Abstract
The obesity epidemic has prompted researchers to find effective weight loss and maintenance tools. Weight loss and subsequent maintenance are reliant on energy balance; the net difference between energy intake and energy expenditure. Negative energy balance, lower intake than expenditure, results in weight loss whereas positive energy balance, greater intake than expenditure, results in weight gain. Resistant starch has many attributes which could promote weight loss and/or maintenance including reduced prostprandial insulinemia, increased release of gut satiety peptides, increased fat oxidation, lower fat storage in adipocytes, and preservation of lean body mass. Retention of lean body mass during weight loss or maintenance would prevent the decrease in basal metabolic rate and, therefore, the decrease in total energy expenditure, that occurs with weight loss. In addition, the fiber-like properties of resistant starch may increase the thermic effect of food thereby increasing total energy expenditure. Due its ability to increase fat oxidation and reduce fat storage in adipocytes, resistant starch has recently been promoted in the popular press as a “weight loss wonder food”. This review focuses on data describing the effects of resistant starch on body weight, energy intake, energy expenditure, and body composition to determine if there is sufficient evidence to warrant these claims.
Keywords: resistant starch, energy balance, energy intake, energy expenditure, body composition, adiposity, lean body mass, weight loss
INTRODUCTION
The ongoing global obesity epidemic has focused researchers on finding novel ways to prevent weight gain or reduce body weight. This goal is imperative as obesity is associated with co-morbidities such as diabetes, cardiovascular disease, and cancer, which are among the greatest causes of death in the Western world. Conversely, weight loss can ameliorate the impact of these co-morbidities (Horton 2009), indicating that weight management is key for the prevention and/or treatment of these diseases.
Basic weight loss theory has always advocated “eat less energy than you burn” which is a proven and effective strategy. Weight maintenance can only be achieved through energy balance, that is, an equivalent amount of energy intake and energy expenditure (EE; (Westerterp 2010). However, weight loss results in a new, lower body weight that is difficult to maintain over time (Teixeira, Going et al. 2004), partly due to cessation of lifestyle changes, but also due to physiological changes that occur post-weight loss which increase hunger and decrease EE, facilitating rapid and efficient weight regain (MacLean, Higgins et al. 2004; MacLean, Higgins et al. 2006). Therefore, it is important to focus on novel and sustainable ways to prevent or minimize this ‘energy gap’ (higher energy intake and lower EE).
Addition of resistant starch (RS) to the diet may offer such a solution. RS may increase EE via effects on the thermic effect of food. RS reduces the caloric density of food due to its indigestibility and has been shown to decrease postprandial glycemia/insulinemia, improve insulin sensitivity, alter secretion and/or expression of gut satiety peptides, incretins, and adipokines, prevent fat deposition in adipocytes, and possibly increase satiety (Higgins 2004). Effects which should, in conjunction, decrease energy ingestion via increased satiety signaling (ghrelin, leptin, insulin, adiponectin, GLP-1, PYY, GIP) and lower caloric intake due to energy dilution of the diet. These effects have been hyped by the popular press, who have labeled resistant starch a “weight loss wonder food”. This review focuses on data describing the effects of RS on body weight, energy intake, EE, and body composition to determine if there is sufficient evidence to warrant these claims.
Resistant Starch
Resistant starch is any starch or starch digestion products that are not digested and absorbed in the upper digestive tract and, so, pass to the large bowel (Asp, Bjorck et al. 1987). Here, RS is a good substrate for fermentation which increases short chain fatty acid concentrations and lowers bowel pH. There are four major categories of RS: RS1 is physically inaccessible to digestive enzymes due to the presence of seed coats, germ, etc. (eg. whole grains); RS2 is inaccessible to enzymes due to starch conformation (eg. high amylose maize starch which is comprised primarily of α-1,4 glycosidic links); RS3 is retrograded starch (eg. cooked then cooled pasta or rice); and RS4 encompasses starches that are chemically modified to be resistant to digestion (Englyst, Liu et al. 2007). Several studies have investigated the effect of RS on energy balance and body weight. Almost all studies compared RS2 from high amylose maize starch with digestible starch (DS) which, for the purposes of this review, is defined as rapidly digestible amylopectin starch. Those that use other forms of RS or control starches will be pointed out.
Energy Expenditure
The key to body weight regulation is the balance between energy intake, or food consumption, and Total EE (TEE). TEE has three components; 1) basal metabolic rate (BMR), 2) the thermic effect of food (TEF; also referred to as diet-induced thermogenesis), and 3) physical activity thermogenesis (Figure 1; see (Ravussin and Bogardus 1992) for a review). BMR is the amount of energy required to sustain basic metabolic functions under fasting conditions. BMR is positively influenced by lean body mass (LBM) and declines with increasing age. TEF is the energy consumed to absorb, break-down, and oxidize or store ingested nutrients. TEF is dependent on meal size and composition. Different forms of fiber have small, discreet effects on TEE via TEF (Elia and Cummings 2007, Smith, Brown et al. 1998). As RS has many properties of insoluble fiber, it would be reasonable to assume that RS may also have a discreet effect on TEE. Physical activity thermogenesis comprises the energy required for activities of daily living (walking to your car, housework, etc) plus planned physical activity (running, gym classes, etc). Regular exercise acts to maintain or increase LBM which can contribute to an overall increase in TEE due its effects on BMR.
Figure 1. Components of Total Energy Expenditure (TEE).
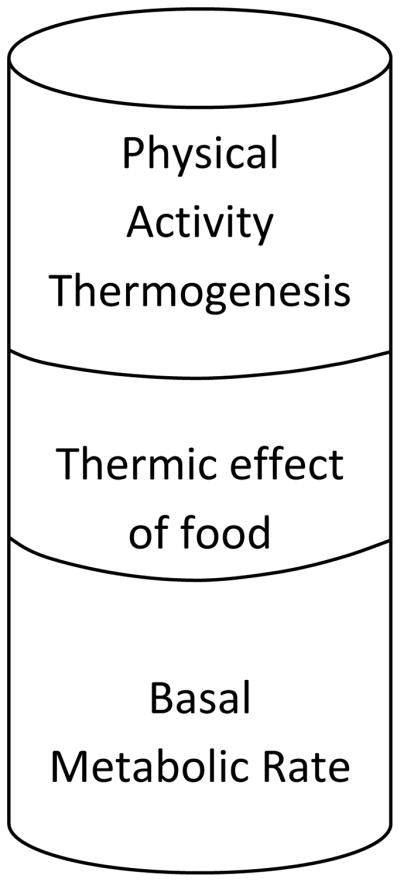
To increase TEE, one of the three components must be directly increased. Basal metabolic rate is associated with fat free mass and age. Thermic effect of food is reliant upon the amount of food ingested and the macronutrient composition of the diet. Therefore, the most predictable way to increase TEE is to increase physical activity.
A) Rodent Data
Body Weight
Studies in rats and mice show that chronic RS2 feeding does not generally influence body weight, food intake, TEE, or TEF although there are some caveats to these data that will be discussed below. An acute rat study comparing RS2, soluble rye fiber, apple pectin, and DS found no difference in TEE between any of the starches tested (Aust, Dongowski et al. 2001). In chronic studies, all but two show that RS had no effect on total body weight (Table 1). The exceptions to the rule are: 1) a study in which the test diets were highly obesogenic (high sugar and fat content) implying that RS aides weight maintenance under obesogenic conditions, and 2) a study in which five weeks on RS2 from mung bean starch significantly decreased the body weight of both healthy and diabetic rats despite ingestion of the same amount of diet in grams, implying that RS increases TEE (Lerer-Metzger, Rizkalla et al. 1996). However, the formulation of this diet did not provide equivalent caloric density between the DS and RS diets as they were based on direct starch replacement. DS starch provides 4 kCal/g whereas the RS would yield around 2.5 kCal/g. Thus, the RS diet had a lower caloric density than the DS diet and consumption of the same volume of diet would equate to lower caloric intake in the RS group which could explain the body weight differences in this study.
Table 1.
Summary of data from chronic rodent studies investigating the effects of RS on body weight and composition (chronological order). All data relative to animals fed DS. A blank cell indicates that this data was not collected.
| RS effect on: | Duration (weeks) | Body Weight | Energy Intake | TEE | RQ | Body Fat/ Fat Pad Wt | LBM | Adipocyte Size | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Lerer-Metzger et al (Lerer-Metzger, Rizkalla et al. 1996) | 5 | ↓ | = (*) | = | ↓ | ||||
|
| |||||||||
| Kabir et al (Kabir, Rizkalla et al. 1998) | 3 | = | = (*) | = | ↓ | ||||
|
| |||||||||
| Shen et al (Shen, Keenan et al. 2009) | 9 | = / = disemb | = | ↓ by 8.3% | |||||
|
| |||||||||
| So et al (So, Yu et al. 2007) | 8 | = | ↓ | ↓ by 33% | ↓ | ||||
|
| |||||||||
| Zhou et al (Zhou, Martin et al. 2008) | |||||||||
| Healthy mice |
|
16 | = / ↓ disemb | ↑ | = | ↓ | ↓ by 24% RS2 ↓ by 36% RS3 |
||
| Obese mice | 16 | = | ↑ | = | ↓ | = | |||
|
| |||||||||
| Pawlak et al (Pawlak, Bryson et al. 2001) | |||||||||
| Expt 1, healthy rats | 18 | = | Controlled | ↓ by 41% | ↑ | ||||
| Expt 2, obese mice | 9 | = | ↓ by 45% | ↑ | |||||
|
| |||||||||
| Pawlak (Pawlak, Kushner et al. 2004) | 9 | = | ↓ by 22% | ||||||
|
| |||||||||
| Scribner et al ((Scribner, Pawlak et al. 2008) | 40 | = | = | = | ↓ | ↓ by 40% | |||
|
| |||||||||
| Coate & Huggins ((Coate and Huggins)2010) | 16 | = | = (*) | ↓ by 24% on LFD not HFD | |||||
|
| |||||||||
| Fukushima et al ((Fukushima, Ohashi et al. 2001)) | 4 | = | = (◆) | ||||||
|
| |||||||||
| Shimotoyodome et al (Shimotoyodome, Suzuki et al.) 2010) | |||||||||
| Experiment 1 | 24 | = (#^) | = (#^) | = (^) ↓ (#) | |||||
| Experiment 2 | 24 | ↓ (^) | = (^) | ↓ (^) | |||||
| Experiment 3 | 0.75 | ↑ (#) | ↓(#) | ||||||
|
| |||||||||
| Higgins et al (Higgins, Jackman et al. 2011) | 9 | ↓ | ↓ for 2 weeks then = | = | ↓ by 20% | ↑ 10% | ↓ | ||
LBM, fat free mass (i.e. lean body mass)
disemb, disemboweled body weights
RQ, respiratory quotient. Lower RQ represents higher fat oxidation.
RS4 effects relative to RS2 NOT DS (TEE and RQ only reported for RS2 vs RS4 not for DS)
RS4 effects relative to DS (RS2 not reported in experiment 2)
Food intake was estimated in grams without taking into consideration the energy of the diets (RS had lower energy density).
RS3 vs DS
All other chronic rodent studies showed no change in total body weight in response to RS feeding. However, this data may be misleading as RS feeding increases the total contents of the bowel, increases the thickness of the lumen, and significantly increases the mass of the microbiome. Thus, total body weight which includes bowel weight may overestimate total body weight in response to RS feeding. Two studies have observed both total body weight and disemboweled body weight with one study showing no difference in disemboweled body weight (Shen, Keenan et al. 2009) whereas the other showed that RS significantly decreased disemboweled body weight relative to a DS diet (Zhou, Martin et al. 2008).
Energy Intake
Given data showing no change in body weight in response to RS feeding, it is reasonable to assume that RS does not change energy intake or TEE. Disregarding studies that measured food consumption by weight without taking into account the energy density of the diet (Kabir, Rizkalla et al. 1998; Kabir, Rizkalla et al. 1998), (Lerer-Metzger, Rizkalla et al. 1996), seven studies examined food intake in response to chronic RS feeding. Four studies showed no change in energy intake, one study showed increased energy intake (Zhou, Martin et al. 2008) whereas two showed decreased energy intake in response to a RS diet (So, Yu et al. 2007; Higgins, Jackman et al. 2011; Table 1). Three of the four studies that show no difference in energy intake between RS and DS rats fed diets of equivalent energy density and rats ate the same volume of food regardless of diet (Scribner, Pawlak et al. 2008, Shen, Keenan et al. 2009, Shimotoyodome, Suzuki et al., 2010). It is notable that the study that observed a lower energy intake in response to RS provided diets, in which the energy density was lower for the RS than the DS diet (So, Yu et al. 2007). In this study, RS animals ate significantly more food but, despite this, energy intake was lower due to the lower energy density of the diet. So, this increase in food intake may be a biological adaptation to the lower caloric density of the diet. In support of this notion, studies that showed equivalent energy intake had diet formulations which were of equal energy density.
These data expose an experimental factor which could influence study outcome measures; diet energy density. To this reviewer’s mind, optimal experimental design would be a single experiment that employs a DS, RS, and DS energy matched (DS-EM; matched to the energy density of RS) diets. One study did just this and found that disemboweled body weight and body fat percentage was lower for the RS2 and RS3 groups relative to DS but not for RS2 vs DS-EM (Zhou, Martin et al. 2008). These data suggest that the effects of RS2 on body weight and adiposity could be due to its capacity to reduce the energy density of the diet to the same extent as the DS-EM diet whereas RS3 has discreet effects that are unrelated to the energy density of the diet.
TEE
There are many fewer studies available that directly measure TEE in response to RS in rodents (Table 1). Three studies found that RS intake did not affect TEE (Scribner, Pawlak et al. 2008, Zhou, Martin et al. 2008, Higgins, Jackman et al. 2011) whereas another found that RS4 increased TEE relative to RS2 (Shimotoyodome, Suzuki et al. 2010). The Shimotoyodome study (2010), however, did not directly measure the REE of any control rats on DS diet so this data is difficult to interpret. In addition, this study was extremely convoluted with up to eight groups of rats per experiment in a total of four experiments. Data reported in the Results and Discussion sections did not always compare RS4 with its direct DS control group which further compounds the difficulty in interpreting this data. Regardless, available data suggests that acute RS4 feeding may increase TEE (Shimotoyodome, Suzuki et al. 2010) but this effect is lost over time as the gut microbiome adapts to RS intake (Scribner, Pawlak et al. 2008), (Zhou, Martin et al. 2008), (Higgins, Jackman et al. 2011). So far, these data are equivocal and it is clear that more data needs to be gathered to draw any conclusion regarding the effects of RS on TEE in rodents.
B) Human Data
Body Weight
Human data regarding body weight and energy intake in response to RS are analogous to data in rodent models. Four to 12 weeks of RS feeding had no effect on total body weight in healthy or insulin resistant subjects (de Roos, Heijnen et al. 1995, Johnston, Thomas et al. 2010, Robertson, Bickerton et al. 2005; Table 2). Given that studies in rodents, over a period of 3 to 40 weeks, showed no difference in body weight in response to RS feeding, it is hardly surprising that no difference can be detected in healthy humans who have a relatively protracted life-span. In addition, healthy humans are those who effectively regulate their body weight over time thereby remaining lean. So, in a free-living situation where RS is substituted for DS as part of, or a supplement to, the habitual diet, one would expect healthy individuals to make subtle adjustments in energy balance and remain weight stable. These adjustments are likely too small to measure in a free-living environment. In this regard, experiments aimed at investigating the effects of diet on body weight might observe greater differences in obese individuals who do not effectively maintain body weight over time. Finally, the caveat discussed for rodent data regarding the weight of total bowel contents being significantly greater in response to RS feeding than DS feeding also holds true for the human data. That is, that total body weight measurements overestimate the weight of RS-fed mammals due to the difference in bowel and bowel content weights.
Table 2.
Summary of data from human studies investigating the effects of RS on body weight. All data relative to a DS meal. A blank cell indicates that this data was not collected.
| RS effect on: | Population | Duration | Energy Intake | Body weight | FM | LBM |
|---|---|---|---|---|---|---|
| De Roos et al (de Roos, Heijnen et al. 1995) | Healthy M | 4 weeks | = (#) | = (#) | ||
| Robertson et al (Robertson, Bickerton et al. 2005) | Healthy M & F | 4 weeks | = | = | = | ↑ |
| Johnson et al (Johnston, Thomas et al.) | Insulin resistant M&F | 12 weeks | = | = |
M, males
F, females
RS2 and RS3 relative to DS
Energy Intake
Table 2 demonstrates that little human data is available regarding the effect on long-term RS consumption on total energy intake. The available data would suggest that, similar to rats, RS intake does not change total energy intake in humans relative to a DS diet. This conclusion is supported by acute human studies, which show that RS causes no change in subjective satiety scores or total energy intake at an ad libitum meal and/or over 24 hours (Table 3). Although RS does not impact energy intake relative to DS, studies have shown that rapidly absorbed carbohydrates (glucose, sucrose, maltodextrin) lower the total amount of food eaten compared with RS ingestion (Anderson, Catherine et al. 2002, Anderson, Cho et al. 2010). This concurs with some rodent data that suggest that food intake is increased to compensate for the diluted energy density of a high RS diet (Zhou, Martin et al. 2008). Human data from RS studies (Keogh, Lau et al. 2007) and other nutritional interventions (Zaveri and Drummond 2009) add credence to the idea that both rats and humans might increase total food intake to compensate for a diet of lower energy density.
Table 3.
Summary of data from acute human studies investigating the effects of RS on energy intake. All data relative to a DS meal*.
| RS effect on*: | Population | Energy Intake | VAS |
|---|---|---|---|
| De Roos et al (de Roos, Heijnen et al. 1995) | Healthy M | = (#) | ↓ for RS2 |
| Bodinham et al (Bodinham, Frost et al.) | Healthy M | ↓ | = |
| Keogh et al (Keogh, Lau et al. 2007) | Healthy F | ↑ | = |
| Anderson et al (Anderson, Catherine et al. 2002) | Healthy M | = | = |
| Anderson et al (Anderson, Cho et al.) | Healthy M | = | = |
For consistency with all other sections in this chapter, data presented here compare RS directly to DS and may therefore differ from conclusions in the published manuscripts that compare RS to a simple carbohydrate, for example.
VAS, visual analog scale measurement of subjective hunger
M, males
F, females
RS2 and RS3 relative to glucose
It is interesting to note that subjective visual analog scale (VAS) ratings of hunger and satiety did not correlate with objective measurement of energy intake in three of the five studies examined (Table 3). This is an important caveat to keep in mind when reviewing all satiety literature and implies that energy intake is influenced by factors other than an individual’s feeling of hunger. It is also important to remember that, in the context of weight management, only energy intake will have an impact on body weight.
TEE
Analogous to rodent data, human studies indicate that RS ingestion has no effect on TEE or TEF in comparison to DS consumption (de Roos, Heijnen et al. 1995, Anderson, Catherine et al. 2002, Anderson, Cho et al. 2010; Table 4). There is an important caveat to this data: it is possible that RS could change TEE via fermentation BUT almost all of the acute studies were too short to capture this effect. In healthy adults, fermentation of RS starts about 6–8 hours following meal ingestion (Sands, Leidy et al. 2009) but all acute studies measured TEE over 5 hours or less. The kinetics of TEE for RS vs DS ingestion are very different. In response to DS, EE peaks 30 minutes post-meal consumption whereas this peak is shifted to the right, at 90 minutes, for RS ingestion (Sands, Leidy et al. 2009). So, it seems important to assess TEE for RS and other indigestible carbohydrates over a more protracted time span in order to glean accurate data regarding the effects of ingestion during the fermentation period.
Table 4.
Summary of data from human studies investigating the effects of RS on TEE. All data relative to a DS meal. A blank cell indicates that this data was not collected.
| RS effect on: | Population | Duration (hours) | Energy Intake | TEF | TEE | RQ |
|---|---|---|---|---|---|---|
| Sands et al ((Sands, Leidy et al. 2009)) | Healthy M & F | 4 | Fixed: 50g available CHO | = | ||
| Ranganthan et al ((Ranganathan 1994)) | Healthy M | 3 RQ=12 |
Fixed: 30g total CHO | = (^) | = (^) | = (^) |
| Keogh et al ((Keogh, Lau et al. 2007)) | Healthy F | 3 | Fixed: SR, MM @ breakfast & lunch | = | = | |
| Tagliabue et al ((Tagliabue, Raben et al. 1995)) | Healthy M | 5 | Fixed:50g total CHO | = | ↓ | ↓ |
| Heijnen et al ((Heijnen, Deurenberg et al. 1995)) | Healthy M | 5 | Fixed: 50g total CHO | ↓ | ||
| Higgins et al ((Higgins, Higbee et al. 2004)) | Healthy M & F | 24 | Fixed: SR, MM | = | ↓ | |
| Howe et al ((Howe, Rumpler et al. 1996)) | Healthy & hyperinsulinemic M | 14 weeks 24h TEE | Fixed: SR, MM 14 weeks, 1g CHO/kg body weight | = | = |
M, males
F, females
CHO, carbohydrate
SR, starch replacement
MM, mixed meal
RS4 effects relative to cellulose (fiber)
RS2 and RS3 relative to DS
Two studies have shown that RS may cause a slight decrease in TEE and TEF (Heijnen, Deurenberg et al. 1995, Tagliabue, Raben et al. 1995)). Heijnen et al (1995) estimated that consumption of 27g of RS per day would decrease TEE by 0.7%. Although this may seem trivial, small changes in TEE over a protracted period of time without compensation in energy intake can profoundly impact weight management. However, average RS intake in the USA is 3–8g per day (Murphy, Douglass et al. 2008) which would not be enough to produce physiologically relevant changes in TEE. In support of this notion and the five studies that showed no difference in TEE in response to RS ingestion, high dose (30g/day) chronic RS feeding/supplement studies have found no change in body weight in response to RS (Johnston, Thomas et al. 2010, Robertson, Bickerton et al. 2005; Table 2). Thus, it is unlikely that RS has any biologically relevant effect on total body weight, TEE, or TEF in healthy humans. However, these parameters have not been examined in obese individuals and such studies must be conducted before any conclusions can be drawn regarding the role of RS consumption as part of a calorie–restricted weight loss or weight maintenance diet. Indeed, the metabolic responses to over- or under-feeding can differ markedly from those during energy balance. Howe et al conducted an overfeeding study requiring healthy men to eat 125% of their daily energy requirements (Howe, Rumpler et al. 1996). TEE was unaffected by increased energy intake or RS content of the diet whereas carbohydrate oxidation was markedly increased and fat oxidation significantly decreased under overfeeding conditions. Protein oxidation was higher in response to DS with overfeeding but did not change from energy balance conditions in response to RS ingestion. Therefore, it is important to study the effects of RS in the context of underfeeding (i.e. caloric restriction) and overfeeding in obese individuals so that the impact of RS in weight loss and weight maintenance can be evaluated. Currently, there is no available data that would inform conclusions of any kind regarding RS and weight loss in obese individuals.
C) Body Composition
Despite numerous observations in rodents and humans describing no change in body weight, energy intake, or TEE in response to RS ingestion, almost every rodent study that has measured body composition finds lower fat mass (FM) and/or higher LBM with RS ingestion than DS ingestion (Shen, Keenan et al. 2009, So, Yu et al. 2007, Zhou, Martin et al. 2008, Pawlak, Bryson et al. 2001, Pawlak, Kushner et al. 2004, Scribner, Pawlak et al. 2008, Coate and Huggins 2010, Shimotoyodome 2010, Suzuki et al. 2010; Table 1). These data have been scrutinized and replicated many times in different experimental paradigms lending credence to the idea that RS causes changes in metabolic flux that act to increase fat oxidation with a concomitant decrease in carbohydrate and protein oxidation. Thus, RS could increase protein accretion (LBM) and reduce the amount of fat available for net storage without changing TEE. In addition, it has been shown that high fiber diets, such as a RS diet, cause lower total metabolizable energy than predicted/measured in vitro due to decreased in vivo digestibility of non-starch polysaccharides, carbohydrate, and fat (Behall and Howe 1995). Thus, there may be less net carbohydrate and fat available for storage in response to a RS diet.
Rodent studies have observed an 8 – 45% reduction in total body fat percentage in response to RS versus DS feeding (Shen, Keenan et al. 2009, So, Yu et al. 2007, Zhou, Martin et al. 2008, Pawlak, Bryson et al. 2001, Pawlak, Kushner et al. 2004, Scribner, Pawlak et al. 2008, (Coate and Huggins 2010, Shimotoyodome, Suzuki et al. 2010; RS4 vs RS2 for (Shimotoyodome, Suzuki et al. 2010). Of note is the fact that the adiposity of visceral fat depots is also decreased by RS consumption. This is a significant finding as visceral fat rather than subcutaneous fat depots seem to be metabolically more harmful and are strongly associated with dyslipidemia, obesity, diabetes, and cardiovascular disease.
These observations regarding decreased adiposity in response to RS ingestion are supported by studies employing radioactive tracers. In humans, RS ingestion at a single meal caused a decrease in the respiratory quotient (RQ), indicative of higher whole body fat oxidation (Higgins, Higbee et al. 2004, Tagliabue, Raben et al. 1995), and simultaneous radioactive tracer administration showed that meal fat oxidation was increased relative to DS ingestion (Higgins, Higbee et al. 2004). During this period of increased fat oxidation, there was a concomitant decrease in carbohydrate oxidation (Tagliabue, Raben et al. 1995). As high fiber diets have been shown to decrease total metabolizable energy due to decreased in vivo digestibility of non-starch polysccharides, carbohydrate, and fat (Behall and Howe 1995), and carbohydrate availability drives its oxidation, a reduction in carbohydrate digestibility/availability could contribute to this increase in fat oxidation observed with RS consumption. However, only two human studies have examined the effects of these metabolic changes on body composition in response to chronic RS ingestion and these found that RS had no effect on total FM (Johnston, Thomas et al. 2010, Robertson, Bickerton et al. 2005)) but increased LBM (Robertson, Bickerton et al. 2005). Further long-term studies are necessary in humans to obtain accurate information regarding the effects of RS on body composition.
In rats, three experiments show that RS feeding caused a decrease in RQ, indicative of higher whole body fat oxidation, with no change in TEE (Zhou, Martin et al. 2008, Scribner, Pawlak et al. 2008, Shimotoyodome, Suzuki et al. 2010). Radioactive tracer studies have shown that RS feeding caused a significant reduction of total lipogenesis in adipocytes but not liver or muscles and had no effect on glycogenesis (Kabir, Rizkalla et al. 1998). Thus, there is sufficient evidence in both humans and rats to suggest that, relative to DS ingestion, RS promotes fat oxidation, decreases carbohydrate oxidation, and prevents fat accumulation specifically in adipocytes.
D) RS Has Adipocyte-Specific Effects
The adipocyte-specificity of changes in lipid metabolism in response to RS feeding might lay in the effects of RS on gene expression and enzyme activity. RS acts to decrease fatty acid synthase (FAS) activity and GLUT4 expression in adipocytes but not in liver (Kabir, Rizkalla et al. 1998). Decreased GLUT4 expression selectively in adipocytes in response to RS ingestion would reduce glucose uptake and availability for storage whereas lower FAS activity would decrease total lipogenesis (lipid synthesis from all carbon sources) in adipocytes. It is important to note that these effects are adipocyte-specific which could account for the smaller adopicyte size in RS-fed animals noted in several rodent studies (Kabir, Rizkalla et al. 1998; Kabir, Rizkalla et al. 1998, So, Yu et al. 2007, Higgins, Jackman et al 2011).
Insulin hypersecretion in the absence of insulin resistance may also contribute to increased fat deposition in DS-fed rats relative RS-fed rats. Studies have demonstrated a marked increase in insulin secretion in DS-fed rats over time whereas RS feeding maintains juvenile levels of insulin secretion for up to 26 weeks (Byrnes, Miller et al. 1995, Higgins, Proctor et al. 1999) with no difference in whole body insulin sensitivity between the DS and RS groups (Pawlak, Bryson et al. 2001). Insulin-sensitive tissues, in response to high insulin concentrations, exhibit markedly blunted fat oxidation and increased glucose and lipid storage.
There is an abundance of evidence from rodents and some supporting human data to conclude that RS consumption reduces adiposity and maintains LBM without any effect on TEE. Thus, one could speculate that RS consumption may not increase the efficacy of a calorie-restricted weight loss diet in which reduction of food intake and/or increased TEE are necessary. However, given RSs effects to decrease adiposity and maintain LBM, it could be a beneficial part of a weight maintenance diet. Weight maintenance requires energy balance, in contrast to a weight loss diet which requires negative energy balance. RS does not promote negative energy balance but, by maintaining LBM, it could prevent the decrease in BMR that is observed with weight loss and prevent weight regain by reducing adiposity in energy balance conditions. The observation that RS increases protein retention during overfeeding (Howe, Rumpler et al. 1996, Higgins, Jackman et al 2011) also indicates that RS could have positive effects on body composition. All of these issues need to be directly addressed by conducting well-designed studies in obese individuals during periods of underfeeding, overfeeding, and energy balance before any meaningful conclusions can be drawn.
E) Role of Fermentation and the Gut Microbiome
Zhou et al (Zhou, Martin et al. 2008) showed that RS improved glucose tolerance and decreased adiposity in healthy but not obese mice. In addition, cecal pH was lower in response to RS feeding in healthy but not obese mice suggesting that obese mice suffer from an impairment in fermentation. It was suggested that fermentation of RS was necessary to observe its effects on body composition. In humans, the glucose response to a standardized breakfast following an evening meal containing RS or DS showed that postprandial glycemia was inversely correlated with fermentation (Nilsson, Ostman et al. 2008). That is, fermentation caused lower postprandial glycemia at a subsequent meal. As circulating insulin concentrations have been implicated in fat deposition in DS-fed animals, higher fermentation of RS, resulting in lower glycemia/insuliemia, may be one mechanism that causes lower adiposity in RS-fed animals.
There is accumulating evidence to suggest that the composition of the gut microbiota plays a crucial role in the absorption and storage of ingested energy. In pigs, after 17 weeks of high fiber feeding, lean animals showed a 13% increase in total fecal bacterial counts whereas obese animals exhibited a 37% decline (Varel, Pond et al. 1982). In addition, distribution of different bacterial phyla and genera seem to be influenced by diet composition. A high fat diet in rats causes higher Lactobacillus and lower Bacteroidetes numbers than a low fat diet (Mozes, Bujnakova et al. 2008). This data taken together with the data showing that RS causes decreased adiposity in rats fed a low fat diet but not a high fat diet (Coate and Huggins 2010) indicates that the high fat diet is changing the gut microbiota in such a way that RS fermentation is decreased and its effects on whole body adiposity are lost as a result.
RS feeding in mice increased indigenous bifidobacteria number which increased the population of Lactobacillus and Bacteroides and decreased the number of coliforms in the colon, increased butyrate production (Wang, Brown et al. 2002, Maathuis, Hoffman et al. 2009 for RS3), and decreased pH (Fukushima, Ohashi et al. 2001). Thus, there is direct evidence that RS feeding can influence the gut microbiota and increase fermentation. This is important as the composition of the gut microbiome has been implicated in the development of obesity in rodents and humans. Obese mice have significantly less Bacteroidetes and more Furmicutes than lean controls which is unrelated to food consumption (Ley, Backhed et al. 2005) but shows the same trends as a high fat diet in rats (Mozes, Bujnakova et al. 2008). In addition, transplantation of obese gut microbiota, containing genes encoding proteins responsible for digestion of indigestible starch thus facilitating the salvage of extra energy from the diet, to clean mice caused the salvage of more energy from the diet and greater adiposity than the microbiota from lean mice (Turnbaugh, Ley et al. 2006). Conversely, germ-free mice with sterile guts are protected from diet induced obesity (Backhed, Manchester et al. 2007) and mice reared in sterile conditions have much lower adiposity than conventionally reared mice with gut microbiome intact, despite equivalent energy intake between the groups (Turnbaugh, Backhed et al. 2008). In humans, obesity is also associated with decreased bacterial diversity which changes the distribution of bacterial genes and metabolic pathways (Turnbaugh, Hamady et al. 2009).
Taken together, these data provide convincing evidence that the gut microbiome can play a crucial role in the absorption and utilization of dietary nutrients as well as exhorting a strong influence the development of obesity. As RS can change the microbiome of the gut, it is reasonable to assume that RS consumption could influence the development of obesity and the success of weight loss/maintenance attempts.
CONCLUSIONS
Five important caveats regarding data interpretation were discussed:
Energy intake may be dependent on the energy density of the diet; future studies should consider this during the experimental design process.
Higher total bowel content, lumen thickness, and mass of the microbiome in response to RS ingestion can cause overestimation of total body weight.
Data in humans is from healthy adults who are able to effectively regulate body weight. It is likely that different effects would be observed in obese subjects.
Acute human studies have been too short to observe any effect of RS fermentation on TEE.
Visual analog scale estimation of hunger may not correlate well with food intake so data from subjective measurements should be interpreted carefully.
It is apparent that ingestion of RS, relative to DS, has no effect on body weight in healthy rodents, no effect on energy intake, although this seems to be dependent on the energy density of the diet, and the effects on TEE are equivocal and require further investigation. However, there is strong evidence demonstrating that RS lowers whole body and visceral adiposity. The magnitude of these changes in adiposity are very large and sufficient to independently improve insulin sensitivity, and reduce the risk of diabetes, CVD, and certain cancers.
Human data corresponds well with that from rats. RS, in comparison to DS, does not seem to have any impact on body weight, although studies in humans would need to be of longer duration in order to observe any such effect, has no effect on energy intake or TEE, and increases fat oxidation. There is a scarcity of data regarding the effect of RS on fat mass and LBM in humans. There is data from mice and humans to demonstrate that RS changes the microbiota in the gut which has been shown to influence energy absorption and the development of obesity.
There is some scant evidence that the metabolic changes that occur in response to RS ingestion may not occur in obese rodents. Clearly, further studies need to be conducted in obese humans and rodent models before any conclusions can be drawn regarding the usefulness of RS in this population. A plethora of metabolic adaptations occur in obesity and in the weight reduced state, including changes to the microbiota that influence energy absorption from the diet and obesity, and it is vital to determine if RS has any biologically relevant effects under these conditions. The observation that RS increases protein retention during overfeeding indicates that RS could have positive effects on body composition and, therefore, on BMR. Clearly, there is evidence that RS has effects, such as increased fat oxidation and reduced fat storage in adipocytes, that imply that it would be a useful weight loss and/or maintenance tool. However, there is no direct data showing that RS has any impact on body weight, energy intake, or energy expenditure. Therefore, it is necessary to conduct well-designed studies, of sufficient duration, in obese individuals during periods of underfeeding, overfeeding, and energy balance before any meaningful conclusions can be drawn.
Acknowledgments
Sources of Support: Supported by NIH/NCRR Colorado CTSI Grant UL1 RR025780 and NIH/NIDDK DK-038088. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
The sole author had responsibility for all parts of this manuscript and has no conflicts of interest to declare.
References
- Anderson GH, Catherine NL, et al. Inverse association between the effect of carbohydrates on blood glucose and subsequent short-term food intake in young men. Am J Clin Nutr. 2002;76(5):1023–1030. doi: 10.1093/ajcn/76.5.1023. [DOI] [PubMed] [Google Scholar]
- Anderson GH, Cho CE, et al. Relation between estimates of cornstarch digestibility by the Englyst in vitro method and glycemic response, subjective appetite, and short-term food intake in young men. Am J Clin Nutr. 2010;91(4):932–939. doi: 10.3945/ajcn.2009.28443. [DOI] [PubMed] [Google Scholar]
- Asp NG, Bjorck I, et al. Enzyme resistant starch fractions and dietary fibre. Scandinavian Journal of Gastroenterology - Supplement. 1987;129:29–32. doi: 10.3109/00365528709095847. [DOI] [PubMed] [Google Scholar]
- Aust L, Dongowski G, et al. Estimation of available energy of dietary fibres by indirect calorimetry in rats. Eur J Nutr. 2001;40(1):23–29. doi: 10.1007/pl00007382. [DOI] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behall KM, Howe JC. Effect of long-term consumption of amylose vs amylopectin starch on metabolic variables in human subjects. American Journal of Clinical Nutrition. 1995;61(2):334–340. doi: 10.1093/ajcn/61.2.334. [DOI] [PubMed] [Google Scholar]
- Bodinham CL, Frost GS, et al. Acute ingestion of resistant starch reduces food intake in healthy adults. Br J Nutr. 1036:917–922. doi: 10.1017/S0007114509992534. [DOI] [PubMed] [Google Scholar]
- Byrnes SE, Miller JC, et al. Amylopectin starch promotes the development of insulin resistance in rats. Journal of Nutrition. 1995;125(6):1430–1437. doi: 10.1093/jn/125.6.1430. [DOI] [PubMed] [Google Scholar]
- Coate KC, Huggins KW. Consumption of a high glycemic index diet increases abdominal adiposity but does not influence adipose tissue pro-oxidant and antioxidant gene expression in C57BL/6 mice. Nutr Res. 2010;30(2):141–150. doi: 10.1016/j.nutres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- de Roos N, Heijnen ML, et al. Resistant starch has little effect on appetite, food intake and insulin secretion of healthy young men. European Journal of Clinical Nutrition. 1995;49(7):532–541. [PubMed] [Google Scholar]
- Elia M, Cummings JH. Physiological aspects of energy metabolism and gastrointestinal effects of carbohydrates. Eur J Clin Nutr. 2007;61(Suppl 1):S40–74. doi: 10.1038/sj.ejcn.1602938. [DOI] [PubMed] [Google Scholar]
- Englyst KN, Liu S, et al. Nutritional characterization and measurement of dietary carbohydrates. Eur J Clin Nutr. 2007;61(Suppl 1):S19–39. doi: 10.1038/sj.ejcn.1602937. [DOI] [PubMed] [Google Scholar]
- Fukushima M, Ohashi T, et al. Low density lipoprotein receptor mRNA in rat liver is affected by resistant starch of beans. Lipids. 2001;36:129–134. doi: 10.1007/s11745-001-0698-4. [DOI] [PubMed] [Google Scholar]
- Heijnen ML, Deurenberg P, et al. Replacement of digestible by resistant starch lowers diet-induced thermogenesis in healthy men. British Journal of Nutrition. 1995;73(3):423–432. doi: 10.1079/bjn19950044. [DOI] [PubMed] [Google Scholar]
- Higgins J. Resistant starch: metabolic effects and potential health benefits. J AOAC Int. 2004;87(3):761–768. [PubMed] [Google Scholar]
- Higgins J, Higbee D, et al. Resistant starch consumption promotes lipid oxidation. Nutr Metab. 2004;1:8. doi: 10.1186/1743-7075-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Proctor D, et al. Aging changes tissue-specific glucose metabolism in rats. Metab. 1999;48:1445–1449. doi: 10.1016/s0026-0495(99)90157-9. [DOI] [PubMed] [Google Scholar]
- Higgins JA, Jackman MR, et al. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutr Metab (Lond) 2011;8:49. doi: 10.1186/1743-7075-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton ES. Effects of lifestyle changes to reduce risks of diabetes and associated cardiovascular risks: results from large scale efficacy trials. Obesity (Silver Spring) 2009;17(Suppl 3):S43–48. doi: 10.1038/oby.2009.388. [DOI] [PubMed] [Google Scholar]
- Howe JC, Rumpler WV, et al. Dietary starch composition and level of energy intake alter nutrient oxidation in “c” arbohydrate-sensitive” men. J Nutr. 1996;126(9):2120–2129. doi: 10.1093/jn/126.9.2120. [DOI] [PubMed] [Google Scholar]
- Johnston KL, Thomas EL, et al. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med. 2010;27(4):391–397. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- Kabir M, Rizkalla SW, et al. Dietary amylose-amylopectin starch content affects glucose and lipid metabolism in adipocytes of normal and diabetic rats. Journal of Nutrition. 1998;128(1):35–43. doi: 10.1093/jn/128.1.35. [DOI] [PubMed] [Google Scholar]
- Kabir M, Rizkalla SW, et al. A high glycemic index starch diet affects lipid storage-related enzymes in normal and to a lesser extent in diabetic rats. Journal of Nutrition. 1998;128(11):1878–1883. doi: 10.1093/jn/128.11.1878. [DOI] [PubMed] [Google Scholar]
- Keogh JB, Lau CW, et al. Effects of meals with high soluble fibre, high amylose barley variant on glucose, insulin, satiety and thermic effect of food in healthy lean women. Eur J Clin Nutr. 2007;61(5):597–604. doi: 10.1038/sj.ejcn.1602564. [DOI] [PubMed] [Google Scholar]
- Lerer-Metzger M, Rizkalla SW, et al. Effects of long-term low-glycaemic index starchy food on plasma glucose and lipid concentrations and adipose tissue cellularity in normal and diabetic rats. British Journal of Nutrition. 1996;75(5):723–732. doi: 10.1079/bjn19960176. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis A, Hoffman A, et al. The effect of the undigested fraction of maize products on the activity and composition of the microbiota determined in a dynamic in vitro model of the human proximal large intestine. J Am Coll Nutr. 2009;28(6):657–666. doi: 10.1080/07315724.2009.10719798. [DOI] [PubMed] [Google Scholar]
- MacLean PS, Higgins JA, et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006;290(6):R1577–1588. doi: 10.1152/ajpregu.00810.2005. [DOI] [PubMed] [Google Scholar]
- MacLean PS, Higgins JA, et al. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R288–R297. doi: 10.1152/ajpregu.00010.2004. [DOI] [PubMed] [Google Scholar]
- Mozes S, Bujnakova D, et al. Developmental changes of gut microflora and enzyme activity in rat pups exposed to fat-rich diet. Obesity (Silver Spring) 2008;16(12):2610–2615. doi: 10.1038/oby.2008.435. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Douglass JS, et al. Resistant starch intakes in the United States. J Am Diet Assoc. 2008;108(1):67–78. doi: 10.1016/j.jada.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Nilsson AC, Ostman EM, et al. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr. 2008;138(4):732–739. doi: 10.1093/jn/138.4.732. [DOI] [PubMed] [Google Scholar]
- Pawlak DB, Bryson JM, et al. High glycemic index starch promotes hypersecretion of insulin and higher body fat in rats without affecting insulin sensitivity. J Nutr. 2001;131(1):99–104. doi: 10.1093/jn/131.1.99. [DOI] [PubMed] [Google Scholar]
- Pawlak DB, Kushner JA, et al. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364(9436):778–785. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- Ranganathan S. Comparative study of the acute effects of resistant starch and dietary fibers on metabolic indexes in men. American Journal of Clinical Nutrition. 1994;59(4):879–883. doi: 10.1093/ajcn/59.4.879. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Bogardus C. A brief overview of human energy metabolism and its relationship to essential obesity. Am J Clin Nutr. 1992;55(1 Suppl):242S–245S. doi: 10.1093/ajcn/55.1.242s. [DOI] [PubMed] [Google Scholar]
- Robertson MD, Bickerton AS, et al. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. American Journal of Clinical Nutrition. 2005;82(3):559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- Sands AL, Leidy HJ, et al. Consumption of the slow-digesting waxy maize starch leads to blunted plasma glucose and insulin response but does not influence energy expenditure or appetite in humans. Nutr Res. 2009;29(6):383–390. doi: 10.1016/j.nutres.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner KB, Pawlak DB, et al. Long-term effects of dietary glycemic index on adiposity, energy metabolism, and physical activity in mice. Am J Physiol Endocrinol Metab. 2008;295(5):E1126–1131. doi: 10.1152/ajpendo.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Keenan MJ, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17(1):40–45. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotoyodome A, Suzuki J, et al. RS4-type resistant starch prevents high-fat diet-induced obesity via increased hepatic fatty acid oxidation and decreased postprandial GIP in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2010;298(3):E652–662. doi: 10.1152/ajpendo.00468.2009. [DOI] [PubMed] [Google Scholar]
- Smith T, Brown JC, et al. Energy balance and thermogenesis in rats consuming nonstarch polysaccharides of various fermentabilities. Am J Clin Nutr. 1998;68(4):802–819. doi: 10.1093/ajcn/68.4.802. [DOI] [PubMed] [Google Scholar]
- So PW, Yu WS, et al. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS One. 2007;2(12):e1309. doi: 10.1371/journal.pone.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A, Raben A, et al. The effect of raw potato starch on energy expenditure and substrate oxidation. Am J Clin Nutr. 1995;61(5):1070–1075. doi: 10.1093/ajcn/61.4.1070. [DOI] [PubMed] [Google Scholar]
- Teixeira PJ, Going SB, et al. Pretreatment predictors of attrition and successful weight management in women. Int J Obes Relat Metab Disord. 2004;28(9):1124–1133. doi: 10.1038/sj.ijo.0802727. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Varel VH, Pond WG, et al. Influence of high-fiber diet on bacterial populations in gastrointestinal tracts of obese- and lean-genotype pigs. Appl Environ Microbiol. 1982;44(1):107–112. doi: 10.1128/aem.44.1.107-112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, I, Brown L, et al. Manipulation of colonic bacteria and volatile fatty acid production by dietary high amylose maize (amylomaize) starch granules. J Appl Microbiol. 2002;93(3):390–397. doi: 10.1046/j.1365-2672.2002.01704.x. [DOI] [PubMed] [Google Scholar]
- Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev. 2010;68(3):148–154. doi: 10.1111/j.1753-4887.2010.00270.x. [DOI] [PubMed] [Google Scholar]
- Zaveri S, Drummond S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J Hum Nutr Diet. 2009;22(5):461–468. doi: 10.1111/j.1365-277X.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Martin RJ, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295(5):E1160–1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


