Abstract
In humans, changing from upright to supine elicits an approximately 10 mmHg increase in cephalic venous pressure caused by the hydrostatic column effect, but episcleral venous pressure (EVP) and intraocular pressure (IOP) rise by only a few mmHg. The dissociation of the small increases in IOP and EVP compared to the larger increase in cephalic venous pressure suggests a regulatory mechanism controlling EVP. The aim of the present study was to determine if the rabbit model is suitable to study the effects of postural changes on EVP despite its short hydrostatic column. In anesthetized rabbits (n= 43), we measured arterial pressure (AP), IOP, and orbital venous pressure (OVP) by direct cannulation; carotid blood flow (BFcar) by transit time ultrasound, heart rate (HR) by digital cardiotachometer, and EVP with a servo null micropressure system. The goal of the protocol was to obtain measurement of supine EVP for ≈ 10 minutes, followed by ≈ 10 minutes of EVP measurement with the rabbit in a head down tilt. The data were analyzed by paired t-tests and the results reported as the mean ± standard error of the mean. In a separate group of animals (n= 35), aqueous flow was measured by fluorophotometry. This protocol entailed measurement of aqueous flow in the supine position for ≈ 60 minutes, followed by ≈ 60 minutes of aqueous flow measurement with the rabbit in a head down tilt. From supine to head down tilt, AP and BFcar were unchanged, IOP increased by 2.3 ± 0.4 mmHg (p< 0.001), EVP increased by 2.4 ± 0.4 mmH (p< 0.001), OVP increased by 2.5 ± 0.2 mmHg (p< 0.001) and HR decreased by 9 ± 3 bpm (p= 0.002). Head down tilt caused no significant change in aqueous flow. Although the hydrostatic column in the rabbit is shorter than humans, the rabbit model permits sufficiently sensitive measurements of the pressures and systemic parameters likely involved in the EVP responses to posture change. The present results indicate directionally similar EVP and IOP responses to tilt as occur in humans and, as in humans, the responses are smaller than would be expected from the change in the hydrostatic column height. Also, as in humans, the model reveals no change in aqueous flow during head down tilt. We conclude the rabbit model is appropriate for studying the mechanisms responsible for the relative immunity of EVP and IOP to posture change.
Keywords: rabbit, episcleral venous pressure, intraocular pressure, blood pressure, posture
Introduction
A potential recurring challenge to IOP homeostasis is posture change with the consequent gravity-dependent changes in cephalic arterial and venous pressures (Krieglstein et al., 1978; Iwabuchi et al., 1983; Bill, 1984; Iwabuchi et al., 1986; Carlson et al., 1987; Gisolf et al., 2004). In humans in the upright position, blood flowing from the heart to the eye and back experiences the force of gravity such that the cephalic arterial and venous pressures are 10 - 15 mmHg lower than in the supine position (Bill, 1984), yet the IOP increase in going from upright to supine is only a few mmHg, much smaller than the changes in cephalic vascular pressures (Krieglstein et al., 1978; Bill, 1984; Carlson et al., 1987).
The modest IOP response to posture change is surprising given the current understanding of steady state IOP as codified in the Goldmann equation:
where IOP is intraocular pressure, F is the rate of aqueous flow through the anterior chamber, U is the rate of aqueous flow exiting the eye by the uveoscleral outflow pathway, C is the trabecular outflow facility (i.e. conductance), and EVP is the episcleral venous pressure (Goldmann, 1951; Barany, 1963; Moses, 1987; Brubaker, 2004).
Based on the Goldmann equation, the normal EVP of 9 mmHg is responsible for 60% of the typical human IOP of 15mmHg and changes in EVP should elicit 1:1 changes in IOP (Goldmann, 1951; Zeimer, 1989; Toris et al., 1999). The arterial inflow and venous outflow of the episcleral circulation communicate directly with the cephalic arterial and venous systems and so EVP and consequently IOP should be influenced by their behavior. If cephalic vascular pressures increase by 10 mmHg when going from upright to supine, then EVP and IOP should also, but they do not.
Previous studies indicate little effect of posture change on aqueous flow, outflow facility, or EVP but only EVP should follow the passive gravity-induced behavior that occurs elsewhere in the cephalic venous circulation.(Krieglstein et al., 1978; Iwabuchi et al., 1983; Grady et al., 1986; Carlson et al., 1987) While this non-passive EVP behavior suggests a regulatory process, the underlying mechanism is unclear. If it is a neural feedback loop analogous to the arterial baroreflex, then the sensor, afferent nerves, site of central integration, efferent nerves and end-organ effector need to be identified along with the set-point and reflex gain. Alternatively, if it is a local mechanism like a myogenic response, then the site or sites of action need to be identified. For technical reasons, these potential mechanisms cannot be studied in humans and so an animal model is necessary. The current study sought to establish a rabbit model for this purpose and test the hypothesis that EVP and IOP respond to posture change in a non-passive manner similar to humans.
Materials and methods
Animal preparation
All animal procedures were approved by the local Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals. At the end of all experiments, the animals were euthanized with an overdose of anesthetic without ever having regained consciousness.
New Zealand white rabbits (n = 78; 2.3 + 0.1 kg) of both genders were housed in the vivarium with food and water ad libitum for 4-7 days prior to the experiment. On the day of the experiment, the animal was anesthetized with pentobarbital (≈ 30 mg/kg, i.v., supplemented every 15-30 min) then placed on a heating pad to maintain body temperature at ≈39° C for the remainder of the experiment. The animal was intubated through a tracheostomy and artificially respired with room air to maintain expired PCO2 (SurgiVet V9004; Sims BCI Inc., Waukesha, WI) in a range of 40-42 mmHg. Once the animal was connected to artificial respiration, blunt dissection was employed to locate the right common carotid. A loose silk ligature was placed around the right common carotid artery to allow for placement of a flow probe later in the experiment. The right central ear artery was then exposed and cannulated with PE-50 tubing connected to a pressure transducer. Next, the animal was placed in a stereotaxic head holder to prevent movement and keep the head position fixed for the duration of the experiment. A transit time ultrasound flow probe (2PSB; Transonic Systems, Ithaca, NY) was then positioned on the right common carotid artery and connected to a flowmeter (TS420; Transonic Systems, Ithaca, NY). Blood flow through the right common carotid artery (BFcar) served as an index of cerebral blood flow and the pulsatile signal used to trigger a digital cardiotachometer (ADInstruments PowerLab Chart v7.1; Colorado Springs, CO) to measure heart rate (HR). The animal was then paralyzed with gallamine triethiodide (1 mg/kg, i.v., supplemented as needed) to minimize eye movement. Next, a 23 gauge needle was inserted into the posterior supraorbital foramen and connected to a pressure transducer to measure orbital venous pressure (OVP) (Reitsamer and Kiel, 2002), after which a 23 gauge needle connected to another pressure transducer was inserted through the sclera at the pars plana and into the vitreous compartment to measure IOP. To prevent the rabbit ocular trauma response (Sears, 1960; Neufeld et al., 1972; Camras and Bito, 1980), the eye was anesthetized with topical lidocaine (1%) prior to cannulation. All pressure transducers were calibrated (Delta-Cal 650-950, Utah Medical Products, Midvale, UT, and a water manometer) and zeroed at the level of the eye. All parameters were digitally recorded (PowerLab/16sp and Chart v5.5.6; ADInstruments, Colorado Springs, CO) on a computer.
EVP was measured with a micropipette-based servonull micropressure system (Model 900A; World Precision Instruments, Sarasota, FL). Prior to the experiment, micropipettes were pulled (P-87 pipette puller; Sutter Instrument Co., Novato, CA) from borosiliate glass capillary tubes (1B100-6, World Precision Instruments, Sarasota, FL), and their tips were beveled in 3 planes to inner diameters of 3-5 μm using a micropipette beveler (BV-10 with plate 104-F; Sutter Instrument Co., Novato, CA). For the experiments, the pipettes were filled with 2M sodium chloride solution and connected to the servonull pressure pod. A reference electrode placed in the skin completed the electrical circuit when the pipette tip was placed inside an episcleral vein. The positive or negative pressure applied to the open end of the pipette that maintained tip resistance constant was recorded as the pressure in the vessel. Episcleral veins in the superior portion of the eye posterior to the limbus were cannulated using a micromanipulator and a surgical microscope (Reitsamer and Kiel, 2002; Zamora and Kiel, 2009; Zamora and Kiel, 2010). Once all parameters were stable, the protocol entailed ≈ 10 minutes of measurements in the horizontal position, the pipette withdrawn, the animal tilted to a head-down position, the vein re-cannulated and EVP measured for ≈ 10 more minutes. In the supine or tilted position, withdrawal and re-insertion of the pipette tip at the same vein location yielded consistent readings of EVP (e.g. in 10 different vessels, before and after re-insertion EVP values were 10.63 ± 0.61 versus 10.47 ± 0.64 mmHg, which were not significantly different).
Aqueous flow was measured by fluorophotometry (FM-2 Laboratory Animal Edition; OcuMetrics, Mountain View, CA). A detailed description of the technique is provided elsewhere. (Brubaker, 1991) For these experiments, 4-6 drops of topical fluorescein (2.5 mg/mL, Flurox; Ocusoft, Richmond, TX) were applied to the right eye approximately 2 hours prior to induction of anesthesia. Surgery was performed as described above, and once the rabbit was mounted in the stereotaxic instrument and the animal's physiology was stable (≈ 3.5 hours after application of topical fluorescein), triplicate fluorophotometric scans were performed at 15 minute intervals for ≈ 60 minutes in the horizontal position, and then for ≈ 90 minutes in head-down tilt.(Kiel et al., 2001) From each scan, the cornea and anterior chamber fluorescein concentrations were determined, and then repeated scans over time used to track the rate of fluorescein disappearance from the eye. From the resultant fluorescein decay curves, the flow of aqueous humor was determined using Brubaker's method.(Topper et al., 1984; Brubaker, 1991)
All data were time-averaged off-line, tabulated in Excel spreadsheets and the control versus tilt data were compared by paired t-tests (GraphPad Prism, La Jolla, CA). Results are reported as mean ± standard error of the mean and p-values less than 0.05 were considered significant. Based on a power analysis (α=0.05, β=0.2) using data from previous studies, the group sizes used had sufficient power to detect 15% changes in aqueous flow and episcleral venous pressure.
Results
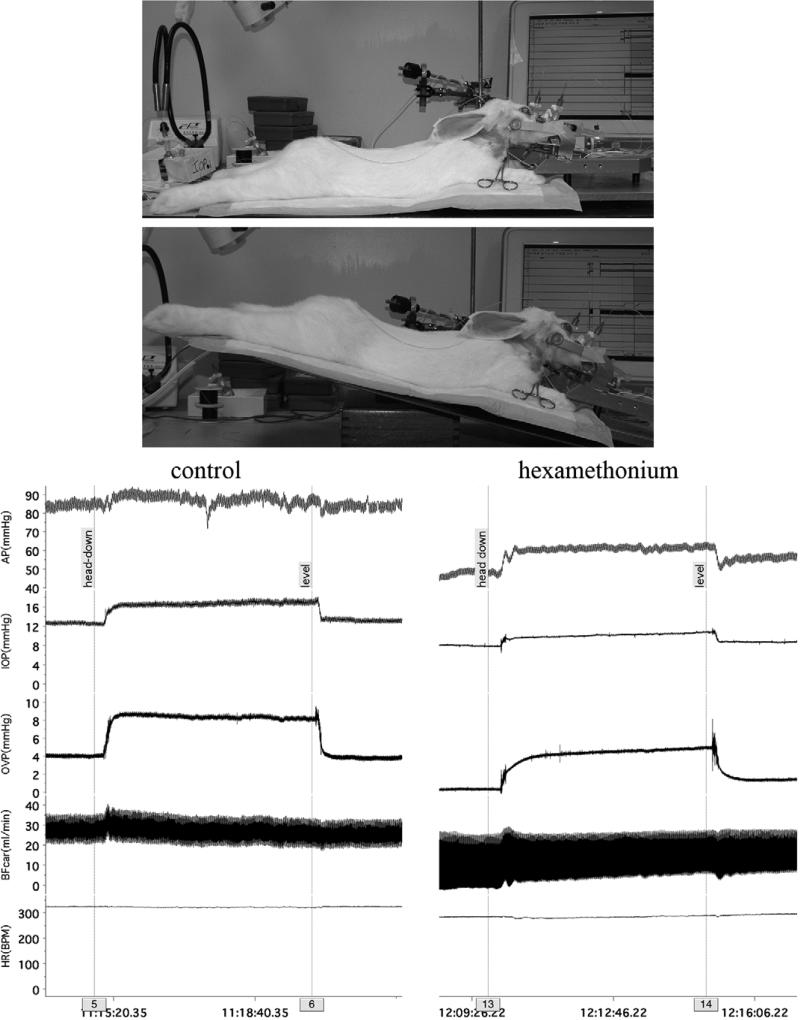
The photographs in Figure 1 show a representative rabbit in the supine and tilted position, and the traces show the responses of the measured variables before (control) and after ganglionic blockade with hexamethonium (25 mg/kg, iv) to blunt the cardiovascular reflexes and reveal the hydrostatic column effect. During control, tilt caused the mean arterial pressure to rise by 3 mmHg; after ganglionic blockade, the mean arterial pressure rose by 12 mmHg.
Figure 1.
Procedure to change animal position (top photographs) and demonstration of the magnitude of the passive hydrostatic column effect on cephalic arterial pressure. (The Chart software used to record the experiments allows for comments to be inserted during the recording. These comments are marked with a faint line through the channels to a numbered grey box on the time bar. Comments “5” and “13” were entered just before the animal was titled to the “head-down” position, and comments “6” and “14” were entered just before the animal was returned to the supine “level” position.)
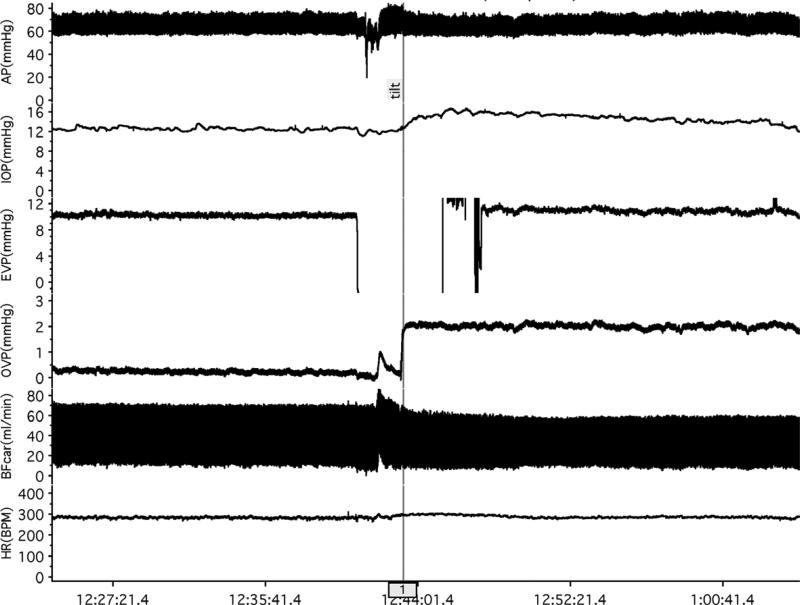
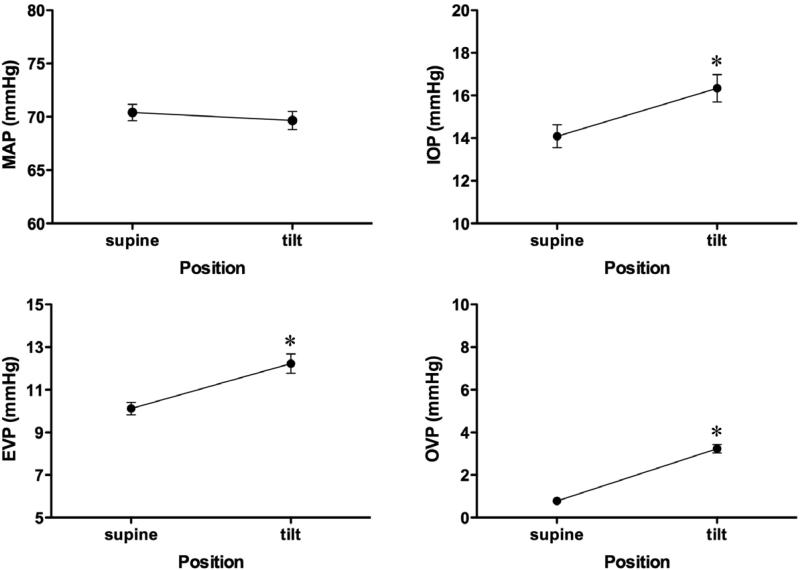
Figure 2 shows traces from a typical experiment in the episcleral venous pressure protocol and Figure 3 shows the mean pressure responses for the group (n=43). Mean arterial pressure was unchanged (70.4 ± 0.8 versus 69.7 ± 0.9 mmHg, p=0.104) while increases occurred in intraocular pressure (14.1 ± 0.5 to 16.3 ± 0.6 mmHg, p<0.001), episcleral venous pressure (10.1 ± 0.3 to 12.3 ± 0.5 mmHg, p<0.001) and orbital venous pressure (0.78 ± 0.1 mmHg to 3.2 ± 0.2, p<0.001). Carotid blood flow was unchanged (28.6 ± 1.5 ml/min versus 27.5 ± 1.3 ml/min, p=0.065) and heart rate decreased slightly (294 ± 4 bpm to 285 ± 5 bpm, p<0.001).
Figure 2.
EVP protocol. Baseline measurements were made in the supine position for ≈10 min. The EVP pipette was then withdrawn from the episcleral vein to prevent breaking the tip while the animal was tilted, then the pipette re-inserted in the vein and measurements continued for ≈10 min in the head-down tilt position.
Figure 3.
EVP protocol pressure responses to head-down tilt. (* p<0.01, supine versus tilt position)
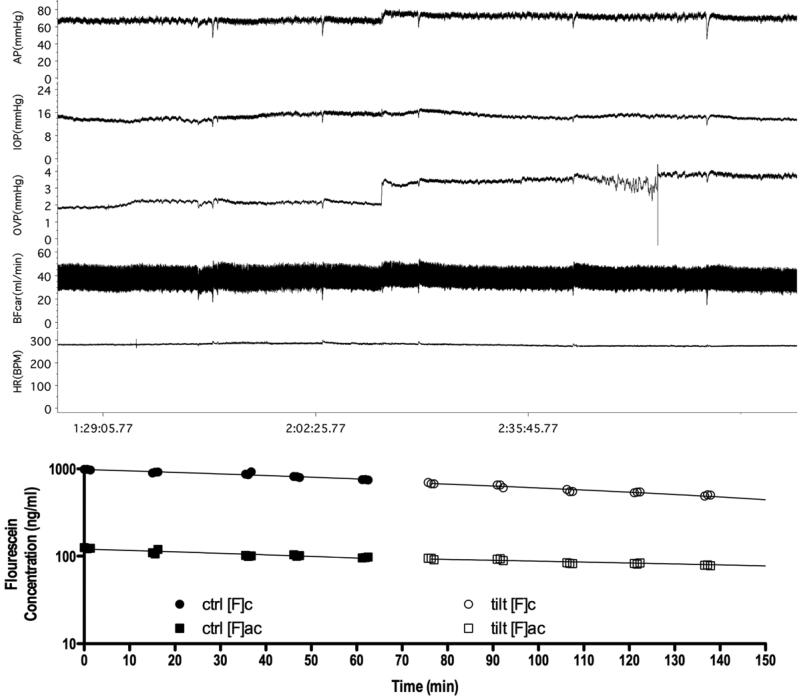
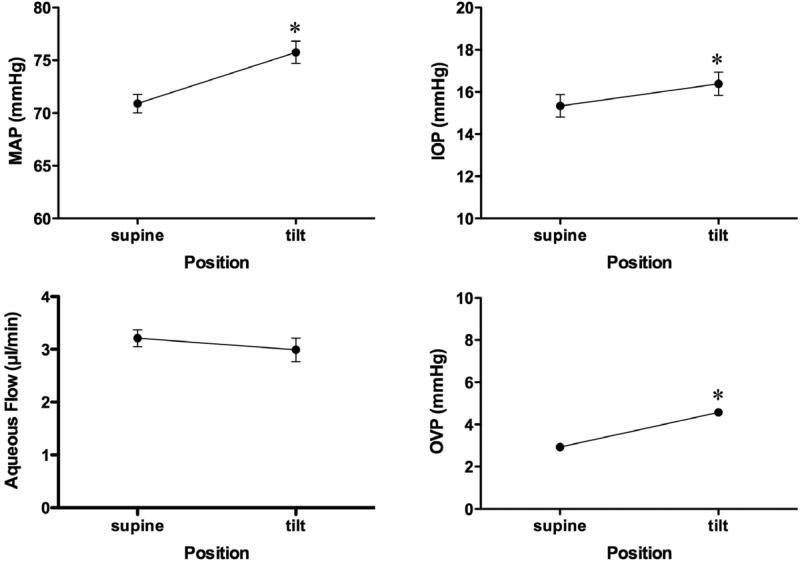
Figure 4 shows traces and the associated fluorescein elimination curves used to calculate aqueous flow from a typical experiment in the aqueous flow protocol. Figure 5 shows the mean pressure and aqueous flow responses for the group (n=35). Aqueous flow was unchanged (3.21 ± 0.2 versus 2.99 ± 0.2 μm/min, p=0.216) while increases occurred in mean arterial pressure (70.9 ± 0.9 to 75.8 ± 1.1 mmHg, p<0.001), intraocular pressure (15.3 ± 0.5 to 16.4 ± 0.6 mmHg, p<0.001) and orbital venous pressure (2.9 ± 0.1 to 4.6 ± 0.2, p<0.001). Carotid blood flow was unchanged (33.3 ± 1.5 versus 34.1 ± 1.6 ml/min, p=0.070) and heart rate decreased slightly (296 ± 4 to 284 ± 4 bpm, p<0.001).
Figure 4.
Aqueous flow protocol. . A typical trace is shown from an aqueous flow experiment. The protocol entailed 60 minutes of measurements with the animal supine. Then the animal was tilted for an additional 60 minutes. Triplicate fluorophotometric scans were performed every 15 min to calculate aqueous flow. (ctrl: control supine period; tilt: head-down tilt period; [F]c: cornea fluorescein concentration; [F]ac: anterior chamber fluorescein concentration)
Figure 5.
Aqueous flow protocol pressure and aqueous flow responses to head-down tilt. (*p<0.01, supine versus tilt positions)
Discussion
The goal of this study was to validate the rabbit as a model for studying EVP and IOP responses to changes in posture, since neither parameter can be measured continuously in humans. Anatomically, the rabbit and primate episcleral circulation share similar features, i.e., both have few capillaries, numerous arterio-venous anastamoses and muscular veins. However, one notable difference in the aqueous outflow pathway between rabbits and humans is that rabbits lack Schlemm's canal (Prince, 1964). Instead, rabbits have an aqueous plexus that drains into the episcleral veins, but aqueous outflow through the trabecular pathway is still assumed to be governed by trabecular facility and the IOP-to-EVP pressure gradient (Funk and Rohen, 1994; Rohen and Funk, 1994; Funk et al., 1996; Selbach et al., 1998)
Despite similar episcleral circulations, the size difference between humans and rabbits is large, and the hydrostatic column in the rabbit is much smaller. For humans going from upright to supine, the hydrostatic column effect is equivalent to ≈ 31 mmHg (assuming a heart to eye distance of 40 cm, blood density of 1.05 g/cm3, and mercury density of 13.6 g/cm3); in the rabbit it is ≈12 mmHg (assuming a heart to eye distance of 15 cm). However, it is not possible to position an anesthetized, paralyzed rabbit mounted in a stereotaxic instrument in completely vertical and horizontal positions. Instead, the animal and stereotaxic instrument were placed on a large metal plate so that the plane of the animal could be tilted by ≈20 degrees. Measurements with a fluid filled tube indicated a hydrostatic challenge with head-down tilt of ≈ 6 mmHg. Measurements of MAP after ganglionic blockade (Fig 1) suggest the hydrostatic challenge was closer to 10 mmHg. In control animals, the rise in arterial pressure with tilt was smaller, likely due to a more efficient baroreflex in those animals. Subtle differences in baroreflex efficacy likely also account for the difference in blood pressure responses to tilt between the two protocol groups. The blood pressure traces in Figures 2 and 4 show returns to baseline consistent with a baroreflex or other reflex compensatory responses over time that is not evident after ganglionic blockade. Humans with baroreflex failure also exhibit pronounced hydrostatic column effects during posture change. (Singleton et al., 2003)
During tilt, EVP and IOP increased by ≈ 2 mmHg, consistent with the 1:1 relationship predicted by the Goldmann equation. Aqueous flow also did not change during tilt. Outflow facility was not measured, but a study in humans indicates no change in outflow facility during posture change (Selvadurai et al., 2010). To our knowledge, uveoscleral outflow has not been measured during posture change, but it seems unlikely that uveoscleral outflow would be altered (i.e., posture change should not alter the iris or ciliary muscle extracellular matrix or change ciliary muscle tension). Taken together, this information suggests that EVP is the pivotal aqueous dynamics variable affected during posture change.
Somewhat similar results were reported in humans. During 15 degree tilt in healthy human subjects, IOP rose by ≈2 mmHg and aqueous flow did not change (Carlson et al., 1987). In another human study, IOP and EVP both rose by ≈ 2 mmHg when subjects were lowered from a 30 degree head up position to supine (Krieglstein et al., 1978). These small, posture-induced increases in EVP and IOP are both less than expected from the change in the hydrostatic column.
EVP and IOP both changed less than the expected 6-10 mmHg increase anticipated for the rabbit passive hydrostatic column response. OVP also rose by only ≈2 mmHg, such that the pressure gradient from the episcleral veins to the orbital venous sinus remained unchanged at ≈8 mmHg. (Reitsamer and Kiel, 2002) It was not possible to measure episcleral blood flow and it is unclear if episcleral resistance was altered. We assume the episcleral circulation is in series with the orbital venous sinus, but it is clear that the interconnections between the eye, orbit and the rest of the cephalic venous circulation are complex. For example, stopping all eye blood flow by raising IOP above systolic blood pressure decreases OVP by <0.5 mmHg (Reitsamer and Kiel, 2002), indicating that the orbital sinus has other connections than the eye and it may be that the episcleral circulation does as well. Consequently, the magnitude of the OVP rise transmitted to the episcleral circulation during tilt is unclear. It is also unclear in humans, since OVP has not been measured in humans in any position.
Conclusions
In conclusion, EVP and other relevant ocular and systemic parameters can be measured in the rabbit during simulated posture change. Moreover, the EVP and IOP responses to tilt are qualitatively similar to those reported in humans and do not seem to be passive. An obvious follow-up question is whether there is a mechanism controlling EVP during posture change. Two possibilities are a neural mechanism (e.g. the baroreflex) or perhaps a local control mechanism (e.g. the myogenic response). Anatomical studies indicate that the episcleral circulation has a complex vascular organization and is richly innervated (Funk and Rohen, 1996; Selbach et al., 2005). Therefore, it seems likely that this circulation is under neural control. However, episcleral veins are atypically muscular, suggesting a myogenic response may also play a role. Further study is needed to determine if EVP is actively regulated during posture change and the mechanisms involved.
Highlights.
EVP and other relevant ocular and systemic parameters can be measured in the rabbit during simulated posture change
EVP and IOP responses to posture change are qualitatively similar to those reported in humans.
As in humans, EVP and IOP responses to posture change do not seem to be passive
Acknowledgements
This work was supported by NIH RO1 EY09702 and NGR, a program of the American Health Assistance Foundation. The technical assistance of Ms Alma Maldonado is also sincerely appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Barany E. A mathematical formulation of intraocular pressure as dependent on secretion, ultrafiltration, bulk outflow, and osmotic reabsorption of fluid. Invest Ophthalmol. 1963;2:584–590. [PubMed] [Google Scholar]
- Bill A. Circulation in the eye. In: Renkin EM, Michel CC, editors. The Microcirculation, Part 2, Handbook of Physiology. Section 2 American Physiological Society; Bethesda: 1984. pp. 1001–1034. [Google Scholar]
- Brubaker RF. Clinical evaluation of the circulation of aqueous humor. In: Tasman W, Jaeger EA, editors. Duane's Clinical Ophthalmology. J.B. Lippincott Co.; Philadelphia, PA: 1991. pp. 1–11. [Google Scholar]
- Brubaker RF. Flow of aqueous humor in humans. Invest. Ophthalmol. Vis. Sci. 1991;32:3145–3166. [PubMed] [Google Scholar]
- Brubaker RF. Goldmann's equation and clinical measures of aqueous dynamics. Exp Eye Res. 2004;78:633–7. doi: 10.1016/j.exer.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Camras CB, Bito LZ. The pathophysiological effects of nitrogen mustard on the rabbit eye. II. The inhibition of the initial hypertensive phase by capsaicin and the apparent role of substance P. Invest Ophthalmol Vis Sci. 1980;19:423–428. [PubMed] [Google Scholar]
- Carlson K, McLaren J, Topper J, Brubaker R. Effect of body position on intraocular pressure and aqueous flow. Invest. Ophthalmol. Vis. Sci. 1987;28:1346–1350. [PubMed] [Google Scholar]
- Funk R, Rohen JW. In vivo observations of the episcleral vasculature in the albino rabbit. J. Glaucoma. 1994;3:44–50. [PubMed] [Google Scholar]
- Funk RH, Rohen JW. Scanning electron microscopic study of episcleral arteriovenous anastomoses in the owl and cynomolgus monkey. Curr Eye Res. 1996;15:321–7. doi: 10.3109/02713689609007627. [DOI] [PubMed] [Google Scholar]
- Funk RHW, Gehr J, Rohen JW. Short-term hemodynamic changes in episcleral arteriovenous anastomoses correlate with venous pressure and IOP changes in the albino rabbit. Curr Eye Res. 1996;15:87–93. doi: 10.3109/02713689609017615. [DOI] [PubMed] [Google Scholar]
- Gisolf J, van Lieshout JJ, van Heusden K, Pott F, Stok WJ, Karemaker JM. Human cerebral venous outflow pathway depends on posture and central venous pressure. J. Physiol. 2004;560:317–27. doi: 10.1113/jphysiol.2004.070409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann H. Abflussdruck, minutenvolumen und widerstand der kammerwasser-stromung des menschen. Doc Ophthalmol. 1951;5-6:278–356. doi: 10.1007/BF00143664. [DOI] [PubMed] [Google Scholar]
- Grady MS, Bedford RF, Park TS. Changes in superior sagittal sinus pressure in children with head elevation, jugular venous compression, and PEEP. J Neurosurg. 1986;65:199–202. doi: 10.3171/jns.1986.65.2.0199. [DOI] [PubMed] [Google Scholar]
- Iwabuchi T, Sobata E, Ebina K, Tsubakisaka H, Takiguchi M. Dural sinus pressure: various aspects in human brain surgery in children and adults. Am J Physiol. 1986;250:H389–96. doi: 10.1152/ajpheart.1986.250.3.H389. [DOI] [PubMed] [Google Scholar]
- Iwabuchi T, Sobata E, Suzuki M, Suzuki S, Yamashita M. Dural sinus pressure as related to neurosurgical positions. Neurosurgery. 1983;12:203–7. doi: 10.1227/00006123-198302000-00012. [DOI] [PubMed] [Google Scholar]
- Kiel JW, Reitsamer HA, Walker JS, Kiel FW. Effects of nitric oxide synthase inhibition on ciliary blood flow, aqueous production and intraocular pressure. Exp Eye Res. 2001;73:355–364. doi: 10.1006/exer.2001.1050. [DOI] [PubMed] [Google Scholar]
- Krieglstein G, Waller W, Leydhecker W. The vascular basis of the positional influence on the intraocular pressure. Albrecht v. Graefes Arch. klin. exp. Ophthal. 1978;206:99–106. doi: 10.1007/BF00414618. [DOI] [PubMed] [Google Scholar]
- Moses R. Intraocular Pressure. In: Moses R, Hart W, editors. Adler's Physiology of the Eye: clinical application. C.V. Mosby Co; St Louis: 1987. pp. 223–245. [Google Scholar]
- Neufeld AH, Jampol LM, Sears ML. Aspirin prevents the disruption of the blood-aqueous barrier in the rabbit eye. Nature. 1972;238:158–9. doi: 10.1038/238158a0. [DOI] [PubMed] [Google Scholar]
- Prince J. The Rabbit in Eye Research. Charles C Thomas Publisher; Springfield, IL: 1964. [Google Scholar]
- Reitsamer HA, Kiel JW. A rabbit model to study orbital venous pressure, intraocular pressure, and ocular hemodynamics simultaneously. Invest Ophthalmol Vis Sci. 2002;43:3728–3734. [PubMed] [Google Scholar]
- Rohen JW, Funk R. Functional morphology of the episcleral vasculature in rabbits and dogs: presence of arteriovenous anastomoses. J Glaucoma. 1994;3:51–57. [PubMed] [Google Scholar]
- Sears ML. Miosis and intraocular pressure changes during manometry. Arch Ophthal. 1960;63:159–166. doi: 10.1001/archopht.1960.00950020709014. [DOI] [PubMed] [Google Scholar]
- Selbach JM, Rohen JW, Steuhl KP, Lutjen-Drecoll E. Angioarchitecture and innervation of the primate anterior episclera. Curr Eye Res. 2005;30:337–44. doi: 10.1080/02713680590934076. [DOI] [PubMed] [Google Scholar]
- Selbach JM, Schonfelder U, W FRH. Arteriovenous anastomomoses of the episcleral vasculature in the rabbit and rat eye. J Glaucoma. 1998;7:50–57. [PubMed] [Google Scholar]
- Selvadurai D, Hodge D, Sit AJ. Aqueous humor outflow facility by tonography does not change with body position. Invest Ophthalmol Vis Sci. 2010;51:1453–7. doi: 10.1167/iovs.09-4058. [DOI] [PubMed] [Google Scholar]
- Singleton CD, Robertson D, Byrne DW, Joos KM. Effect of posture on blood and intraocular pressures in multiple system atrophy, pure autonomic failure, and baroreflex failure. Circulation. 2003;108:2349–54. doi: 10.1161/01.CIR.0000097114.11038.26. [DOI] [PubMed] [Google Scholar]
- Topper JE, McLaren J, Brubaker RF. Measurement of aqueous humor flow with scanning ocular fluorophotometers. Curr Eye Res. 1984;3:1391–95. doi: 10.3109/02713688409000834. [DOI] [PubMed] [Google Scholar]
- Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999;127:407–12. doi: 10.1016/s0002-9394(98)00436-x. [DOI] [PubMed] [Google Scholar]
- Zamora D, Kiel J. Episcleral Venous Pressure Responses to Topical Nitroprusside and N-nitro-L-arginine methyl ester. Invest Ophthalmol Vis Sci. 2010;51:1614–1620. doi: 10.1167/iovs.09-4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora DO, Kiel JW. Topical proparacaine and episcleral venous pressure in the rabbit. Invest Ophthalmol Vis Sci. 2009;50:2949–52. doi: 10.1167/iovs.08-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimer R. Episcleral venous pressure. In: Ritch R, Shields M, Krupin T, editors. The Glaucomas. C.V. Mosby Company; St Louis: 1989. pp. 249–255. [Google Scholar]