Neurological involvement in Behçet's disease (BD), namely, neuro-Behçet's disease (NBD), causes devastating central nervous system (CNS) complications and is present in 5% to 30% of patients with BD.1 Immunological and molecular pathways are involved in NBD and include the release of interleukin (IL)-1, -6 and -8, and TNF-α and IFN-γ into cerebrospinal fluid (CSF),2 which reflects a nonspecific inflammatory pattern that is compatible with auto-inflammatory disease pathways. IL-33 is an unconventional member of the IL-1 family that has been recently implicated in several inflammatory and autoimmune diseases.3 We document here, for the first time, that IL-33 is upregulated in the CNS of patients with NBD. We studied the IL-33 level and IL-33 mRNA expression in CSF and their correlation with the levels of the IP-10 and MCP-1 chemokines in 20 NBD patients, compared with those of 12 age-matched, non-inflammatory neurological disease (NIND) patients and 10 patients with headache attributed to Behçet's disease (HaBD). IL-33 was elevated in the CSF from NBD patients compared with those of disease controls. At the mRNA level, IL-33 was highly expressed in NBD. In parallel, nuclear factor κB (NF-κB), which mediates IL-33 transcription, was also elevated when compared with disease controls. Chemokine mRNA (IP-10 and MCP-1) was highly expressed in NBD compared with the disease controls. In summary, IL-33 levels are elevated in the central nervous system of patients with NBD, implicating IL-33 in BD neurological lesions.
BD is a complex, multisystem inflammatory disorder of unknown etiology. This disease typically manifests as recurrent oral and genital ulcerations and uveitis that are variably accompanied by symptoms affecting the skin, large vessels, gastrointestinal system and CNS. The precise mechanisms of tissue destruction in BD have not been fully elucidated. A number of viruses, including hepatitis viruses, parvovirus B19 and herpes simplex virus, has been implicated in the etiology of BD. All studied patients fulfilled the diagnostic criteria of the International Study Group for BD.4 Of the 22 NBD patients, 10 had parenchymal involvement (subacute neurological syndrome; brainstem involvement; hemispheric involvement with spinal cord involvement; bilateral pyramidal signs) and 12 patients had cerebral vein and arterial thrombosis.5 The treatment modalities consisted of immunosuppressive agents in combination with high oral doses or intravenous pulses of glucocorticosteroids.
The disease controls included two groups. The first was composed of BD patients suffering from HaBD, a symptom that is not considered a neurological manifestation of BD. The second control group included 12 patients with NIND, such as stroke or dementia. The Ethics Committee of Medicine at the University of Tunis approved the project, and informed consent was obtained from all participants. Blood and CSF were collected from NBD, HaBD and NIND patients; CSF was obtained at the time of initial presentation. IL-33 was measured in serum and CSF by sandwich ELISAs, as recently reported.6 NF-κB DNA-binding activity was analyzed using the TransAM NF-κB p65 transcription factor assay kit (Active Motif, Carlsbad, CA, USA).6
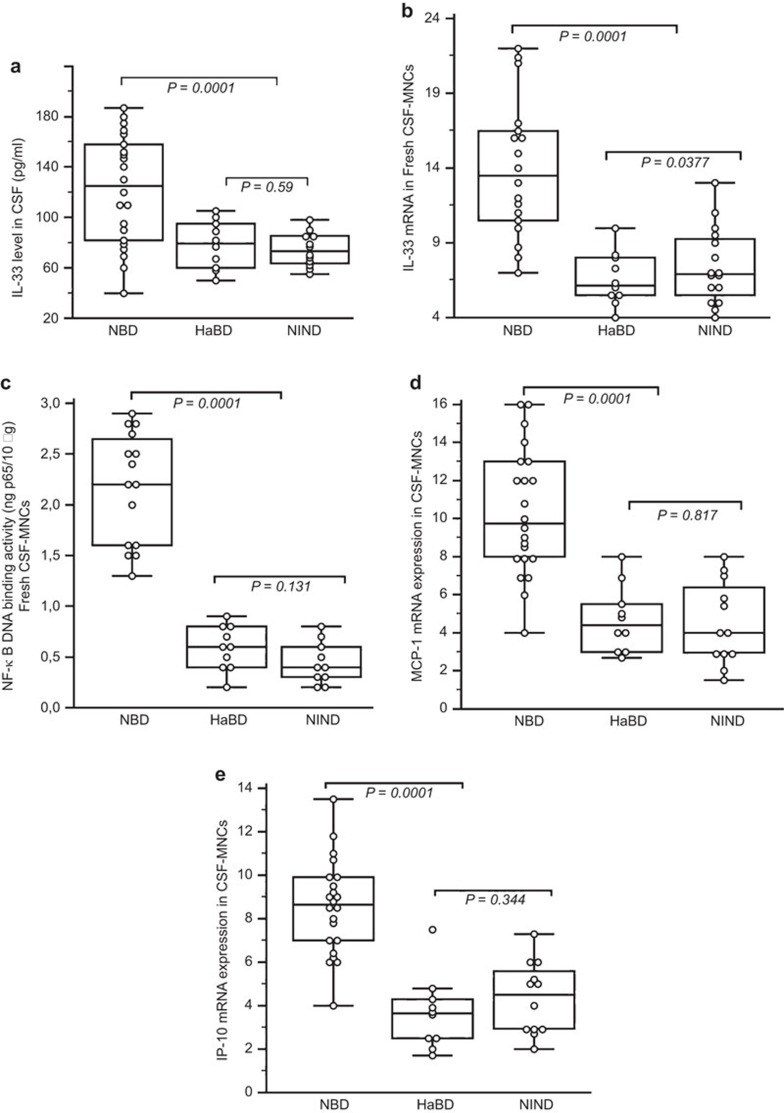
CSF from NBD patients had significantly higher levels of IL-33 (122±43.43 pg/ml; range: 40–187 pg/ml) than that of HaBD (78.30±19.05 pg/ml; range: 50–105 pg/ml; P=0.005) and NIND (74.50±13.45 pg/ml; range: 55–98 pg/ml; P=0.0001) patients (Figure 1a). Moreover, CSF IL-33 levels from NBD patients were increased compared to those in serum from NBD patients (84.63±27.74 pg/ml; P=0.0015). Increased levels of IL-33 mRNA transcripts were detected in NBD patients (13.92±4.55) compared with disease controls (HaBD: 6.59±1.78; NIND: 7.41±2.51; P<0.0001) (Figure 1b). A significant correlation was observed between IL-33 mRNA expression and the IL-33 protein level in NBD patients (r=0.820; P<0.0001).
Figure 1.
Expression of IL-33 in cerebrospinal fluid cells from patients with NBD. (a, b) The IL-33 level in CSF and IL-33 mRNA expression in CSF MNCs of NBD patients. The relative mRNA levels of IL-33 are normalized to GAPDH and expressed in AUs. IL-33 in CSF and IL-33 mRNA were highly expressed in NBD patients compared to HaBD and NIND patients. Pearson correlation test showed an association between the CSF IL-33 level and IL-33 mRNA expression (r=0.820; P<0.0001). (c) NF-κB activation in CSF MNCs from NBD patients and control diseases. The NF-κB DNA binding activity is reported as ng of bound p65 protein per 10 µg of total protein in nuclear extracts. NF-κB activation correlated with IL-33 mRNA expression in NBD patients (r=0.739; P=0.00016). (d, e) MCP-1 and IP-10 chemokine mRNA expression in CSF MNCs of NBD patients (15 patients tested). The MCP-1 and IP-10 mRNAs were highly expressed in NBD compared to HaBD (10) and NIND (10) patients. Significant correlations were observed between IL-33 mRNA expression and MCP-1 (r=0.689; P=0.0015) and IL-33 mRNA expression and IP-10 (r=0.753; P=0.0003) in NBD patients. In the figures, the median is indicated by a line inside each box, the 25th and 75th percentiles are indicated by box limits, and the lower and upper error bars represent the 10th and 90th percentiles, respectively. The data in the text are presented as the mean±s.d. Values were compared using Kruskal–Wallis analysis with Dunn's correlation for multiple testing. AU, arbitrary unit; CSF, cerebrospinal fluid; HaBD, headache attributed to Behçet's disease; MNC, mononuclear cell; NBD, neuro-Behcet's disease; NF-κB, nuclear factor κB; NIND, non-inflammatory neurological disease; s.d., standard deviation.
To examine the possible mechanisms of elevated IL-33 expression in leukocytes of NBD patients, we quantified the activation of the transcription factor NF-κB, which has been shown to mediate IL-33 induction.7 Nuclear extracts from freshly isolated CSF leukocytes were prepared and allowed to bind to an NF-κB consensus oligonucleotide sequence. Bound NF-ou was then detected by a p65 (RelA)-specific antibody and quantified based on calibration using purified p65 recombinant protein. NF-κB DNA binding activity was significantly increased in NBD patients (2.166±0.54) compared to NIND (0.44±0.20; P=0.0001) and HaBD (0.59±0.21; P=0.0001) patients (Figure 1c). Furthermore, NF-κB activation correlated with IL-33 mRNA expression in NBD patients (r=0.739; P=0.00016).
The mRNA expression of the IP-10 and MCP-1 chemokines was quantified to find a correlation with IL-33 that reflected the degree of inflammation in the CNS. Chemokine mRNAs were highly expressed in NBD CNS mononuclear cells compared to the disease controls (P=0.0001; P=0.0001) (Figure 1d and e). We did not observe significant differences between the MCP-10 (P=0.817) and IP-10 (P=0.344) levels in NIND and HaBD patients, but significant correlations were observed between the mRNA expression of IL-33 and MCP-1 (r=0.689; P=0.0015) and IL-33 and IP-10 (r=0.753; P=0.0003) in NBD patients.
IL-33 is a tissue-derived cytokine that is released in damaged tissues or necrotic cells, and acts as an alarmin3 in the host defense against pathogens. For BD patients with neurological manifestations, IL-33 levels were remarkably higher in CSF than in serum, suggesting that it may be a predominant mediator that is locally induced when neurological damage occurs. Indeed, recent reports confirmed that IL-33 is expressed in human CNS tissues, indicating that IL-33 may act as a potentially critical regulator of innate immune responses in the CNS, similar to what was reported for IP-10 and MCP-1.8
MCP-1 and IP-10 mRNA levels positively correlate with IL-33 mRNA expression in NBD patients. Unfortunately, because obtaining sufficient numbers of eligible CSF samples from healthy controls is difficult, we could not compare the CSF levels of these factors between NBD patients and healthy controls. Because chemokines are produced by CNS cells within hours after injury and are thought to mediate the recruitment and activation of mononuclear phagocytes,9 we suppose that increased chemokine levels in the CSF of NBD patients may mediate early regulation and recruitment of protective immunity to reduce severe tissue damage.
The major role of IL-33 in the CSN of our patients is reflected by the positive correlation that was observed between the mRNA expression of IL-33 and its protein level in the CSF. Our data suggest that in our patients, IL-33 expression in the CSF is intrinsic to CNS inflammatory lesions. Considering the hypothesis of an infectious trigger in BD pathogenesis, IL-33, together with additional inflammatory mediators such as MCP-1 and IP-10, could be critically required for fighting the hypothetical pathogen. In the same way, the activity of NF-κB, which has been shown to mediate IL-33 induction, was significantly increased in NBD patients and correlated with IL-33 mRNA expression. However, IL-33 is also considered a transcriptional repressor that dampens NF-κB activity.10
Our data pointed to a possible inflammatory role of IL-33 in the CNS of NBD patients that could contribute to immune cell activation and augment signaling pathways that mediate oligodendrocyte and neuronal injury. IL-33 and chemokines secreted during disease are key properties to evaluate because they can help us to understand disease development and inform us of ways to develop treatments and cures. Further studies with a wider range of patient samples are needed to determine the impact of treatment on disease progression and the secretion of these mediators. Furthermore, the target for IL-33 in NBD is another key, unanswered question that requires histological studies, as both microglia and astrocytes express IL-33 receptors. Future studies investigating if and how the IL-33 protein plays a beneficial or detrimental role in the CNS injury of NBD are needed.
Acknowledgments
This work was supported by grants from the Ministry of Research and the Ministry of Health of Tunisia (Unit Research 12SP15 ‘Molecular expression of cellular interactions and modes of communication').
The authors have no financial conflicts of interest.
References
- Farahangiz S, Sarhadi S, Safari A, Borhani-Haghighi A. Magnetic resonanceimaging findings and outcome of neuro-Behçet's disease: the predictive factors. Int J Rheum Dis. 2012;15:e142–e149. doi: 10.1111/1756-185X.12013. [DOI] [PubMed] [Google Scholar]
- Saruhan-Direskeneli G, Yentür SP, Akman-Demir G, Işik N, Serdaroğlu P. Cytokines and chemokines in neuro-Behçet's disease compared to multiple sclerosis and other neurological diseases. J Neuroimmunol. 2003;145:127–134. doi: 10.1016/j.jneuroim.2003.08.040. [DOI] [PubMed] [Google Scholar]
- Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- International Study Group for Behçet's disease Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- Borhani Haghighi A. Treatment of neuro-Behçet's disease: an update. Expert Rev Neurother. 2009;9:565–574. doi: 10.1586/ern.09.11. [DOI] [PubMed] [Google Scholar]
- Hamzaoui K, Kaabachi W, Fazaa B, Zakraoui L, Mili-Boussen I, Haj-Sassi F. Serum IL-33 levels and skin mRNA expression in Behçet's disease. Clin Exp Rheumatol. 2013;31 3 Suppl 77:6–14. [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Hudson CA, Christophi GP, Gruber RC, Wilmore JR, Lawrence DA, Massa PT. Induction of IL-33 expression and activity in central nervous system glia. J LeukocBiol. 2008;84:631–643. doi: 10.1189/jlb.1207830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2005;288:L3–L15. doi: 10.1152/ajplung.00405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Mohs A, Thomas M, Klare J, Ross R, Schmitz ML, et al. The dual function cytokine IL-33 interacts with the transcription factor NF-κB to dampen NF-κB-stimulated gene transcription. J Immunol. 2011;187:1609–1616. doi: 10.4049/jimmunol.1003080. [DOI] [PubMed] [Google Scholar]