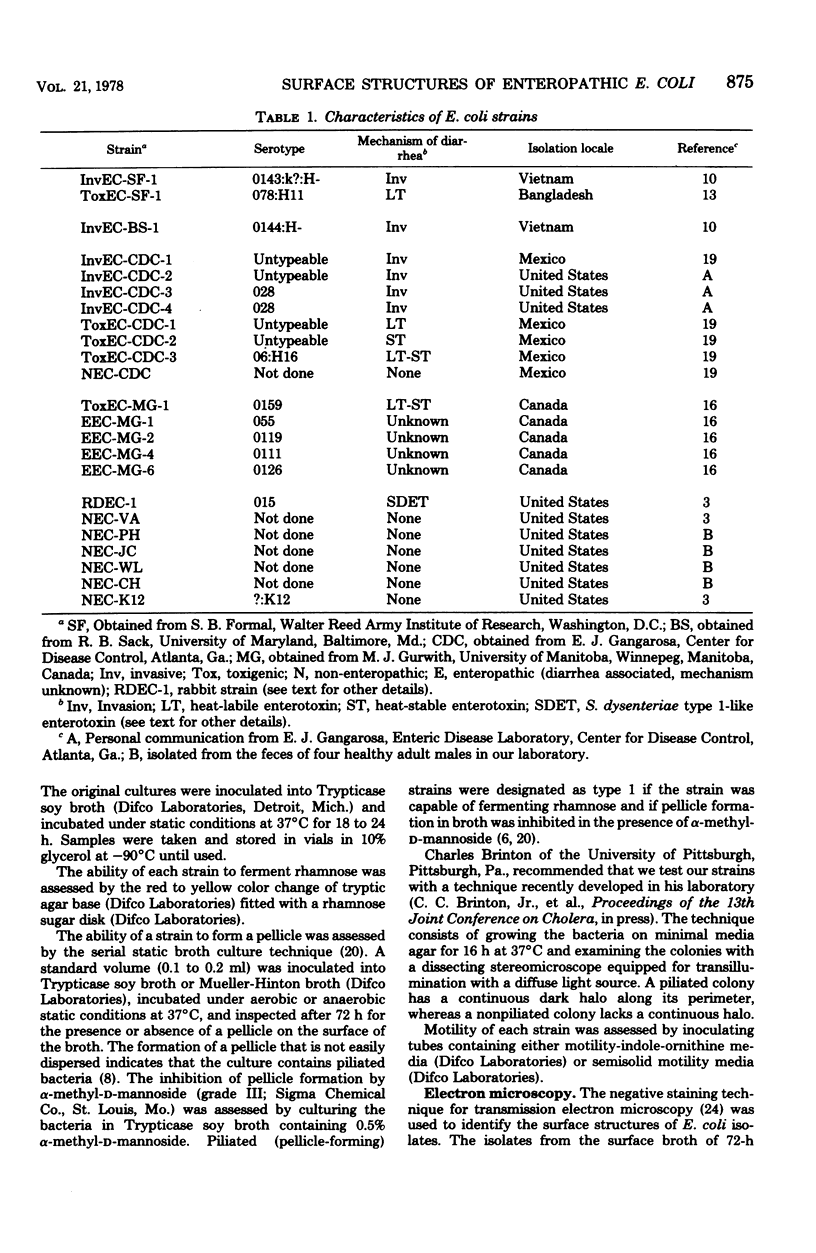
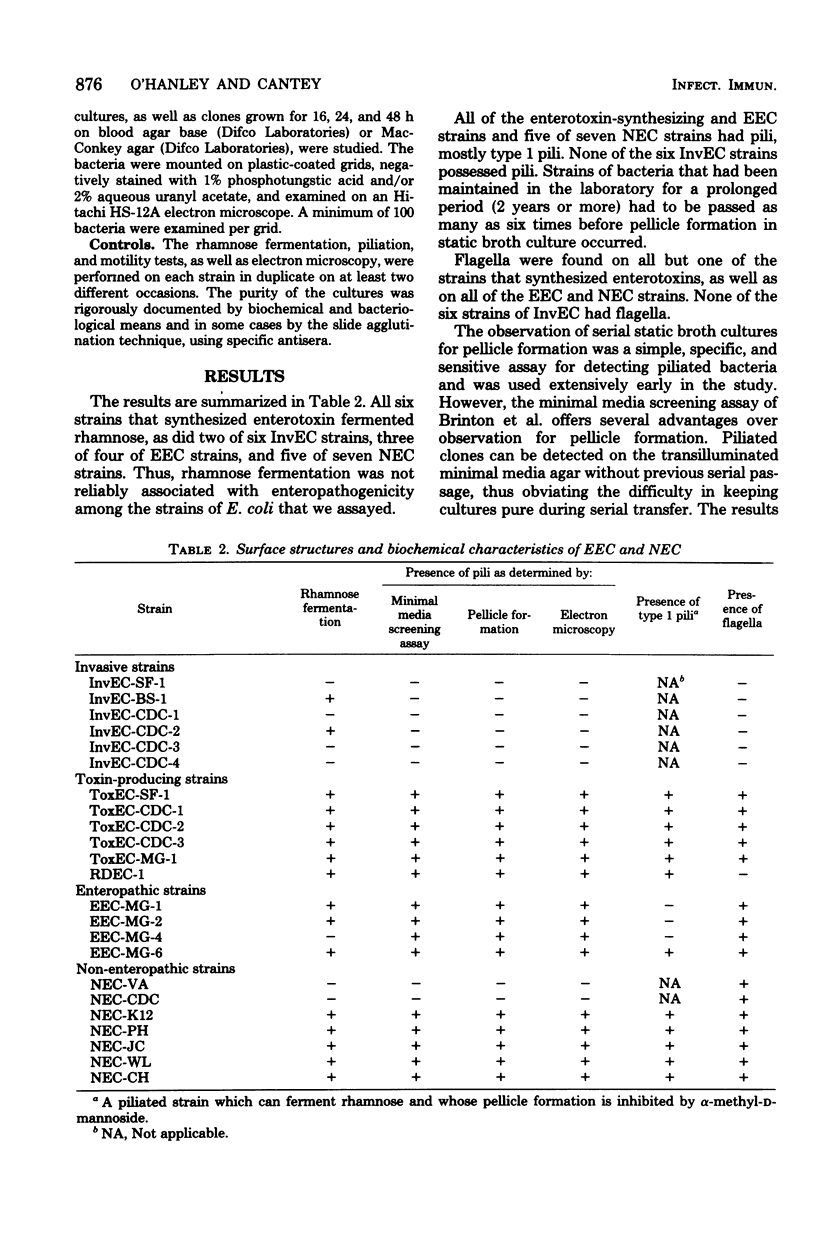
Strains of Escherichia coli, mostly of human origin, were obtained from several different investigators who had isolated them from patients with diarrhea from many different parts of the world. The mechanisms by which these E. coli were thought to have caused diarrhea included: (i) synthesis of labile, stable, or Shigella dysenteriae-like enterotoxins; (ii) invasion of the intestinal mucosa; and (iii) unknown. Each strain was carefully examined for pili or flagella to correlate the presence or absence of such surface structures with a particular mechanism of diarrhea. The presence of pili was determined by colony morphology on minimal media, pellicle formation in static broth culture, and transmission electron microscopy. The pili were categorized as type 1 if the bacteria fermented rhamnose and if pellicle formation was inhibited by α-methyl-d-mannoside. The presence of flagella was confirmed in motility media and by transmission electron microscopy. Six invasive E. coli strains lacked pili and flagella. Ten E. coli strains that synthesized enterotoxins or produced diarrhea by an unknown mechanism were piliated (seven with type 1 pili), and all but one had flagella. Pili and flagella seem to be associated with strains of E. coli that produce diarrhea by enterotoxin synthesis or unknown mechanisms. Strains that produce diarrhea by mucosal invasion lack both types of surface structures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allweiss B., Dostal J., Carey K. E., Edwards T. F., Freter R. The role of chemotaxis in the ecology of bacterial pathogens of mucosal surfaces. Nature. 1977 Mar 31;266(5601):448–450. doi: 10.1038/266448a0. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., O'Hanley P. D., Blake R. K. A rabbit model of diarrhea due to invasive Escherichia coli. J Infect Dis. 1977 Nov;136(5):640–648. doi: 10.1093/infdis/136.5.640. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- DuPont H. L., Formal S. B., Hornick R. B., Snyder M. J., Libonati J. P., Sheahan D. G., LaBrec E. H., Kalas J. P. Pathogenesis of Escherichia coli diarrhea. N Engl J Med. 1971 Jul 1;285(1):1–9. doi: 10.1056/NEJM197107012850101. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Darekar M. R., Wheater D. W. Fimbriae and infectivity in Salmonella typhimurium. J Med Microbiol. 1976 Nov;9(4):459–473. doi: 10.1099/00222615-9-4-459. [DOI] [PubMed] [Google Scholar]
- Duguid J. P. The function of bacterial fimbriae. Arch Immunol Ther Exp (Warsz) 1968;16(2):173–188. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. S., DuPont H. L. Detection and characterization of colonization factor of enterotoxigenic Escherichia coli isolated from adults with diarrhea. Infect Immun. 1978 Feb;19(2):727–736. doi: 10.1128/iai.19.2.727-736.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Sep;8(3):322–328. doi: 10.1128/iai.8.3.322-328.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstl F., Semenitz E. Zur Morphologie der Geisseln und Fimbrien von Escherichia-Stämmen. Zentralbl Bakteriol Orig A. 1975 Jun;232(1):48–54. [PubMed] [Google Scholar]
- Guentzel M. N., Field L. H., Eubanks E. R., Berry L. J. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect Immun. 1977 Feb;15(2):539–548. doi: 10.1128/iai.15.2.539-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwith M. J., Wiseman D. A., Chow P. Clinical and laboratory assessment of the pathogenicity of serotyped enteropathogenic Escherichia coli. J Infect Dis. 1977 May;135(5):735–743. [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai G. J., Burrows W. The titration of cholera toxin and antitoxin in the rabbit ileal loop. J Infect Dis. 1966 Dec;116(5):606–614. doi: 10.1093/infdis/116.5.606. [DOI] [PubMed] [Google Scholar]
- Merson M. H., Morris G. K., Sack D. A., Wells J. G., Feeley J. C., Sack R. B., Creech W. B., Kapikian A. Z., Gangarosa E. J. Travelers' diarrhea in Mexico. A prospective study of physicians and family members attending a congress. N Engl J Med. 1976 Jun 10;294(24):1299–1305. doi: 10.1056/NEJM197606102942401. [DOI] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNLEY M. J., HORNE R. W. Electron microscope observations on the structure of fimbriae, with particular reference to Klebsiella strains, by the use of the negative staining technique. J Gen Microbiol. 1962 Apr;28:51–56. doi: 10.1099/00221287-28-1-51. [DOI] [PubMed] [Google Scholar]


