Abstract
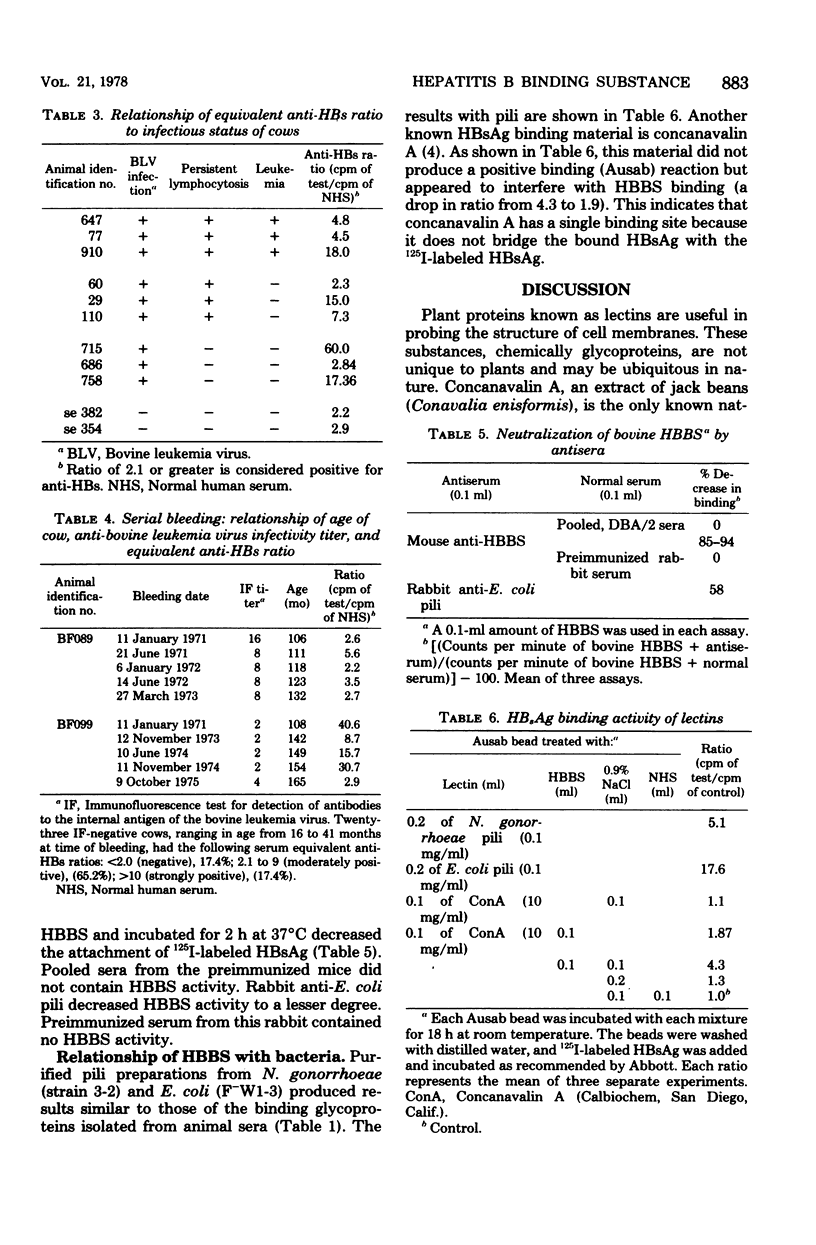
Sera from certain animal species contain a substance(s) which binds hepatitis B surface antigen. The hepatitis B binding substance found in animals is not antibody, but appears to be a glycoprotein which reacted with antigen-coated beads and produced a "false positive" test for antibody. This glycoprotein could be selectively and quantitatively removed by reaction with purified hepatitis B surface antigen and centrifugation. Pili fractions isolated from Neisseria gonorrhoeae and Escherichia coli bound to hepatitis B surface antigen and produced false positive anti-hepatitis B surface antigen reactions. Mouse anti-bovine hepatitis B binding substance and rabbit anti-E. coli pili were capable of neutralizing bovine hepatitis B binding substance.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTRAM U., HALBERG P. A SPECIFIC ANTIBODY AGAINST THE EPITHELIUM OF THE SALIVARY DUCTS IN SERA FROM PATIENTS WITH SJOEGREN'S SYNDROME. Acta Allergol. 1964;19:458–466. doi: 10.1111/j.1398-9995.1964.tb03284.x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- CURTAIN C. C., PYE J. A mucoprotein from bovine submaxillary glands with restricted inhibitory action against influenza virus haemagglutination. Aust J Exp Biol Med Sci. 1955 Jun;33(3):315–322. doi: 10.1038/icb.1955.32. [DOI] [PubMed] [Google Scholar]
- Cawley L. P. Reaction between concanavalin A and the Australia antigen. Am J Clin Pathol. 1972 Feb;57(2):253–253. doi: 10.1093/ajcp/57.2.253. [DOI] [PubMed] [Google Scholar]
- Eckert E. A., Lanni F., Beard D., Beard J. W. Effect of Swine Influenza Virus on the Viscosity of the Egg-white Inhibitor of Hemagglutination. Science. 1949 May 6;109(2836):463–464. doi: 10.1126/science.109.2836.463. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Carbohydrate residue of a urine mucoprotein inhibiting influenza virus haemagglutination. Nature. 1952 Oct 18;170(4329):662–663. doi: 10.1038/170662a0. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A., MURPHY W. H. Studies on mucoproteins. IV. The linkage of the prosthetic group to the protein core in ovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1961 Jan 1;46:81–90. doi: 10.1016/0006-3002(61)90648-5. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. The structure of the prosthetic group of bovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1957 Jun;24(3):649–650. doi: 10.1016/0006-3002(57)90267-6. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO Y., HASHIMOTO S., PIGMAN W. PURIFICATION AND PROPERTIES OF PORCINE SUBMAXILLARY MUCIN. Arch Biochem Biophys. 1964 Feb;104:282–291. doi: 10.1016/s0003-9861(64)80015-1. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING J. S., Jr, FIELDEN M. L., GOODMAN H. O., BOYCE W. H. Total nondialyzable solids in human urine. X. Isolation and characterization of nonultrafiltrable material with blood-group substance activity. Arch Biochem Biophys. 1961 Nov;95:310–315. doi: 10.1016/0003-9861(61)90151-5. [DOI] [PubMed] [Google Scholar]
- McCREA J. F. Studies on influenza virus receptor-substance and receptor-substance analogues. I. Preparation and properties of a homogeneous mucoid from the salivary gland of sheep. Biochem J. 1953 Aug;55(1):132–138. doi: 10.1042/bj0550132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950 May;74(1):106–108. [PubMed] [Google Scholar]
- Weng L. K., Bayer M. E., London W. T. Interaction of hepatitis B surface antigen (Australia antigen) with membrane vesicles of Pseudomonas aeruginosa. Infect Immun. 1975 Jul;12(1):180–186. doi: 10.1128/iai.12.1.180-186.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


