Abstract
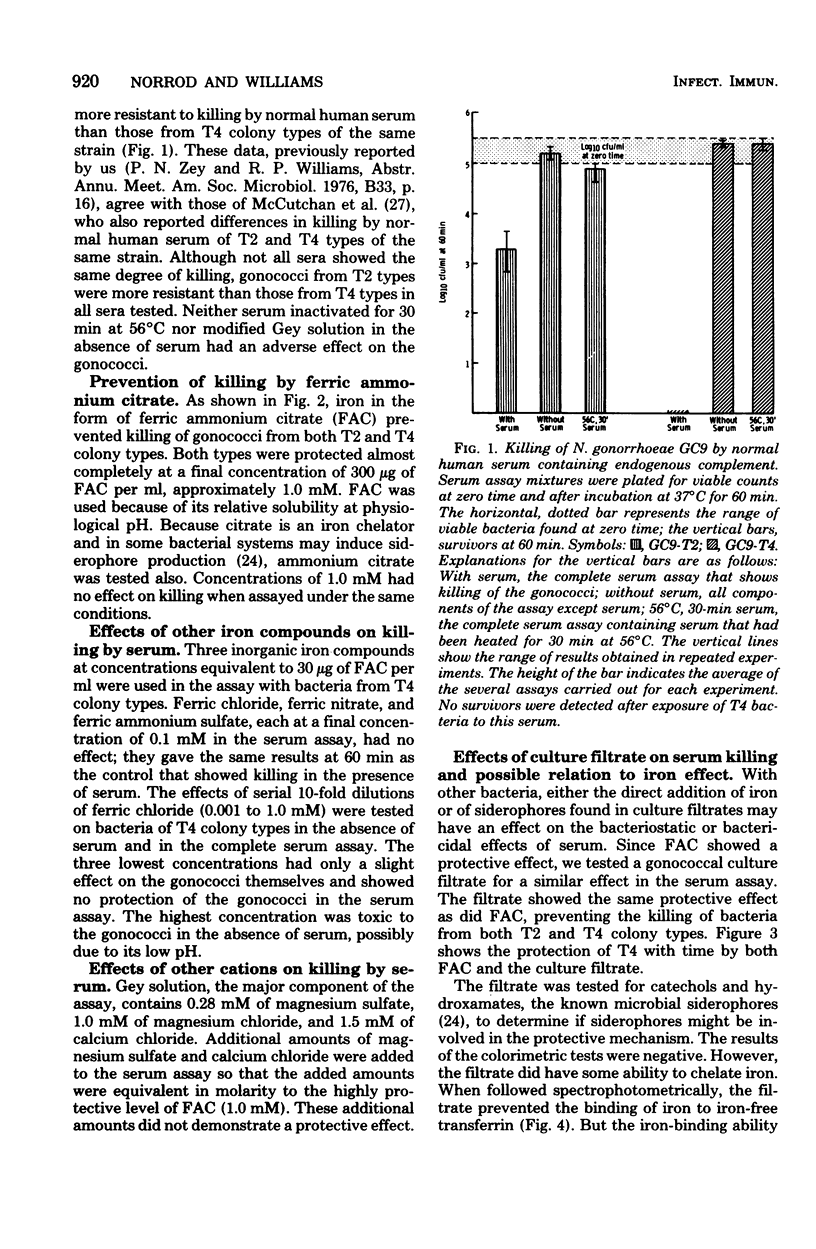
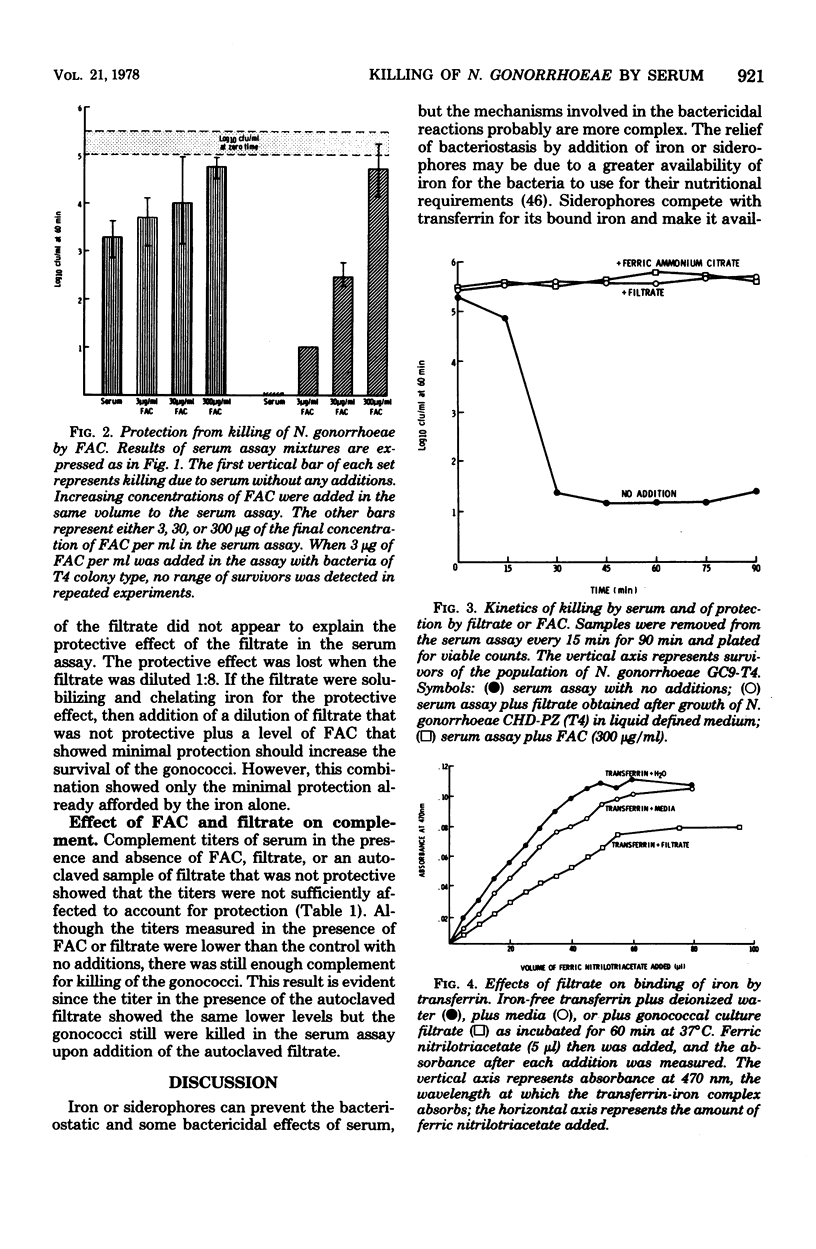
Neisseria gonorrhoeae GC9, both colony types T2 and T4, were killed by normal human serum, although populations of colony type T4 were more susceptible. Ferric ammonium citrate prevented the killing of populations of both T2 and T4 colony types. Other iron compounds tested showed no protective effect, nor did ammonium citrate or the divalent cations magnesium or calcium. A filtrate from cultures of an N. gonorrhoeae strain grown in a liquid defined medium showed a similar protective effect in the serum assay. The filtrate appeared to chelate iron, as measured by decreased ability of iron-free transferin to bind iron in the presence of the filtrate. However, the two effects did not appear to be related. Neither ferric ammonium citrate nor the culture filtrate sufficiently inactivated complement to account for protection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Booij J. Some divergent results of protein standardization. Clin Chim Acta. 1972 May;38(2):355–362. doi: 10.1016/0009-8981(72)90126-x. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Elmros T., Burman L. G., Bloom G. D. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 May;126(2):969–976. doi: 10.1128/jb.126.2.969-976.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J. The effect of iron and transferrin on the killing of Escherichia coli in fresh serum. Immunology. 1971 Apr;20(4):493–500. [PMC free article] [PubMed] [Google Scholar]
- Galley C. B., Walker R. I., Ledney G. D., Gambrill M. R. Evaluation of biologic activity of ferric chloride-treated endotoxin in mice. Exp Hematol. 1975 Jun;3(3):197–204. [PubMed] [Google Scholar]
- Gibson F., Magrath D. I. The isolation and characterization of a hydroxamic acid (aerobactin) formed by Aerobacter aerogenes 62-I. Biochim Biophys Acta. 1969 Nov 18;192(2):175–184. doi: 10.1016/0304-4165(69)90353-5. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths E. Abnormal phenylalanyl-tRNA found in serum inhibited Escherichia coli, strain 0111. FEBS Lett. 1972 Sep 1;25(1):159–164. doi: 10.1016/0014-5793(72)80476-9. [DOI] [PubMed] [Google Scholar]
- Griffiths E. Mechanism of action of specific antiserum on Pasteurella septica. Selective inhibition of net macromolecular synthesis and its reversal by iron compounds. Eur J Biochem. 1971 Nov 11;23(1):69–76. doi: 10.1111/j.1432-1033.1971.tb01593.x. [DOI] [PubMed] [Google Scholar]
- Hafiz S., McEntegart M. G., Jephcott A. E. Reversion of Kellogg's colonial types of Neisseria gonorrhoeae in liquid medium. J Med Microbiol. 1977 Aug;10(3):377–380. doi: 10.1099/00222615-10-3-377. [DOI] [PubMed] [Google Scholar]
- Hafiz S., McEntegart M. G. Prolonged survival of Neisseria gonorrhoeae in a new liquid medium. Br J Vener Dis. 1976 Dec;52(6):381–383. doi: 10.1136/sti.52.6.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. N., Knox J. M., Williams R. P. Attachment of gonococci to sperm. Influence of physical and chemical factors. Br J Vener Dis. 1976 Apr;52(2):128–135. doi: 10.1136/sti.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvach J. T., Wiles T. I., Mellencamp M. W., Kochan I. Use of transferrin-iron enterobactin complexes as the source of iron by serum-exposed bacteria. Infect Immun. 1977 Nov;18(2):439–445. doi: 10.1128/iai.18.2.439-445.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D. V., James A. N., Williams R. P. Radiolabeling of and macromolecular syntheses in Neisseria gonorrhoeae types 1 and 4. Appl Environ Microbiol. 1977 Feb;33(2):328–333. doi: 10.1128/aem.33.2.328-333.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- O'Reilly R. J., Welch B. G., Kellogg D. S., Jr An indirect fluorescent-antibody technique for study of uncomplicated gonorrhea. II. Selection and characterization of the strain of Neisseria gonorrhoeae used as antigen. J Infect Dis. 1973 Jan;127(1):77–83. doi: 10.1093/infdis/127.1.77. [DOI] [PubMed] [Google Scholar]
- Odugbemi T. O., Dean B. Iron contents of different colonial types of Neisseria gonorrhoeae. J Gen Microbiol. 1978 Jan;104(1):161–163. doi: 10.1099/00221287-104-1-161. [DOI] [PubMed] [Google Scholar]
- Odugbemi T. O., Hafiz S. The effects of iron chelators on the colonial morphology of Neisseria gonorrhoeae. J Gen Microbiol. 1978 Jan;104(1):165–167. doi: 10.1099/00221287-104-1-165. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: role of iron in virulence. Infect Immun. 1975 Dec;12(6):1313–1318. doi: 10.1128/iai.12.6.1313-1318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. The critical role of iron in host-bacterial interactions. J Clin Invest. 1978 Jun;61(6):1428–1440. doi: 10.1172/JCI109062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Ferric iron and the antibacterial effects of horse 7S antibodies to Escherichia coli O111. Immunology. 1976 Mar;30(3):425–433. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink W. W., Keefer C. S. STUDIES OF GONOCOCCAL INFECTION. I. A STUDY OF THE MODE OF DESTRUCTION OF THE GONOCOCCUS IN VITRO. J Clin Invest. 1937 Mar;16(2):169–176. doi: 10.1172/JCI100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink W. W., Keefer C. S. STUDIES OF GONOCOCCAL INFECTION. II. THE BACTERIOLYTIC POWER OF THE WHOLE DEFIBRINATED BLOOD OF PATIENTS WITH GONOCOCCAL ARTHRITIS. J Clin Invest. 1937 Mar;16(2):177–183. doi: 10.1172/JCI100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Artenstein M. S. Cross-reactivity of Neisseria gonorrhoeae and Neisseria meningitidis and the nature of antigens involved in the bactericidal reaction. J Infect Dis. 1974 Sep;130(3):240–247. doi: 10.1093/infdis/130.3.240. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: relationship between autolysis in buffer and the hydrolysis of peptidoglycan. Infect Immun. 1977 Oct;18(1):210–219. doi: 10.1128/iai.18.1.210-219.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Welch B. G., O'Reilly R. J. An indirect fluorescent-antibody technique for study of uncomplicated gonorrhea. I. Methodology. J Infect Dis. 1973 Jan;127(1):69–76. doi: 10.1093/infdis/127.1.69. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D., Lankford C. E. Production by Salmonella typhimurium of 2,3-dihydroxybenzoylserine, and its stimulation of growth in human serum. J Infect Dis. 1970 Feb;121(2):129–136. doi: 10.1093/infdis/121.2.129. [DOI] [PubMed] [Google Scholar]
- Wong K. H., Arko R. J., Logan L. C., Bullard J. C. Immunological and serological diversity of Neisseria gonorrhoeae: gonococcal serotypes and their relationship with immunotypes. Infect Immun. 1976 Dec;14(6):1297–1301. doi: 10.1128/iai.14.6.1297-1301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


