Abstract
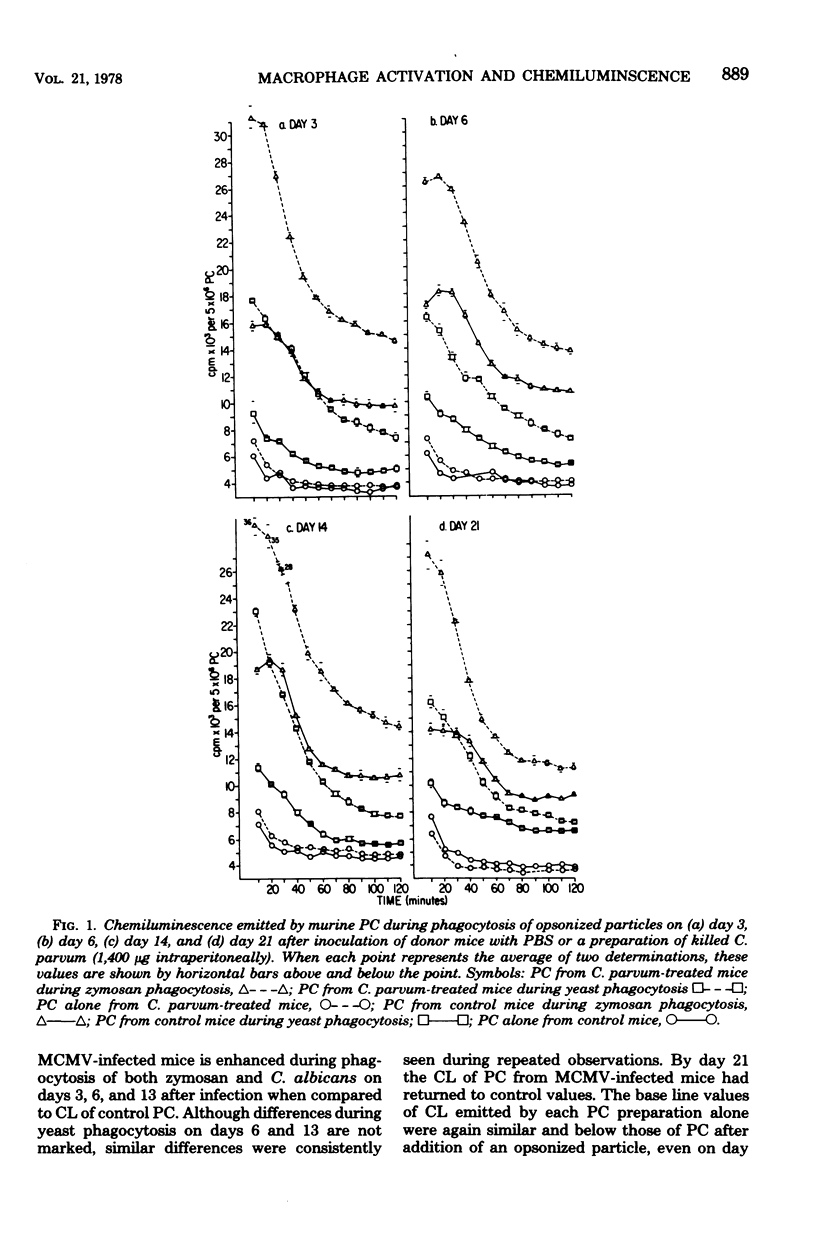
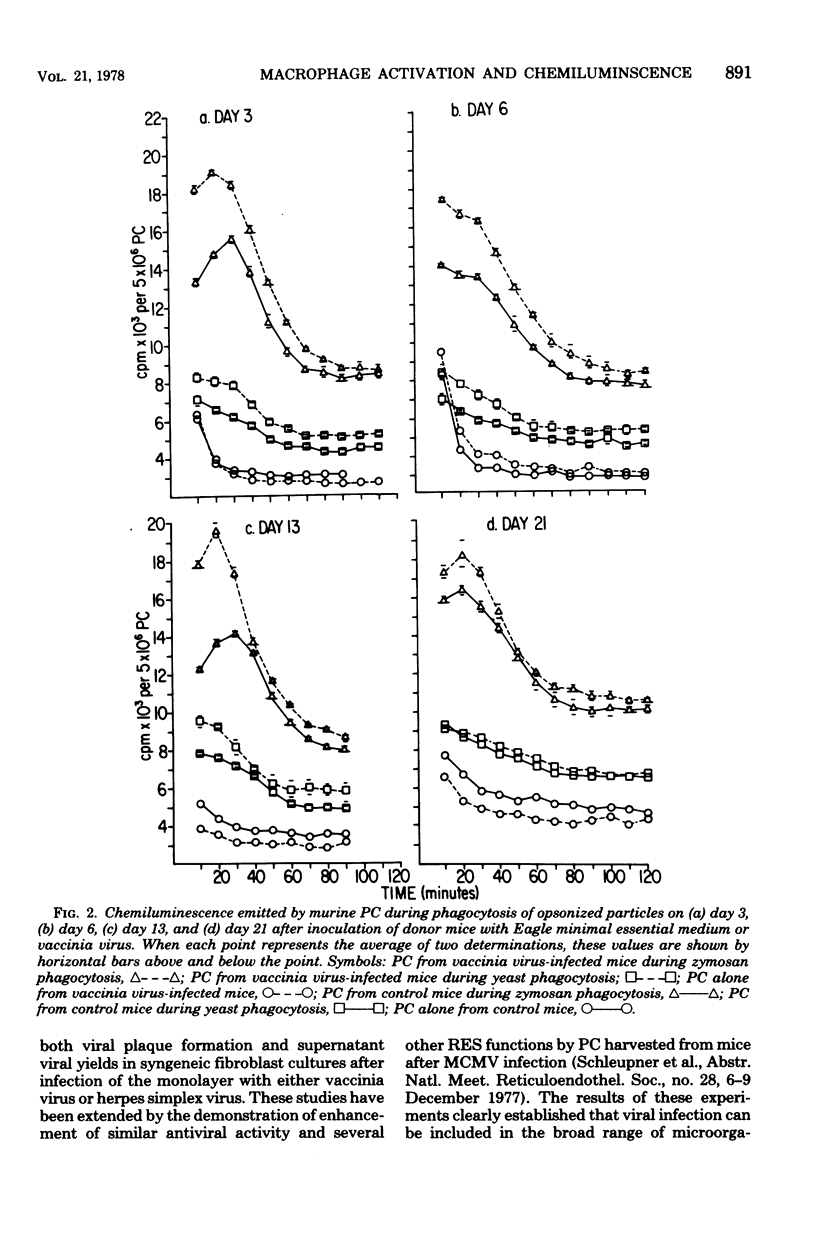
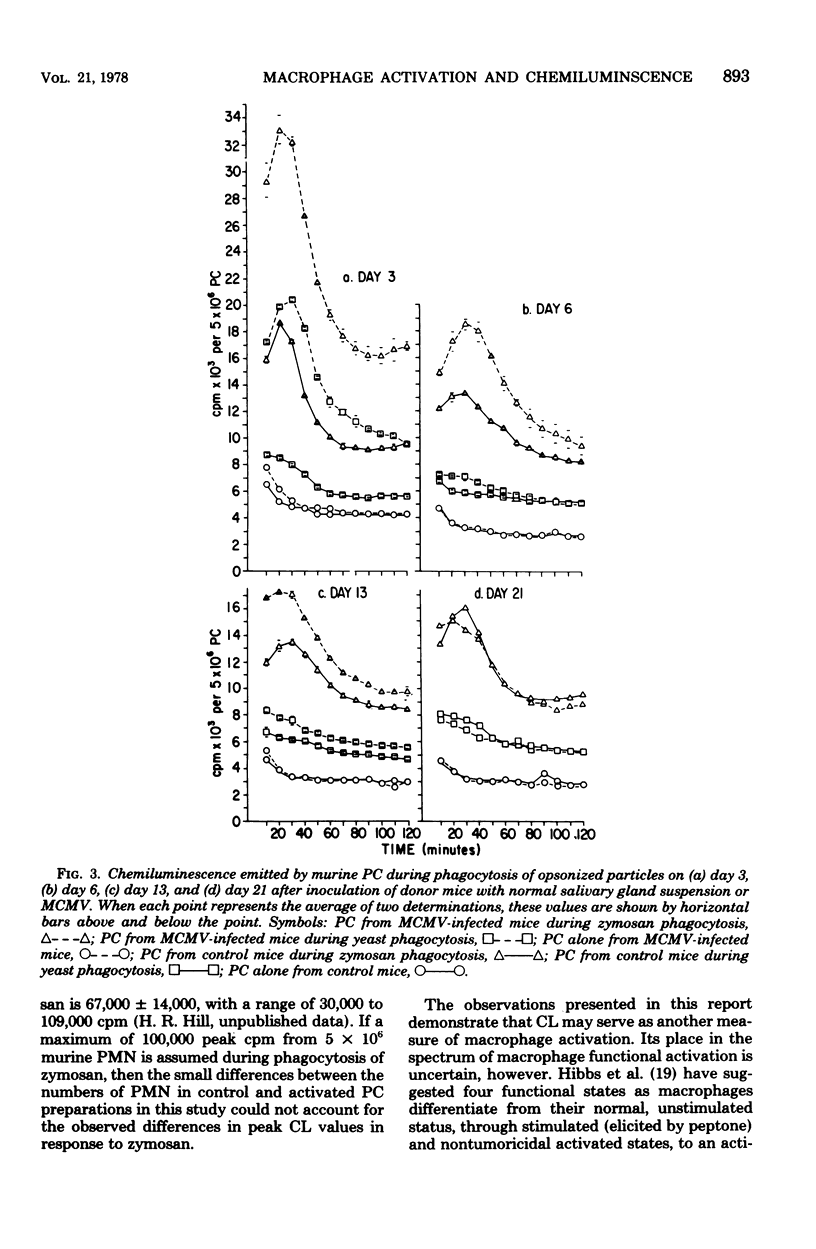
A number of studies have demonstrated the ability of various bacterial preparations, protozoa, and chemicals to activate macrophages and concomitantly to enhance host resistance to both tumors and infections. Recently, viral infections have been shown to have a similar effect upon macrophage function. To better define the metabolic state of activated macrophages, we have evaluated the ability of peritoneal cells (PC) from vaccinia virus- or murine cytomegalovirus-infected or Corynebacterium parvum-treated mice to emit chemiluminescence (CL) during phagocytosis of zymosan particles or yeasts. PC from C. parvum-treated mice (1,400 microgram intraperitoneally) emitted enhanced CL over controls on days 3, 6, 14, and 21 after treatment, thereby establishing the emission of CL as a correlate of metabolic activation. Previous evidence for activation of PC from vaccinia virus-infected mice (10(8) plaque-forming units) was confirmed by demonstration of enhanced levels of CL on days 3, 6, and 13 after murine infection. Likewise, PC from mice infected with murine cytomegalovirus (10(5) plaque-forming units) 3, 6, or 13 days previously demonstrated augmented levels of CL over controls. Opsonized virus particles (vaccinia virus or murine cytomegalovirus) failed to induce the emission of CL with PC from mice infected with the isologous virus. Our data further demonstrate the immunomodulationinduced by virus infections and suggest that the detection of CL is an easily quantitated correlate of macrophage activation which may be helpful in defining metabolic alterations induced during activation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlam C., Broughton E. S., Scott M. T. Enhanced resistance of mice to infection with bacteria following pre-treatment with Corynebacterium parvum. Nat New Biol. 1972 Feb 16;235(59):219–220. doi: 10.1038/newbio235219a0. [DOI] [PubMed] [Google Scholar]
- Adlam C., Scott M. T. Lympho-reticular stimulatory properties of Corynebacterium parvum and related bacteria. J Med Microbiol. 1973 Aug;6(3):261–274. doi: 10.1099/00222615-6-3-261. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Loose L. D. Phagocytic activation of a luminol-dependent chemiluminescence in rabbit alveolar and peritoneal macrophages. Biochem Biophys Res Commun. 1976 Mar 8;69(1):245–252. doi: 10.1016/s0006-291x(76)80299-9. [DOI] [PubMed] [Google Scholar]
- Beall G. D., Repine J. E., Hoidal J. R., Rasp F. L. Chemiluminescence by human alveolar macrophages: stimulation with heat-killed bacteria or phorobol myristate acetate. Infect Immun. 1977 Jul;17(1):117–120. doi: 10.1128/iai.17.1.117-120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mims C. A. Macrophage activation in mice infected with ectromelia or lymphocytic choriomeningitis viruses. Aust J Exp Biol Med Sci. 1973 Jun;51(3):393–398. doi: 10.1038/icb.1973.35. [DOI] [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Scott M. T. Effect of Corynebacterium parvum treatment on the growth of Salmonella enteritidis in mice. Infect Immun. 1974 May;9(5):863–869. doi: 10.1128/iai.9.5.863-869.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Mulikin D., McCall C. E. The generation of superoxide anion by various types of phagocyte. J Infect Dis. 1975 Apr;131(4):443–446. doi: 10.1093/infdis/131.4.443. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber P. A., Glasgow L. A. Effect of Corynebacterium acnes on interferon production in mice. Infect Immun. 1972 Sep;6(3):272–276. doi: 10.1128/iai.6.3.272-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A., Fischbach J., Bryant S. M., Kern E. R. Immunomodulation of host resistance to experimental viral infections in mice: effects of Corynebacterium acnes, Corynebacterium parvum, and Bacille calmette-guérin. J Infect Dis. 1977 May;135(5):763–770. doi: 10.1093/infdis/135.5.763. [DOI] [PubMed] [Google Scholar]
- Halpern B. N., Biozzi G., Stiffel C., Mouton D. Inhibition of tumour growth by administration of killed corynebacterium parvum. Nature. 1966 Nov 19;212(5064):853–854. doi: 10.1038/212853a0. [DOI] [PubMed] [Google Scholar]
- Hamilton J. R., Overall J. C., Glasgow L. A. Synergistic effect on mortality in mice with murine cytomegalovirus and Pseudomonas aeruginosa, Staphylococcus aureus, or Candida albicans infections. Infect Immun. 1976 Oct;14(4):982–989. doi: 10.1128/iai.14.4.982-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Discrimination between neoplastic and non-neoplastic cells in vitro by activated macrophages. J Natl Cancer Inst. 1974 Nov;53(5):1487–1492. doi: 10.1093/jnci/53.5.1487. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Resistance to murine tumors conferred by chronic infection with intracellular protozoa, Toxoplasma gondii and Besnoitia jellisoni. J Infect Dis. 1971 Dec;124(6):587–592. doi: 10.1093/infdis/124.6.587. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Role of activated macrophages in nonspecific resistance to neoplasia. J Reticuloendothel Soc. 1976 Sep;20(3):223–231. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Chapman H. A., Jr, Weinberg J. B. Macrophage tumor killing: influence of the local environment. Science. 1977 Jul 15;197(4300):279–282. doi: 10.1126/science.327547. [DOI] [PubMed] [Google Scholar]
- JACKSON J. F. Supravital blood studies, using acridine orange fluorescence. Blood. 1961 May;17:643–649. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J., Drath D., Harper A. Biochemical characteristics of activated macrophages. Ann N Y Acad Sci. 1975 Jun 13;256:266–274. doi: 10.1111/j.1749-6632.1975.tb36053.x. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Glasgow L. A., Overall J. C., Jr Antiviral activity of an extract of Brucella abortus: induction of interferon and immunopotentiation of host resistance. Proc Soc Exp Biol Med. 1976 Jul;152(3):372–376. doi: 10.3181/00379727-152-39399. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- McBride W. H., Jones J. T., Weir D. M. Increased phagocytic cell activity and anaemia in Corynebacterium parvum treated mice. Br J Exp Pathol. 1974 Feb;55(1):38–46. [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Glasgow L. A., Crane J. L., Jr, Kern E. R. Comparison of antiviral and antitumor activity of activated macrophages. Cell Immunol. 1977 Feb;28(2):404–415. doi: 10.1016/0008-8749(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Kern E. R., Glasgow L. A. Immunomodulator-induced resistance against herpes simplex virus. Proc Soc Exp Biol Med. 1977 Apr;154(4):615–620. doi: 10.3181/00379727-154-39730. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Mills E. L., Simmons R. L., Quie P. G. Chemiluminescence response of phagocytizing human monocytes. Infect Immun. 1976 Jul;14(1):129–134. doi: 10.1128/iai.14.1.129-134.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P. N., Rao G. V., Tompkins W. A. In vitro cellular immunity to unrelated pathogens in chickens infected with fowlpox virus. Infect Immun. 1974 Jul;10(1):34–41. doi: 10.1128/iai.10.1.34-41.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda S. J., White D. O. Cytotoxic macrophages: a rapid nonspecific response to viral infection. J Immunol. 1976 Dec;117(6):2067–2072. [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. T. Corynebacterium parvum as an immunotherapeutic anticancer agent. Semin Oncol. 1974 Dec;1(4):367–378. [PubMed] [Google Scholar]
- Sljivić V. S., Watson S. R. The adjuvant effect of Corynebacterium parvum: T-cell dependence of macrophage activation. J Exp Med. 1977 Jan 1;145(1):45–57. doi: 10.1084/jem.145.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzberg J. E., Krahenbuhl J. L., Remington J. S. Dichotomy between macrophage activation and degree of protection against Listeria monocytogenes and Toxoplasma gondii in mice stimulated with Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1037–1043. doi: 10.1128/iai.12.5.1037-1043.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle R. L., North R. J. Mechanisms of antitumor action of Corynebacterium parvum: the generation of cell-mediated tumor specific immunity. J Reticuloendothel Soc. 1976 Sep;20(3):197–208. [PubMed] [Google Scholar]
- Yashphe D. J. Immunological factors in nonspecific stimulation of host resistance to syngeneic tumors. A review. Isr J Med Sci. 1971 Jan;7(1):90–107. [PubMed] [Google Scholar]


