Systemic inflammation is a feature of chronic obstructive pulmonary disease (COPD). Defects in T cell-mediated anti-inflammatory pathways such as cytotoxic T lymphocyte antigen-4 (CTLA-4) may promote damaging inflammation. This study provides novel data implicating the impaired induction of an anti-inflammatory molecule, CTLA-4 in the elevated inflammation observed in COPD patients. Low induction of CTLA-4 in COPD patients paralleled increased markers of systemic inflammation ex vivo and increased T-cell responses to a bacterial superantigen, staphylococcal enterotoxin-B (SEB) in vitro. This mechanism may explain the increased inflammation in COPD patients.
COPD is a leading cause of mortality and morbidity worldwide, affecting mostly smokers and the elderly. COPD is characterized by irreversible inflammation and destruction of lung tissue, leading to airflow obstruction.1 It has been recognized as a multisystemic inflammatory disorder associated with cachexia, vascular and ischaemic heart disease.2 Systemic biomarkers of chronic inflammation such as soluble tumour-necrosis factor receptor (sTNFR) and the activation of T cells (HLA-DR expression) are elevated in this condition.3,4
It is recognized that T-cells play a key role in regulating inflammation in COPD. This is evidenced by the infiltration of cytotoxic CD8+ T cells into the airways of COPD patients and the presence of activated T cells in the lungs of COPD patients who have ceased smoking.4,5 Accumulation of interleukin (IL)-17-producing T cells in the bronchial submucosa and alveolar wall of COPD patients may promote the neutrophilic airway inflammation characteristic of COPD.6,7
Despite evidence of the role of T cells, mechanisms underlying T-cell dysregulation in COPD are unclear. CTLA-4 is an important negative regulator of T-cell activity. Proliferation and activation of T cells are enhanced by binding of the CD28 coreceptor to the CD80/86 complex on antigen presenting cells. CTLA-4 binds CD80/86 with higher affinity, thereby inhibiting T cell-mediated inflammation. Polymorphisms in the CTLA-4 gene (rs231775 and rs5742909) are linked to reduced inhibitory function of CTLA-4, impaired immunosuppression and increased susceptibility to COPD and chronic bronchitis.8,9 We therefore hypothesized that reduced CTLA-4 production may lead to increased inflammation and COPD. Here, CTLA-4 responses of COPD patients (n=11) were compared with age-matched non-smoking healthy controls (n=13).
Eleven ex-smokers (>15 pack-years and ceased smoking >5 years earlier) with stable COPD were recruited from a dedicated COPD clinic. The diagnosis of COPD was established by a respiratory physician and disease severity was characterized according to the Global Initiative for Chronic Obstructive Lung Disease criteria. All COPD patients studied also met the criteria for chronic bronchitis. All COPD patients had been treated with anticholinergic drugs, long-acting beta agonists and inhaled corticosteroids for at least 3 months prior to participating in the study. No patients were receiving systemic corticosteroids or had diabetes, neuromuscular, allergic or rheumatological disease. Comorbidities included hypertension (n=4), osteoporosis (n=2) and ischaemic heart disease (n=1). Thirteen healthy non-smokers with no evidence of COPD and normal spirometry were included as controls (Supplementary Table 1). The study was approved by the Ethics Committee at Royal Perth Hospital and all participants gave informed consent.
Peripheral blood samples were collected in lithium heparin tubes. After centrifugation, plasma was collected and stored at −80 °C. Levels of sTNFR1 (R&D Systems, Minneapolis, MN, USA) were measured in plasma by ELISA. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient centrifugation and cryopreserved in 10% dimethyl sulfoxide/fetal calf serum. To quantify CD4+ T-cells with an activated (HLA-DR+) phenotype, unstimulated PBMC (0.5×106) were stained with CD3-APC-H7, CD4-PerCP-Cy5.5 and HLA-DR-APC antibodies (BD Biosciences, San Jose, CA, USA). In total, 250 000 events were acquired using a BD FACSCanto II cytometer (BD Biosciences) and analysed with FlowJo v5.7.2 software (Tree Star, Ashland, OR, USA).
Expression of CTLA-4 in CD4+ T cells was measured by flow cytometry following stimulation of PBMC with SEB to achieve T-cell receptor-mediated activation of all T cells in vitro and to mimic the activation caused by an inflammatory stimulus in COPD. PBMC were cultured at 1×106 cells/ml for 6 h in polypropylene tubes at a 5° incline in 10% fetal calf serum/RPMI alone or with 1 µg/ml SEB (Sigma-Aldrich, Sydney, Australia). Brefeldin-A (BD Biosciences) was added 2 h after the start of culture. Cells were washed in 1% bovine serum albumin/phosphate-buffered saline (PBS) and surface stained with CD3-APC-H7 and CD4-PerCP-Cy5.5 (BD Biosciences). Intracytoplasmic staining was performed using the BD Pharmingen Human Foxp3 buffer set and CTLA-4-PE antibody (Coulter Immunotech, Marseille, France). In total, 250 000 events were acquired and analysed as described above. IL-17 was measured in culture supernatants from SEB-stimulated PBMC after 40 h by ELISA (eBioscience, San Diego, CA, USA).
Statistical analyses were performed using non-parametric Mann–Whitney tests to compare groups or Spearman's rank tests to assess correlations.
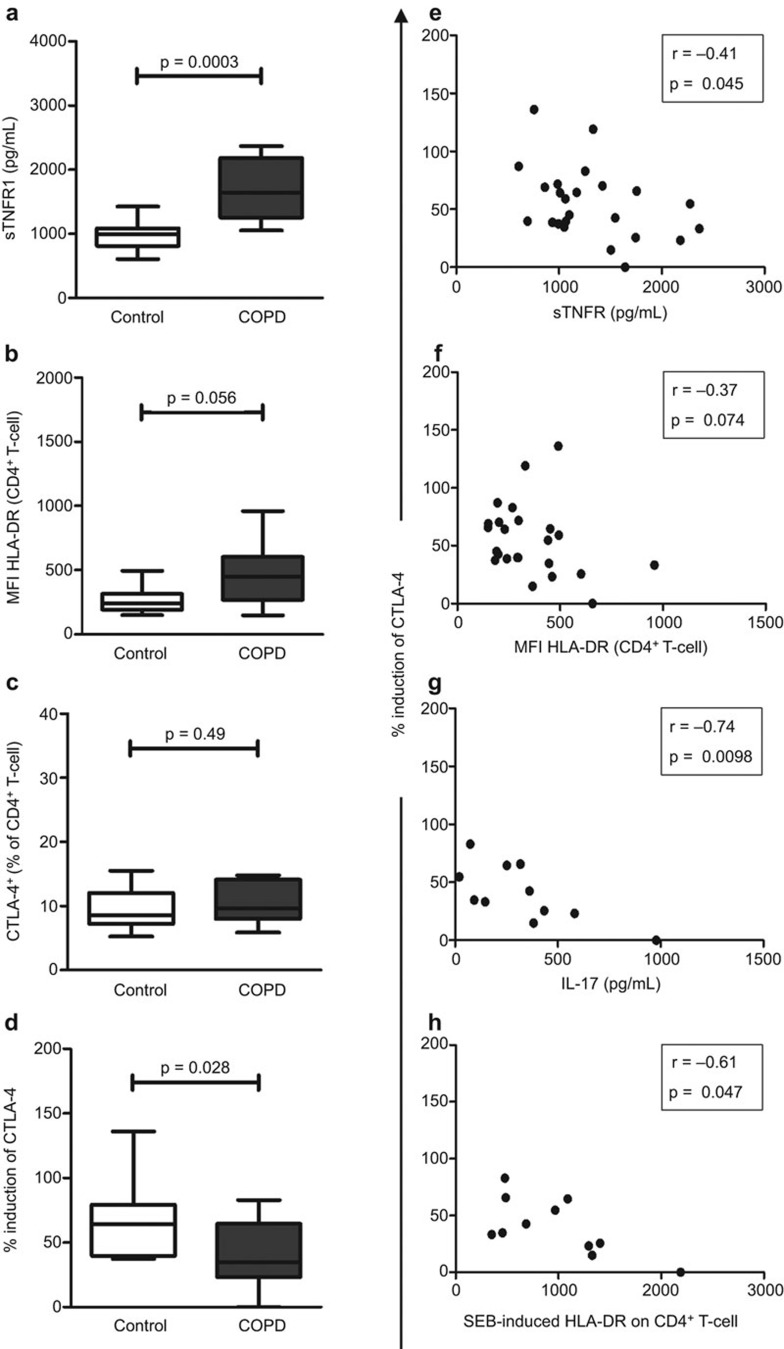
Increased systemic inflammation in COPD patients was evidenced by higher levels of sTNFR1 in plasma (P<0.001; Figure 1a) and HLA-DR expression on CD4+ T cells (P=0.056; Figure 1b) compared to controls. In response to inflammation, sTNFR1 is shed from cell surfaces and is increased in the sputum and blood of COPD patients.3 Elevated T-cell activation (including HLA-DR expression) has also been demonstrated in COPD patients.4
Figure 1.
COPD patients exhibited higher levels of systemic inflammation but lower induction of CTLA-4 on CD4+ T cells compared to controls. (a) Plasma concentration of sTNFR1 was measured by ELISA. (b) Mean fluorescence intensity (MFI) of HLA-DR expression on CD4+ T cells and ex vivo proportions of (c) CTLA-4+CD4+ T cells were quantified by flow cytometry. (d) Induction of CTLA-4 was calculated as a percentage relative to expression seen in unstimulated CD4+ T cells. Induction of CTLA-4 was correlated inversely with (e) plasma levels of sTNFR1, (f) ex vivo expression of HLA-DR on CD4+ T cells and (g) SEB-induced production of IL-17 and (h) activation of CD4+ T cells. Data are presented as median (bar within box) with interquartile (box) and min–max range (whiskers). COPD, chronic obstructive pulmonary disease; CTLA, cytotoxic T lymphocyte antigen; SEB, staphylococcal enterotoxin-B; sTNFR, soluble tumour-necrosis factor receptor.
Notably the proportion of CTLA-4+CD4+ T-cells was similar in unstimulated cells from patients and controls (P=0.49; Figure 1c). However, following stimulation with SEB there was lower induction of CTLA-4 in CD4+ T cells from COPD patients compared to cells from controls (P=0.028; Figure 1d). Hence, impaired induction of CTLA-4 may compromise anti-inflammatory mechanisms in T-cells of COPD patients.
Low induction of CTLA-4 was associated with increased levels of inflammatory markers ex vivo including plasma sTNFR1 (r=−0.41, P=0.045; Figure 1e) and HLA-DR expression on CD4+ T cells (r=−0.37, P=0.074; Figure 1f). This inverse relationship has two possible explanations. Immune activation may limit the production of CTLA-4 in response to an infective stimulus and predispose COPD patients to ongoing inflammation following infections. Alternatively, inherently low induction of CTLA-4 may promote immune activation.
Following SEB stimulation of PBMC, increased production of IL-17 (r=−0.74, P=0.01; Figure 1g) and HLA-DR expression on CD4+ T cells (r=−0.61, P=0.047; Figure 1h) from COPD patients also correlated with the lower induction of CTLA-4. Increased IL-17 levels associated with reduced CTLA-4 induction. IL-17 is associated with increased mucus production and airway inflammation with production of the neutrophil chemoattractant CXCL-8 and neutrophilia.6,7 In a metastatic melanoma model, blocking of CTLA-4 increased numbers of circulating Th17 cells and the production of IL-17.10 Hence, poor induction of CTLA-4 may promote Th17-mediated inflammation in patients with COPD or chronic bronchitis.
In conclusion, this study is the first to show impaired induction of CTLA-4 in COPD patients and to associate this with increased production of IL-17, activation of CD4+ T cells and markers of systemic inflammation. Overall, this suggests a mechanism by which defective regulation of T cell-mediated inflammation may contribute to the immune pathogenesis of COPD.
Acknowledgments
This work was supported by an Ada Bartholomew Grant from the Faculty of Medicine, Dentistry and Health Science, University of Western Australia and a Young Investigator Grant from the Medical Research Foundation of Royal Perth Hospital. We thank Dr Neil Misso for editorial support, the staff at the COPD Linkage Clinic, Ms Elizabeth Hyde and Ms Leah Christie for patient recruitment, and the patients and controls who volunteered for this study.
Footnotes
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary Information
References
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- Vernooy JH, Kucukaycan M, Jacobs JA, Chavannes NH, Buurman WA, Dentener MA, et al. Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am J Respir Crit Care Med. 2002;166:1218–1224. doi: 10.1164/rccm.2202023. [DOI] [PubMed] [Google Scholar]
- Roos-Engstrand E, Ekstrand-Hammarstrom B, Pourazar J, Behndig AF, Bucht A, Blomberg A. Influence of smoking cessation on airway T lymphocyte subsets in COPD. COPD. 2009;6:112–120. doi: 10.1080/15412550902755358. [DOI] [PubMed] [Google Scholar]
- Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Zhong X, Zhang J, Lao Q, He Z, Bai J. The expression of Foxp3 and ROR gamma t in lung tissues from normal smokers and chronic obstructive pulmonary disease patients. Int Immunopharmacol. 2011;11:1780–1788. doi: 10.1016/j.intimp.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang WB, Gao LB, Pan XM, Chen TY, Wang YY, et al. CTLA4 and CD86 gene polymorphisms and susceptibility to chronic obstructive pulmonary disease. Hum Immunol. 2010;71:1141–1146. doi: 10.1016/j.humimm.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Zhu G, Agusti A, Gulsvik A, Bakke P, Coxson H, Lomas DA, et al. CTLA4 gene polymorphisms are associated with chronic bronchitis. Eur Respir J. 2009;34:598–604. doi: 10.1183/09031936.00141808. [DOI] [PubMed] [Google Scholar]
- von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B, et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.