Abstract
The ovary is not an immunologically privileged organ, but a breakdown in tolerogenic mechanisms for ovary-specific antigens has disastrous consequences on fertility in women, and this is replicated in murine models of autoimmune disease. Isolated ovarian autoimmune disease is rare in women, likely due to the severity of the disease and the inability to transmit genetic information conferring the ovarian disease across generations. Nonetheless, autoimmune oophoritis is often observed in association with other autoimmune diseases, particularly autoimmune adrenal disease, and takes a toll on both society and individual health. Studies in mice have revealed at least two mechanisms that protect the ovary from autoimmune attack. These mechanisms include control of autoreactive T cells by thymus-derived regulatory T cells, as well as a role for the autoimmune regulator (AIRE), a transcriptional regulator that induces expression of tissue-restricted antigens in medullary thymic epithelial cells during development of T cells. Although the latter mechanism is incompletely defined, it is well established that failure of either results in autoimmune-mediated targeting and depletion of ovarian follicles. In this review, we will address the clinical features and consequences of autoimmune-mediated ovarian infertility in women, as well as the possible mechanisms of disease as revealed by animal models.
Introduction: infertility and its consequences
Infertility is defined as a lack of pregnancy over a period of 12 months, despite unprotected intercourse each month with the same partner. It impacts nearly 50 million couples globally, with approximately a third of these due to female-only factors.1,2 Estimates in 2010 revealed that as many as 6%, or 1.5 million, of married, reproductive age women are infertile within the United States alone.3 Perhaps surprisingly, some of this is due to increasing rates of sexually transmitted diseases that negatively impact fertility,4 as well as the trend of women to delay childbearing for personal and professional reasons. Yet despite the increasing awareness and availability of effective clinical treatments for age-related female infertility, the emotional and financial burden on these individuals and on society is immense. Compounding these burdens is the uncertainty surrounding the potential long-term impact on the health of children born after assisted reproductive treatment.5
Female fertility can be impaired by any condition that reduces or prevents ovulation, and ovulatory disorders are the most common known causes of female factor infertility.6 The World Health Organization has classified female anovulatory infertility to include three unique categories. Normogonadotropic normogonadism is the most common cause of infertility in women, accounting for about 80% of oligo- or anovulatory women. These women nearly always present with polycystic ovary syndrome: a complex genetic and endocrine disease associated with elevated androgen levels, obesity and hirsuitism. Polycystic ovary syndrome is thought to result in excessive recruitment of preovulatory follicles that fail to respond to physiological levels of follicle-stimulating hormone, ultimately leading to failed development of the Graafian follicle. Hypogonadotropic hypogonadism is characterized by lower than normal levels of pituitary and ovarian hormones, and accounts for roughly 15% of anovulatory disorders. Clinically, this disorder is commonly caused by very low body weight, extended periods of strenuous physical activity, or genetic disruptions resulting in failed release of gonadotropin releasing hormone.
Hypergonadotropic hypogonadism, on the other hand, occurs when ovarian follicles are lost prematurely, i.e., prior to the age of 40. While follicle depletion eventually occurs in all women naturally as they age, premature follicular depletion is an important cause of infertility in women under 40. Although still sometimes referred to as primary ovarian failure or premature menopause, it is generally agreed that the term primary ovarian insufficiency (POI) is more scientifically accurate, as this disease encompasses a wide clinical spectrum, occurs gradually over time, and does not necessarily represent a complete void of follicles.7 POI affects 1 in 100 women under the age of 40 and is defined clinically as 4–6 months of amenorrhea with elevated follicle-stimulating hormone and low estrogen levels.8 Its symptoms and signs are heterogeneous and its etiology is multifactorial, to include genetic and chromosomal abnormalities, infection, iatrogenic causes (e.g., chemotherapy) and autoimmune disease.7
Unfortunately, women experiencing POI have little recourse for establishing a natural pregnancy. The natural fecundity rate (ability to carry a pregnancy to term) of women diagnosed with POI is only 4%–8%.9 Because these women respond poorly to gonadotropin stimulation,10,11 assisted reproductive technologies are unlikely to remedy POI-associated infertility. Early detection of POI, prior to elevation of gonadotropins to menopausal levels, is therefore key if women desire pregnancy; egg donation, surrogacy, and adoption are the primary alternatives once follicular reserves are depleted.
Apart from the emotional stress of infertility, POI places women at elevated risk for osteoporosis, cognitive decline, cardiovascular disease, some types of cancers and importantly, mortality of any cause.12 Premenopausal estrogen maintains bone density in women and premature loss of this estrogen due to accelerated menopause elevates risk for osteoporosis and bone fractures.13,14 Although hormone replacement therapy can correct this, it may also place women at increased risk of coronary heart disease, stroke, pulmonary embolism, and invasive breast cancer.15 Overall, accelerated reproductive senescence due to depletion of ovarian reserves is associated with reduced quality of life and decreased lifespan.16
Autoimmune oophoritis as a cause for primary ovarian insufficiency
Autoimmune oophoritis is one of a larger group of autoimmune endocrinopathies in which immunological self-tolerance to hormone-producing organs fails. Currently, the histopathological analysis of the ovary is the only way to definitively diagnose autoimmune oophoritis, although due to general inaccessibility of the ovaries, this is often not attainable.17,18 Instead, the majority of women with POI are diagnosed based on presence of antibodies reactive against ovarian tissue. Further, autoimmune POI is almost always associated with autoimmunity against other organs, in particular, autoimmune Addison's disease and the presence of serum autoantibodies targeting the adrenal gland.19,20 Thus, the incidence of autoimmune POI that is associated with these autoantibodies is approximately 4%.21
More than 80% of patients with autoimmune Addison's disease possess serum antibodies that recognize the adrenal cortex (adrenal cortex antibodies, ACAs).22 All women identified to date with histological evidence of autoimmune oophoritis possess ACA; paradoxically, the major target of ACA, 21-hydroxylase (CYP21A2),22,23,24 is not expressed in the ovary. In addition to autoantibodies to CYP21A2, autoantibodies to two other steroidogenic enzymes, P450 side chain cleavage (CYP11A1) and 17α-hydroxylase (CYP17), which convert cholesterol to pregnenolone, and progesterone to 17-hydroxyprogesterone, respectively, are present.21,25,26,27 Since these latter enzymes, unlike CYP21A2, are expressed in the ovary, the presence of these antibodies may signify the actual autoimmune targets of the ovary. Antibodies targeting P450ssc and CYP17 are termed ‘steroid cell' (SC) antibodies, since in addition to binding the adrenal cortex, they bind placental syncytiotrophoblast cells, Leydig cells of the testes, and theca and luteal cells of the ovary. Some reports also include 3β-hydroxysteroid dehydrogenase as a target of SC antibodies; however, others do not support this premise.26,28,29
Although diagnostic markers of POI for use in the clinic have not been developed to date, adrenal cortex autoantibodies are predictive of autoimmune oophoritis.21 Conversely, a patient presenting with POI who is found to possess SC autoantibodies should be closely monitored for emergent adrenal insufficiency because of its overlap with, and the life-threatening nature of, Addison's disease.21
Pathology
Grossly, 60% of patients with POI present with enlarged, multicystic ovaries, while 33% and 7% show normal or small ovarian size, respectively.21,30 Ovarian histopathology, when available, reveals infiltration of CD4+ and CD8+ T lymphocytes and plasma cells into the theca interna and externa layers of the follicle and within the corpus luteum, and occasionally the granulosa cell layer is also involved.31 Lymphocytic infiltration seems to occur only in women with serum ACA and SC antibodies.21,32 Additionally, it is reported that primordial follicles are spared, while growing preantral and antral follicles are either destroyed and/or undergo cystic luteinization of granulosa cells.7,19,20,31,33 (see Figures 1 and 4 for histological features of the ovary).
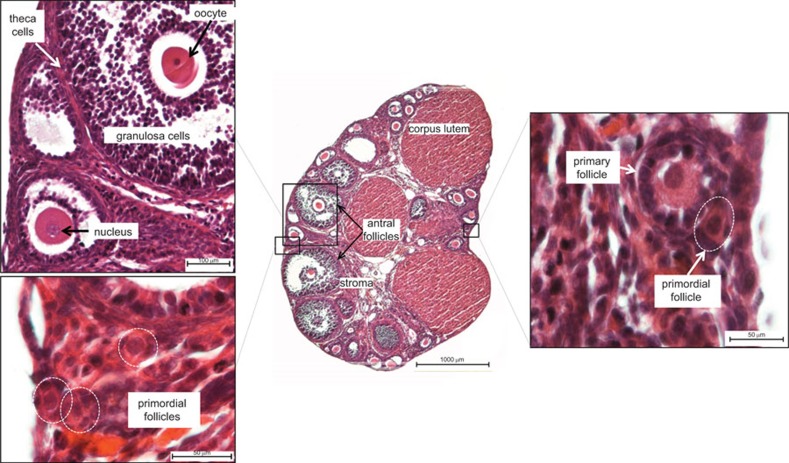
Figure 1.
Histological features of the normal post-pubertal ovary. These images show the structures of a post-pubertal mouse ovary; salient features of the human ovary are essentially the same. Major structures include follicles, which house the oocytes, and the post-ovulatory correlate of the follicle, the corpus luteum. Multiple follicles at various stages of development are present within the pre-senescent ovary at any given time. Several stages of follicles can be seen in this section, including the developmentally earliest stages, primordial and primary follicles (lower left and right insets), and the more advanced antral follicles (center and upper left inset). Oocytes are encased in follicular steroidogenic cells, the inner granulosa and the outer theca cell layers (upper left inset); theca interna and theca externa are not clearly depicted in this image but can be seen in Figure 4. Mature follicles and corpora lutea produce estrogen and progesterone, respectively, which are required sequentially for uterine preparation and maintenance of pregnancy. The sample is stained with hematoxylin and eosin. Scale bars: 100 µm (upper left); 50 µm (lower left); 1000 µm (center); 50 µm (right).
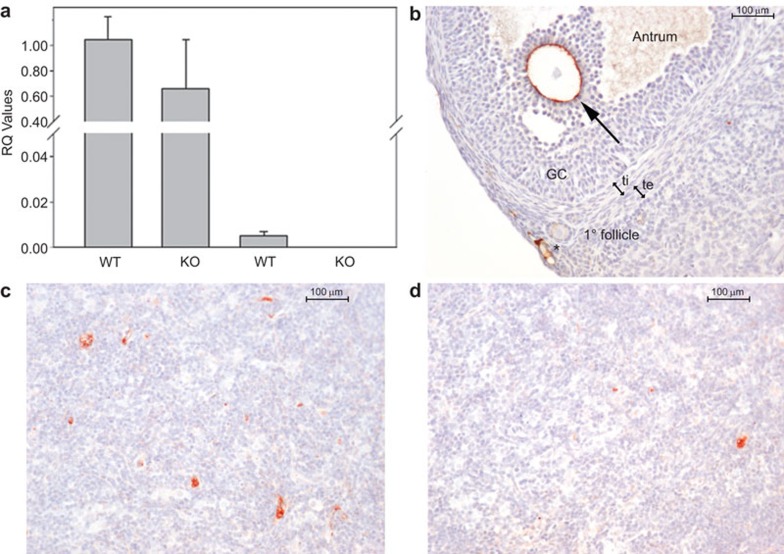
Figure 4.
ZP3 expression in the murine thymic medulla is mediated by Aire. (a) ZP3 mRNA is abundant in the ovaries of both WT and Aire-deficient mice (KO) (right two bars), and is detectable in WT thymi but not KO thymi (left two bars). n=3–4 mice per condition. RQ, relative quantity with respect to a WT mouse ovary sample. (b–d) Immunohistochemical localization of ZP3 in the ovary and thymic medulla of a WT and a KO mouse. In the ovary (b; positive control), ZP3 immunoreactivity is observed surrounding the oocyte of an antral follicle (arrow) and a primary follicle. Nonspecific staining was also observed in the vicinity of vessels (*). In WT thymic medulla (c), ZP3 immunoreactivity is seen in large cells with abundant cytoplasm, presumably mTEC. Immunoreactivity was rare in thymi of Aire-deficient mice (d), although occasional positive cells were observed. Counterstain, hematoxylin. Scale bars=100 µm. AIRE, autoimmune regulator; GC, granulosa cells; ti, theca interna; te, theca externam TEC, medullary thymic epithelial cell; KO, knockout; WT, wild-type.
Proposed mechanism of autoimmune POI in women
La Marca and colleagues34 offered a possible mechanism that integrates the endocrine profiles observed in women with autoimmune POI and histopathological findings, particularly in cases of coincident adrenal disease. Theca cells appear to be the target of autoimmune destruction within the ovary, possibly because of their expression of antigenic enzymes, CYP11A1 and CYP17, and as evidenced by lymphocytic infiltration into this layer.31 However, steroid-producing adrenal cells may often represent the initial target with targeting of theca cells occurring secondarily, since autoimmune POI rarely occurs in isolation, yet is nearly always associated with preclinical or clinical adrenal autoimmune disease and the presence of ACA. Theca cell destruction will result in a failure of these cells to supply androgens to granulosa cells for estradiol synthesis. The resulting drop in serum estradiol likely releases negative feedback to the hypothalamus, leading to elevated levels of follicle-stimulating hormone and luteinizing hormone. Despite the impaired production of estradiol, granulosa cells can remain viable and functional, explaining the high levels of inhibins seen in these patients,31,35 a distinguishing feature of autoimmune POI. The sparing of primordial follicles often observed aligns with the fact that they lack the theca layer of cells, and with the potential recoverability of follicular function following immunosuppressive corticosteroid therapy if pregnancy is desired.36,37,38,39 Indeed, women with autoimmune POI appear to maintain a preserved follicular pool as evidenced by maintenance of serum anti-Mullerian hormone, sometimes for years after diagnosis; however, growing follicles eventually appear to be completely depleted.40
It is possible that primordial follicles are also eventually depleted, either due to increased rate of recruitment or to immune-mediated destruction. Since anti-Mullerian hormone controls the rate of transition of primordial to growing follicles,41 its decline, caused by loss of growing follicles, might accelerate their recruitment into the pool of growing follicles. Newly recruited follicles might then express target antigens and become targets themselves, disallowing any substantial rise in anti-Mullerian hormone. This cycle might then be repeated, leading to a feed-forward loop of rapid follicular recruitment and subsequent destruction. Alternatively, primordial follicles may undergo immunologically mediated loss—either as a result of eventual antigen-specific immunity, or, more likely, nonspecifically as bystanders in an inflammatory environment.
Cases of autoimmune POI that occurs in the absence of adrenal disease and SC antibodies are rare, yet they exist and their presence provides unique insights into ovarian autoimmune POI. In these instances, antibodies against the thyroid gland or acetylcholine receptor have been observed.21,27,42 The autoimmune diseases typically associated with these non-SC antibody-associated POI cases include thyroid disease, myasthenia gravis, and autoimmune polyglandular syndrome (APS) types I and II.43,44,45,46 It seems likely that as with SC antibodies, multiple and shared antigens among endocrine organs raises susceptibility of involvement of multiple organs, including the ovary.
APS-1 is caused by mutation of the autoimmune regulator (AIRE) gene
Autoimmune-mediated ovarian failure is associated with APS type 1 (APS-1), an autosomal recessive disorder caused by mutations in the AIRE gene that leads to a varied display of destructive immune reactions against both endocrine and non-endocrine organs. The most commonly affected organs include the adrenal and parathyroid glands, the pancreas, gastric parietal cells and gonads (male and female). Atypical presentations are more common in young patients and can include dry eye, hyperkalemia, nephritis and rash.47 APS-1 is diagnosed based on the presence of two of three clinical signs: adrenal insufficiency, hypoparathyroidism and chronic mucocutaneous candidiasis.48 More recently, an additional criterion has been proposed based on the presence of neutralizing autoantibodies against type I interferons and interleukin (IL)-17A, IL-17F and/or IL-22 in nearly all APS-1 patients.49,50 The disease usually manifests in childhood with chronic candidiasis; all three of the main clinical manifestations are present by age 20 in about a third of the patients.51 However, the clinical course and phenotype of the disease vary widely and although there is some association with certain human leukocyte antigen class II haplotypes, the specific mutation in the AIRE gene does not seem to dictate clinical phenotype.52,53
Gonadal involvement in APS-1 patients is diagnosed by the presence of hypergonadotropic hypogonadism, and appears in more than half of women by age 20 (prepubertal girls are not usually tested); half of these patients present with primary amenorrhea.51,54 Like other cases of autoimmune POI that are not associated with APS-1, adrenal insufficiency most often precedes hypogonadism. Furthermore, autoantibodies against CYP11A1, CYP21A2 and CYP17 are frequently found.25
While APS-1 is rare globally, in several populations the frequency is increased (1∶9000 in Iranian Jews; 1∶14 000 in Sardinians; 1∶25 000 in Finns). APS-1 was identified as a monogenic disease caused by function-disrupting mutations in the AutoImmuneREgulator gene (AIRE).55,56 More than 80 nonsense, splicing, deletion, and insertion mutations in AIRE have been identified (http://www.hgmd.cf.ac.uk); the most common of these are R257X nonsense and the 964del13 deletion mutations.57,58
Promiscuous gene expression in thymic medullary epithelial cells
The identification of AIRE as the gene responsible for APS-1 in 199755,56 was a pivotal finding of what turned out to be a paradigm shift in our basic understanding of the mechanisms that shape immunological self-tolerance. Mechanisms of immune tolerance had traditionally been categorized into central tolerance, in which developing T cells undergo selection in the thymus such that they recognize only foreign (non-self) antigens presented by major histocompatibility complex molecules, and peripheral tolerance, in which self-reactive T cells that escape thymic central tolerance encounter tissue-specific antigens in the periphery and are induced towards tolerance. In a seminal paper, Derbinski and colleagues59 succinctly summarized the state of knowledge at the time, paraphrased below:
…during the intrathymic development of T cells … the scope of T cell tolerance [is restricted] to those self-antigens that are presented by thymic antigen-presenting cells. It is hardly conceivable that this set of self-antigens should encompass all the self-antigens expressed by parenchymal organs. Accordingly, the thymus is regarded as the site of induction of tolerance to ubiquitously expressed proteins and…blood-borne self-antigens. In contrast, …tolerance to self-antigens that are confined to specific tissues … is facilitated by post-thymic peripheral mechanisms60 or … ignored by the immune system.61
However, some accounts suggested that the restriction of the thymus to ubiquitous self-antigen tolerance was not necessarily true. Perhaps the earliest observation of this was reported by Jolicoeur et al.,62 who used transgenic mice expressing the viral oncogene SV40 T antigen (Tag) under the control of the rat insulin promoter (RIP). These ‘RIP-Tag' mice develop pancreatic β-cell tumors at ∼10 weeks of age, and there was prior evidence of impaired immune surveillance to Tag-expressing tumors. These mice were immunologically tolerant to Tag as evidenced by impaired systemic antibody and T-cell responses; this prompted investigation into whether the transgene was ectopically expressed in the thymus. Surprisingly, Tag mRNA was detectable in thymi of transgenic mice, but apart from robust (and expected) expression in the pancreas and testis, was absent in nearly all of the 15 other tissues examined. Importantly, the authors also demonstrated that thymic expression of Tag under the insulin promoter mirrors expression of endogenous insulin in the thymus, ruling out the possibility of artifactual ectopic expression of the transgene.62 This observation, among others, provided the basis for a novel concept that central tolerance could account for immune tolerance to antigens that are otherwise only expressed in a select few tissues.
Additional reports followed that demonstrated thymic expression of myelin components, which are otherwise limited to the central nervous system; that this expression is sufficient for induction of immune tolerance was demonstrated for a splice variant of proteolipid protein, the principal component of myelin.63,64 Notably, when thymic expression of tissue-restricted antigens was examined in situ, proteins and transcripts were found to be distributed sparsely among medullary thymic epithelial cells (mTECs).65 Further studies were carried out on purified and sorted mTEC from mouse, and then human thymus, using targeted RT-PCR as well as microarray analyses. Remarkably, the list of thymus-expressed tissue-restricted antigens was expanded to include tissues representing virtually every organ system, including both male and female reproductive tissues59,66,67,68 (Tables 1 and 2).
Table 1. Reproductive tissue-restricted antigens highly expressed in human mTECa.
| Placenta | Ovary | Uterus | Testis | Prostate | Pituitary | Hypothalamus | |||
|---|---|---|---|---|---|---|---|---|---|
| 3–10×Meanb | ARHGAP8 | LAMB2 | HF1 | LAMB2 | RNASE1 | ACPP | KRT19 | INSM1 | |
| CEBPB | NID1 | RBP1 | FXYD3 | CRABP1 | ARHGAP8 | NEFH | LOH11CR2A | ||
| CRIP2 | NPC1 | DLK1 | GSN | CTSF | BZRP | P24B | NTS | ||
| DAF | PRSS11 | HXB | PPFIBP2 | CCND1 | SCNN1A | GCGA | |||
| DLX5 | RDC1 | IGFBP7 | HSPA2 | CKMT1 | TPM2 | CSH2 | |||
| DUSP5 | RNASE1 | MYH11 | PRAME | CLDN4 | TSPAN-1 | ||||
| ERBB3 | S100A11 | APM2 | SERPINA5 | CLDN7 | VRP | ||||
| FSTL3 | SLC9A3R1 | VRP | STHM | DBI | |||||
| HDAC5 | SPP1 | SCGB2A1 | |||||||
| INDO | TM4SF1 | VRP | |||||||
| KRT19 | TRIM29 | ||||||||
| KRT7 | |||||||||
| 10–30×Mean | DLK1 | CHI3L1 | CCNA1 | CLDN3 | CHGB | HDC | |||
| GH1 | CYR61 | GSTM3 | ID1 | DLK1 | |||||
| CSF2RB | RAMP1 | ZNF165 | KLK11 | ||||||
| FN1 | TPM2 | SPAG6 | KRT15 | ||||||
| KRT18 | SCGB2A1 | SORD | |||||||
| PLAU | APM2 | ||||||||
| EFS2 | EFS2 | ||||||||
| SPINT1 | |||||||||
| >30×Mean | CGA | HSD17B2 | IGSF1 | ||||||
| CSH2 | PRG2 | GH1 | |||||||
| AOC1 | SDC1 | ||||||||
| CDKN2A | SERPINB2 | ||||||||
| GABRE | TFAP2A |
Abbreviation: mTEC, medulary thymic epithelial cell.
Data represent genes that are restricted in expression to five or fewer tissues among more than 70 tissues and cells, as determined by microarray analysis. Data from Ref. 68 were compared to the gene expression database BioGPS (http://www.BioGPS.org).
Genes included were separated into categories of 3- to 10-fold, 10- to 30-fold and greater than 30-fold above the mean expression across all tissues and cell lines examined.
Table 2. Reproductive tissue-restricted antigens highly expressed in mature mTEC in the mousea.
| UC | Placenta | Ovary | Uterus | MG-NL | MG-L | Testis | Prostate | Pituitary | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 3–10×Meanb | Edn1 | Alb | F2c | Bhmt | Krt13 | Psp | Mup3 | Bhmt | ||
| Klk11 | Csn1s1 | Fgg | Sprr1b | |||||||
| Krt16 | Csn2 | Igf2 | ||||||||
| Tmem45a | Csn1s2a | Kng1 | ||||||||
| Ear1 | Krt13 | |||||||||
| Ear2 | Plagl1 | |||||||||
| 10–30×Mean | Asprv1 | Apoc2 | Zp2 | Mmp7 | Tnnc1 | Fbp11 | Mmp7 | Plagl1 | ||
| Cdkn1c | Cdkn1c | Zp3 | Ldh3 | Presp18 | ||||||
| Crct1 | Hbb-y | |||||||||
| Lgals7 | Krt25 | |||||||||
| Plagl1 | Procr | |||||||||
| Tac2 | Tnfrsf11b | |||||||||
| >30×Mean | Alox12b | Apoa4 | Krt16 | Areg | Csn1s1 | Ubxn11 | Pbp | Pomc1 | ||
| Calm4 | Apob | Csn2 | ||||||||
| Hbb-y | Csn1s2a | |||||||||
| Igf2 | ||||||||||
| Krt10 | ||||||||||
| Sprr1b |
Abbreviations: MG-L, mammary gland - lactating; MG-NL, mammary gland - nonlactating; mTEC, medulary thymic epithelial cell; UC, umbilical cord.
Data from Ref. 67 were compared to the online gene expression database BioGPS. Genes listed represent those expressed by CD80hi mTEC (vs. CD80lo mTEC) that are restricted in expression to 5 or fewer tissues among more than 60 tissues and cells.
Genes included were separated into categories of 3- to 10-fold, 10- to 30-fold and greater than 30-fold above the mean expression across all tissues and cell lines examined.
Genes in italic font are regulated independently of Aire.
AIRE regulates promiscuous gene expression in mTEC
The relevance of thymic expression of tissue-restricted antigens was immediately recognized as a potential mechanism by which central tolerance could contribute to tolerance to tissue-specific antigens, complementary to mechanisms of peripheral tolerance. Further, the observation that expression of AIRE parallels that of many tissue-restricted antigens in mTEC prompted the hypothesis that AIRE drives expression of these genes within the thymus in order to confer establishment of self-tolerance to these antigens.66 Indeed, targeted deletion of Aire in mice replicates the multiorgan autoimmune disease in humans, which is characterized by tissue- and cell-specific autoantibodies and T lymphocyte infiltration.66,69 Moreover, comparison between mTEC from wild-type and Aire-knockout mice revealed the misregulation of many tissue-restricted antigens in mTEC of the knockouts, confirming the notion that these antigens are regulated by Aire.66 That Aire-regulated, promiscuously expressed genes in the thymus are essential for immune tolerance to corresponding tissues has been shown experimentally: in mice, even the loss of thymic expression of single tissue-restricted antigens (interphotoreceptor binding protein in the eye, mucin 6 in the stomach and insulin in pancreatic β cells) renders them targets of peripheral tissue-specific autoimmune disease.70,71,72 In some cases, there is a direct correlation between a particular thymically expressed, tissue-restricted antigen as the target of autoimmunity; however, in many cases, the correlation has not been demonstrated.73 Still, the tissues that are targeted as a result of AIRE mutation in mice and humans mirror those that are represented by tissue-restricted gene expression in mTEC and that are regulated by AIRE.
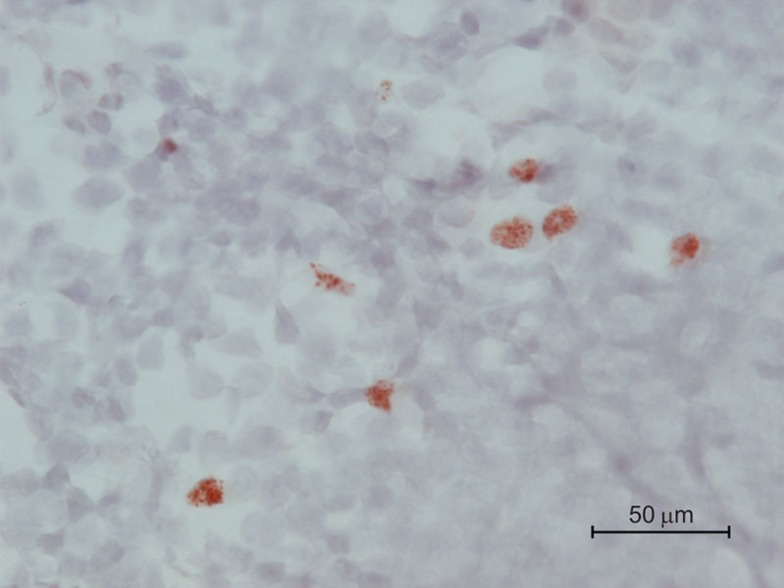
The molecular mechanism of how the ∼58-kDa AIRE protein ensures transcription of particular genes has been reviewed.74 Briefly, AIRE was originally considered a transcription factor based on its nuclear localization, it ability to bind DNA and the presence of a SAND (Sp100, AIRE-1, NucP41/75, DEAF-1) functional domain.75,76,77 However, AIRE also contains two PHD (Plant HomeoDomain)-type zinc fingers that confer its ability to associate with numerous protein partners and that are essential for regulation of gene expression.78,79,80 These PHD domains associate with histone 3 at unmethylated lysine 4 residues (H3K4me0), which are typically within transcriptionally inactive chromatin regions, and in combination with DNA-dependent protein kinase, appear to promote subsequent transcription.81,82,83 Consistent with this model of transcriptional regulation, AIRE-regulated genes are chromosomally clustered and stochastically expressed.68,84,85,86 Additionally, immunochemical localization suggests that AIRE associates with the nuclear matrix in a distinctive speckling pattern (Figure 2).87
Figure 2.
AIRE expression in mTEC of mouse thymic medulla. Immunohistochemical analysis of thymic medulla of a Balb/c mouse using a monoclonal anti-mouse Aire antibody. mTEC, although not universally Aire+, are distinguished by high cytoplasm/nucleus ratio; thymocytes are smaller cells with low cytoplasm/nucleus ratio. Red/brown stain indicates nuclear staining of Aire protein. Counterstain, hematoxylin. Scale bar=50 µm. AIRE, autoimmune regulator; mTEC, medullary thymic epithelial cell.
Based on its potential mechanism of action and the consequences of loss of AIRE function in mice and humans, a model of AIRE-mediated immune tolerance and autoimmune disease in the absence of AIRE can be proposed. AIRE, together with its cofactors, promotes expression of tissue-restricted antigens in mTEC by recognition and recruitment to areas of inactive transcription. Expression of tissue-restricted proteins leads to at least two possible outcomes that culminate in immune tolerance. First, mTEC may present expressed tissue-restricted antigens directly, in the context of either major histocompatibility complex class I or class II. Supporting this model, AIRE itself has been suggested to enhance antigen presentation by mTEC, independently of its effects on expression of tissue-restricted antigens.88 A second possible outcome is that AIRE-expressing cells undergo apoptosis and are phagocytosed, along with their promiscuously expressed antigens, by resident thymic dendritic cells; the antigens are in turn cross-presented by the dendritic cells in the context of major histocompatibility complex class II, to developing CD4+ cells.89 Developing thymocytes that have successfully but randomly rearranged their T-cell receptors in the thymic cortex enter the medulla; should they efficiently recognize self-antigen presented by either mTEC or dendritic cells, they are eliminated and thus blocked from entering the periphery. On the other hand, lack of AIRE function due to genetic deletion or mutation prevents promiscuous gene expression, prevents the negative selection/deletion of autoreactive CD4+ and CD8+ T cells that recognize dominant, high affinity epitopes.90 Further studies suggest that AIRE-expressing mTEC can promote the development of antigen-specific immunosuppressive regulatory T cells.91,92,93 Collectively, potentially destructive autoreactive effector T cells escape deletional tolerance in the thymus, and together with a shortage of regulatory T cells, precipitate autoimmunity and disease upon encountering their cognate antigen in the periphery. Experiments in mice have verified the importance of autoreactive T cells; adoptive transfer of these CD4+ T cells, but not CD8+ or B cells/serum alone, from AIRE-deficient mice confers autoimmune disease in immunodeficient animals.94,95
Aire and ovarian autoimmune disease
Gonadal insufficiency, albeit not life-threatening in itself, is one of the most common manifestations in female APS-1 patients. Likewise, female mice with targeted Aire deficiency display varying degrees of infertility depending on the genetic background. The reason for this variation is likely due to differences in major histocompatibility haplotype; this could similarly contribute to variation in human phenotype, although this point has been debated.77,96,97 In our hands, Aire-deficient mice on the Balb/c genetic background display delayed onset of puberty (as defined by vaginal opening), and although mating behavior is not impaired, 50% of female Aire-deficient mice fail to yield litters at 6–8 weeks of age.98 This appears to be the result of early-to-mid-gestation loss, as all mice undergo at least initial gain of weight following mating; however, only half of the mice continue to gain weight and deliver overtly healthy litters of normal size. The cause of this loss could be either ovarian disease and/or targeting of the embryo/placenta. Data strongly support ovarian insufficiency: as early as 4 weeks of age, evidence of oophoritis as seen by lymphocytic infiltration begins, and by 8 weeks, a dramatic loss of ovarian follicles becomes obvious. By 20 weeks of age, 60% of the mice contain virtually no follicles; instead, residual ovarian tissue contains large, eosinophilic cells and an absence of lymphocytes, presumably due to scattering of the cells after elimination of the antigenic target(s). T lymphocyte infiltration can be observed surrounding healthy and degenerating follicles, within corpora lutea, and even in close contact with the oocyte (Figure 3). A role for the immune system in follicular loss, rather than a role for endogenously expressed ovarian Aire,66 is supported by transplantation experiments. Transfer of wild-type ovaries into Aire-deficient mice results in rapid lymphocyte infiltration reminiscent of a second-set immune response, particularly if the endogenous ovary in the recipient is also depleted of follicles.98 In addition, adoptive transfer of splenocytes from Aire-deficient, but not wild-type mice, into recombinase-activating gene-deficient mice, which lack T and B cells, is followed by lymphocytic infiltration into the ovaries.94
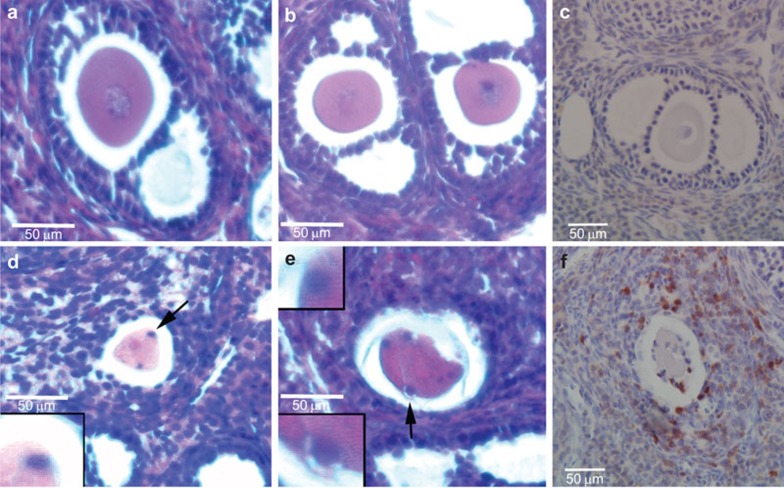
Figure 3.
T lymphocyte infiltration into ovarian follicles of Aire-deficient mice. (a–c) Follicles from WT mice; (d–f) Follicles from Aire-deficient mice. (a, b, d, e) Hematoxylin and eosin stain; (c, f) Immunohistochemistry using anti-CD3 pan-T-cell antibody and hematoxylin counterstain. WT follicles lack detectable lymphocytes, while follicles from Aire-deficient mice are surrounded by T cells, including those in close contact with the degenerating oocyte. Arrowheads in d and e identify putative lymphocytes surrounding oocyte; CD3-positive cells can similarly be seen in proximity to the egg in f. AIRE, autoimmune regulator; WT, wild-type.
Though cross-referencing of publicly available database (BioGPS) together with the published literature,67,68 we compiled a list of genes restricted to tissues relevant to male and female reproductive organs that are expressed within human and murine mTEC (Table 1 and 2). Ovarian antigens are represented in the murine thymus, as evidenced by the expression of oocyte-specific sperm-binding glycoproteins zona pelucida (zp)2, 3 and betaine-homocysteine methyltransferase (bhmt), which apart from the ovary and testis is expressed only in the kidney and liver. Aire-regulated ZP3 mRNA and protein expression in the murine thymus can be verified (Figure 4). It is important to note, however, that microarray analysis may under-represent the number of mTEC-specific, AIRE-regulated tissue-restricted antigens, since RT-PCR studies have confirmed expression of a number of antigens that are not detectable by microarray. Nonetheless, the results suggest that these and/or other ovary-specific proteins could be antigenic targets in mice lacking proper tolerogenic mechanisms. Indeed, ZP3 is a well-known antigen that can not only elicit antibody production, but also effect infertility by blocking sperm binding.99
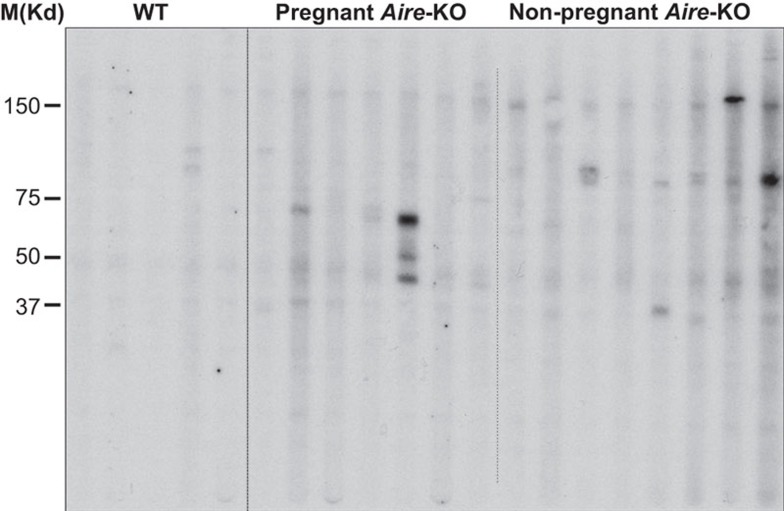
Using sera from mated pregnant and non-pregnant mice to probe ovarian tissue lysates, it is clear that Aire-deficient mice, even at a young age, can generate autoantibodies against a variety of antigenic targets (Figure 5). Cheng et al.100 have reported similar findings; as mice age, they produce autoantibodies that target an early, prominent target of 60 kD. Interestingly, these targets may vary depending on whether the animal has experienced a pregnancy (Figure 5); histological studies using sera reveal the presence of autoantibodies against not only the oocyte, but also against steroid-producing follicular and luteal cells.98 Although the identities of the ovarian targets are not known, the molecular sizes of murine ZP2 (120 kD), ZP3 (83 kD) and BHMT (45 kD) proteins are each within the range of band sizes observed by western immunoblotting.
Figure 5.
Anti-ovary serum autoantibodies are produced by Aire-deficient Balb/c mice. WT (n=5) and Aire-KO (n=15) mice were bred at approximately 6 weeks of age to intact WT Balb/c males. Presence of a copulation plug was confirmed visually, and females were euthanized 7–10 days later. All five WT mice and seven of 15 Aire-KO mice were confirmed pregnant. Sera were collected and used to probe a western blot of ovarian lysate from RAG2−/− mice, fitted with a Mini-Protean II Multiscreen apparatus. Although band size and intensity varied between animals, nearly all Aire-deficient mice displayed serum autoreactivity against ovarian antigens. Prominent bands included ∼70 kD and ∼90 kD antigens in pregnant and non-pregnant animals, respectively. AIRE, autoimmune regulator; RAG, recombinase-activating gene; WT, wild-type.
The day 3 thymectomized mouse model of autoimmune disease has been informative on the mechanisms of ovarian autoimmune disease and requirements for gonadal immune tolerance. Day 3 thymectomized mice develop fulminant ovarian autoimmune disease within 4 weeks, the cause of which has been carefully worked out to be a deficiency in regulatory T cell control over pathogenic autoreactive T cells within the lymph nodes that drain the ovary.101,102,103 Intriguingly, the major antigenic target that has been identified is a maternal-effect gene essential for embryogenesis, NLRP5 (NACHT, LRR and PYD domains-containing protein 5, also called NALP5 or MATER).104,105 Importantly, NLRP5, which is also highly restricted in its tissue distribution, is targeted as a parathyroid antigen in APS-1 patients, many of whom also displayed hypogonadism.106 Whether the same autoantigen is targeted in Aire-deficient mice is uncertain, although in our hands, an antigen of the predicted size for murine NLRP5 (121 kD) was not detected.
Future directions
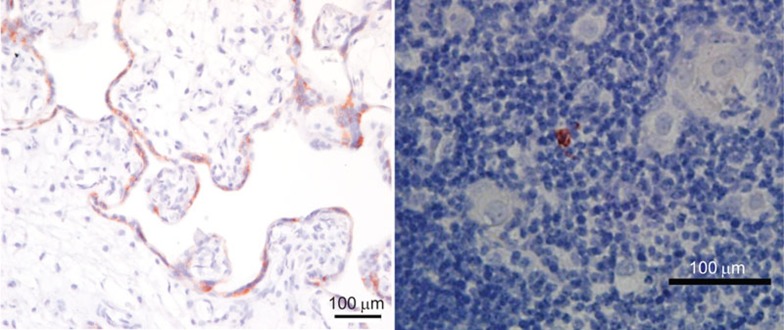
While the ovary is an important target of autoimmune disease, strong cases can also be made with regard to potential roles for central tolerance in other reproductive processes. Tables 1 and 2 clearly show that a plethora of reproductive antigens are expressed in mTEC of both human and murine thymi, including those restricted to other organs of the female reproductive tract and the male reproductive tract. Prostate-specific antigens are prominently represented in the mouse and human thymus, and indeed, Aire-deficient mice experience dramatic onset of prostatitis.107 Seminal vesicles, testis and epididymis are also severely affected in Aire-deficient males on the Balb/c genetic background, which almost completely infertile (Warren and Petroff, manuscript in preparation). One of the most strongly represented organs in both murine and human thymus is the placenta; indeed, chorionic gonadotropin (CGA) protein is confirmed in the human thymus (Figure 6), which raises the intriguing possibility that central tolerance is critical for tolerance to placenta-specific antigens.
Figure 6.
Human CGA expression in the third trimester (term) human placenta (a) (positive control) and thymus (b). Human thymus immunoreactivity (red/brown stain) for CGA averaged 2–3 positive cells per high-powered field, and was only observed in epithelial cells in the medulla. Counterstain, hematoxylin. Scale bars=100 µm. CGA, chorionic gonadotropin A.
Acknowledgments
The authors thank the many members of their labs who have contributed valuably to the work in this article through discussion and interaction. We are grateful to Matahb Fakhari for technical work with ovarian immunohistochemistry. The Aire-deficient mice were a generous donation from the laboratory of Christoph Benoist and Diane Mathis at Harvard Medical School. This work was supported by NIH grants R21HD062879 and R01HD045611 to MGP.
References
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti G, Krausz C. Evaluation and treatment of the infertile couple. J Clin Endocrinol Metab. 1998;83:4177–4188. doi: 10.1210/jcem.83.12.5296. [DOI] [PubMed] [Google Scholar]
- Chandra A, Copen CE. Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of Family Growth. Natl Health Stat Rep. 2013;67:1–19. [PubMed] [Google Scholar]
- Braxton J, Carey D, Davis D, Footman A, Flagg E, Grier L, et al. Sexually Transmitted Disease Surveillance 2012. Atlanta, GA: Department of Health and Human Services: National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; 2013. [Google Scholar]
- Brison DR, Roberts SA, Kimber SJ. How should we assess the safety of IVF technologies. Reprod Biomed Online. 2013;27:710–721. doi: 10.1016/j.rbmo.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Acosta AA, Anand Kumar TC, Andino N, Cooke ID, DeCherney AH, Qin-Sheng G, et al. Recent Advances in Medically Assisted Conception. Geneva: World Health Organization; 1992. [Google Scholar]
- Welt C. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol. 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;76:604–606. [PubMed] [Google Scholar]
- van Kasteren Y, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- Farhi J, Homburg R, Ferber A, Orvieto R, Ben Rafael Z. Non-response to ovarian stimulation in normogonadotrophic, normogonadal women: a clinical sign of impending onset of ovarian failure pre-empting the rise in basal follicle stimulating hormone levels. Hum Reprod. 1997;12:241–243. doi: 10.1093/humrep/12.2.241. [DOI] [PubMed] [Google Scholar]
- Nikolaou D, Lavery S, Turner C, Margara R, Trew G. Is there a link between an extremely poor response to ovarian hyperstimulation and early ovarian failure. Hum Reprod. 2002;17:1106–1111. doi: 10.1093/humrep/17.4.1106. [DOI] [PubMed] [Google Scholar]
- Valdez KE, Petroff BK. Potential roles of the aryl hydrocarbon receptor in female reproductive senescence. Reprod Biol. 2004;4:243–258. [PubMed] [Google Scholar]
- Bagur AC, Mautalen CA. Risk of developing osteoporosis in untreated premature menopause. Calcif Tissue Int. 1992;51:4–12. doi: 10.1007/BF00296207. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barret-Connor E. Early menopause, number of reproductive years and bone mineral density in postmenopausal women. Am J Public Health. 1993;83:983–991. doi: 10.2105/ajph.83.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. J Am Med Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Silbergeld ED, Flaws JA. Chemicals and menopause: effects on age at menopause and on health status in the postmenopausal period. J Women's Health. 1999;8:227–234. doi: 10.1089/jwh.1999.8.227. [DOI] [PubMed] [Google Scholar]
- Kalantaridou SN, Braddock DT, Patronas NJ, Nelson LM. Treatment of autoimmune premature ovarian failure: case report. Hum Reprod. 1999;14:1777–1782. doi: 10.1093/humrep/14.7.1777. [DOI] [PubMed] [Google Scholar]
- Silva CA, Yamakami LY, Aikawa NE, Araujo DB, Carvalho JF, Bonfa E. Autoimmune primary ovarian insufficiency. Autoimmun Rev. 2014;13:427–430. doi: 10.1016/j.autrev.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Bannatyne P, Russell P, Shearman RP. Autoimmune oophoritis: a clinicopathologic assessment of 12 cases. Int J Gynecol Pathol. 1990;9:191–207. doi: 10.1097/00004347-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Sedmak DD, Hart WR, Tubbs RR. Autoimmune oophoritis: a histopathologic study of involved ovaries with immunologic characterization of the mononuclear cell infiltrate. Int J Gynecol Pathol. 1987;6:73–81. [PubMed] [Google Scholar]
- Bakalov VK, Anasti JN, Calis KA, Vanderhoof VH, Premkumar A, Chen S, et al. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2005;84:958–965. doi: 10.1016/j.fertnstert.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Brandao Neto RA, de Carvalho JF. Diagnosis and classification of Addison's disease (autoimmune adrenalitis) Autoimmun Rev. 2014;13:408–411. doi: 10.1016/j.autrev.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Goudie RB, Gray KG, Timbury GC. Autoantibodies in Addison's disease. Lancet. 1957;1:1123–1124. doi: 10.1016/s0140-6736(57)91687-2. [DOI] [PubMed] [Google Scholar]
- Winqvist O, Karlsson FA, Kämpe O. 21-hydroxylase, a major autoantigen in idiopathic Addison's disease. Lancet. 1992;339:1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]
- Chen S, Sawika J, Betterle C, Powell M, Prentice L, Volpato M, et al. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison's disease, and premature ovarian failure. J Clin Endocrinol Metab. 1996;81:1871–1876. doi: 10.1210/jcem.81.5.8626850. [DOI] [PubMed] [Google Scholar]
- Falorni A, Laureti S, Candeloro P, Perrino S, Coronella C, Bizzarro A, et al. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertil Steril. 2002;78:270–279. doi: 10.1016/s0015-0282(02)03205-3. [DOI] [PubMed] [Google Scholar]
- Dal Pra C, Chen S, Furmaniak J, Smith B, Pedini B, Moscon A, et al. Autoantibodies to steroidogenic enzymes in patients with premature ovarian failure with and without Addison's disease. Eur J Endocrinol. 2003;148:565–570. doi: 10.1530/eje.0.1480565. [DOI] [PubMed] [Google Scholar]
- Arif S, Varela-Clvino R, Conway GS, Peakman M. 3β hydroxysteroid dehydrogenase autoantibodies in patients with idiopathic premature ovarian failure target N- and C-terminal epitopes. J Clin Endocrinol Metab. 2001;86:5892–5897. doi: 10.1210/jcem.86.12.eg1201.8111. [DOI] [PubMed] [Google Scholar]
- Arif S, Vallian S, Farzaneh F, Zanone MM, James SL, Pietropaolo M, et al. Identification of 3 beta-hydroxysteroid dehydrogenase as a novel target of steroid cell autoantibodies: association of autoantibodies with endocrine autoimmune disease. J Clin Endocrinol Metab. 1996;81:4439–4445. doi: 10.1210/jcem.81.12.8954056. [DOI] [PubMed] [Google Scholar]
- Lonsdale RN, Roberts PF, Trowell JE. Autoimmune oophoritis associated with polycystic ovaries. Histopathology. 1991;19:77–81. doi: 10.1111/j.1365-2559.1991.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Welt C, Falorni A, Taylor A, Martin K, Hall J. Selective theca cell dysfunction in autoimmune oophoritis results in multifollicular development, decreased estradiol, and elevated inhibin B levels. J Clin Endocrinol Metab. 2005;90:3069–3076. doi: 10.1210/jc.2004-1985. [DOI] [PubMed] [Google Scholar]
- Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–134. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- Gloor E, Hurlimann J. Autoimmune oophoritis. Am J Clin Pathol. 1984;81:105–109. doi: 10.1093/ajcp/81.1.105. [DOI] [PubMed] [Google Scholar]
- La Marca A, Brozzetti A, Giovanna S, Stefania M, Annibale V, Alberto F. Primary ovarian insufficiency: autoimmune causes. Curr Opin Obstet Gynecol. 2010;22:277–282. doi: 10.1097/GCO.0b013e32833b6c70. [DOI] [PubMed] [Google Scholar]
- Tsigkou A, Marzotti S, Borges L, Brozzetti A, Reis F, Candeloro P, et al. High serum inhibin concentration discriminates autoimmune oophoritis from other forms of primary ovarian insufficiency. J Clin Endocrinol Metab. 2008;93:1263–1269. doi: 10.1210/jc.2007-1675. [DOI] [PubMed] [Google Scholar]
- Cowchock FS, McCabe JL, Montgomery BB. Pregnancy after corticosteroid administration in premature ovarian failure (polyglandular endocrinopathy syndrome) Am J Obstet Gynecol. 1988;158:118–119. doi: 10.1016/0002-9378(88)90791-0. [DOI] [PubMed] [Google Scholar]
- Barbarino-Monnier P, Gobert B, Guillet-May F, Béné MC, Barbarino A, Foliguet B, et al. Ovarian autoimmunity and corticotherapy in an in-vitro fertilization attempt. Hum Reprod. 1995;10:2006–2007. doi: 10.1093/oxfordjournals.humrep.a136225. [DOI] [PubMed] [Google Scholar]
- Corenblum B, Rowe T, Taylor PJ. High-dose, short-term glucocorticoids for the treatment of infertility resulting from premature ovarian failure. Fertil Steril. 1993;59:988–991. doi: 10.1016/s0015-0282(16)55915-9. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z, Halachmi S, Peretz BA, Shmuel Z, Golan D, Makler A, et al. Premature ovarian failure—the prognostic application of autoimmunity on conception after ovulation induction. Fertil Steril. 1993;59:750–755. doi: 10.1016/s0015-0282(16)55854-3. [DOI] [PubMed] [Google Scholar]
- Falorni A, Brozzetti A, Aglietti MC, Esposito R, Minarelli V, Tomaro ES, et al. Progressive decline of residual follicle pool after clinical diagnosis of autoimmune ovarian insufficiency. Clin Endocrinol. 2012;77:453–458. doi: 10.1111/j.1365-2265.2012.04387.x. [DOI] [PubMed] [Google Scholar]
- Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, et al. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinology. 2007;148:2301–2308. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- Falsetti L, Scalchi S, Villani MT, Bugari G. Premature ovarian failure. Gynecol Endocrinol. 1999;13:189–195. doi: 10.3109/09513599909167554. [DOI] [PubMed] [Google Scholar]
- Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- Forges T, Monnier-Barbarino P, Faure GC, Bene MC. Autoimmunity and antigenic targets in ovarian pathology. Hum Reprod Update. 2004;10:163–175. doi: 10.1093/humupd/dmh014. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997;89:777–779. doi: 10.1016/s0029-7844(97)00077-x. [DOI] [PubMed] [Google Scholar]
- Ryan MM, Jones HR. Myasthenia gravis and premature ovarian failure. Muscle Nerve. 2004;30:231–233. doi: 10.1002/mus.20067. [DOI] [PubMed] [Google Scholar]
- Perheentupa J. Autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- Betterle C, Greggio NA, Volpato M. Clinical Review 93: autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83:1049–1055. doi: 10.1210/jcem.83.4.4682. [DOI] [PubMed] [Google Scholar]
- Wolff AS, Sarkadi AK, Maródi L, Kärner J, Orlova E, Oftedal BE, et al. Anti-cytokine autoantibodies preceding onset of autoimmune polyendocrine syndrome type I features in early childhood. J Clin Immunol. 2013;33:1341–1348. doi: 10.1007/s10875-013-9938-6. [DOI] [PubMed] [Google Scholar]
- Meloni A, Furcas M, Cetani F, Marcocci C, Falorni A, Perniola R, et al. Autoantibodies against type I interferons as an additional diagnostic criterion for autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2008;93:4389–4397. doi: 10.1210/jc.2008-0935. [DOI] [PubMed] [Google Scholar]
- Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- Gylling M, Tuomi T, Bjorses P, Kontiainen S, Prartanen J, Christie MR, et al. SS-cell autoantibodies, human leukocyte Antigen II alleles, and type 1 diabetes in autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy*. J Clin Endocrinol Metab. 2000;85:4434–4440. doi: 10.1210/jcem.85.12.7120. [DOI] [PubMed] [Google Scholar]
- Halonen M, Eskelin P, Myher AG, Perheentupa J, Husebye ES, Kampe O, et al. AIRE mutations and human leukocyte antigen genotypes as determinants of the autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy phenotype. J Clin Endocrinol Metab. 2002;87:2568–2574. doi: 10.1210/jcem.87.6.8564. [DOI] [PubMed] [Google Scholar]
- Perheentupa J. APS-1/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am. 2002;31:295–320. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Aaltonen J, Bjorses P, Perheentupa J, Horelli-Kuitunen N, Palotie A, Peltonen L, et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- Heino M, Peterson P, Kudoh J, Shimizu N, Antonarakis SE, Scott HS, et al. APECED mutations in the autoimmune regulator (AIRE) gene. Hum Mutat. 2001;18:205–211. doi: 10.1002/humu.1176. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–265. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- Miller JFAP, Heath WR. Self-ignorance in the peripheral T-cell pool. Immunol Rev. 1993;133:131–150. doi: 10.1111/j.1600-065x.1993.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur C, Hanahan D, Smith KM. T-cell tolerance in transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AC, Nicholson LB, Legge KL, Turchin V, Zaghouani H, Kuchroo VK. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- Klein L, Roettinger B, Kyewski B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur J Immunol. 2001;31:2476–2486. doi: 10.1002/1521-4141(200108)31:8<2476::aid-immu2476>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Immunol. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, et al. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavanescu I, Kessler B, Ploegh H, Benoist C, Mathis D. Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc Natl Acad Sci USA. 2007;104:4583–4587. doi: 10.1073/pnas.0700259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyewski B, Taubert R. How promiscuity promotes tolerance: the case of myasthenia gravis. Ann NY Acad Sci. 2008;1132:157–162. doi: 10.1196/annals.1405.026. [DOI] [PubMed] [Google Scholar]
- Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat Rev Immunol. 2008;8:948–957. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C, Bukrinsky A, Peltonen L. Systematic mutagenesis of the functional domains of AIRE reveals their role in intracellular targeting. Hum Mol Genet. 2002;11:3299–3308. doi: 10.1093/hmg/11.26.3299. [DOI] [PubMed] [Google Scholar]
- Kumar PG, Laloraya M, Wang CY, Ruan QG, Davoodi-Semiromi A, Kao KJ, et al. The autoimmune regulator (AIRE) is a DNA-binding protein. J Biol Chem. 2001;276:41357–41364. doi: 10.1074/jbc.M104898200. [DOI] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- Oven I, Brdičková N, Kohoutek J, Vaupotič T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmarinen T, Kangas H, Kytömaa T, Eskelin P, Saharinen J, Seeler JS, et al. Functional interaction of AIRE with PIAS1 in transcriptional regulation. Mol Immunol. 2008;45:1847–1862. doi: 10.1016/j.molimm.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Meloni A, Fiorillo E, Corda D, Incani F, Serra ML, Contini A, et al. DAXX I is a new AIRE-interacting protein. J Biol Chem. 2010;285:13012–13021. doi: 10.1074/jbc.M109.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Org T, Chignola F, Hetényi C, Gaetani M, Rebane A, Liiv I, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–376. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Org T, Rebane A, Kisand K, Laan M, Haljasorg U, Andreson R, et al. AIRE activated tissue specific genes have histone modifications associated with inactive chromatin. Hum Mol Genet. 2009;18:4699–4710. doi: 10.1093/hmg/ddp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žumer K, Low AK, Jiang H, Saksela K, Peterlin BM. Unmodified histone H3K4 and DNA-dependent protein kinase recruit autoimmune regulator to target genes. Mol Cell Biol. 2012;32:1354–1362. doi: 10.1128/MCB.06359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnnidis JB, Venanzi ES, Taxman DJ, Ting JP, Benoist CO, Mathis DJ. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl Acad Sci USA. 2005;102:7233–7238. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci USA. 2008;105:15854–15859. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Pinto S, Rösch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci USA. 2008;105:657–662. doi: 10.1073/pnas.0707486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderle C, Christensen HM, Schweiger S, Lehrach H, Yaspo ML. AIRE encodes a nuclear protein co-localizing with cytoskeletal filaments: altered sub-cellular distribution of mutants lacking the PHD zinc fingers. Hum Mol Genet. 1999;8:277–290. doi: 10.1093/hmg/8.2.277. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- Hansenne I, Louis C, Martens H, Dorban G, Charlet-Renard C, Peterson P, et al. Aire and Foxp3 expression in a particular microenvironment for T cell differentiation. Neuroimmunomodulation. 2009;16:35–44. doi: 10.1159/000179665. [DOI] [PubMed] [Google Scholar]
- Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoss JJ, Shum AK, Johannes KP, Lu W, Krawisz AK, Wang P, et al. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J Immunol. 2008;181:4072–4079. doi: 10.4049/jimmunol.181.6.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavanescu I, Benoist C, Mathis D. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: a therapeutic approach for APECED patients. Proc Natl Acad Sci USA. 2008;105:13009–13014. doi: 10.1073/pnas.0806874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekalainen E, Miettinen A, Arstila TP.Does the deficiency of Aire in mice really resemble human APECED Nat Rev Immunol 20077: 1. [DOI] [PubMed] [Google Scholar]
- Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod. 2012;86:110. doi: 10.1095/biolreprod.111.097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagavant H, Fusi FM, Baisch J, Kurth B, David CS, Tung KS. Immunogenicity and contraceptive potential of a human zona pellucida 3 peptide vaccine. Biol Reprod. 1997;56:764–770. doi: 10.1095/biolreprod56.3.764. [DOI] [PubMed] [Google Scholar]
- Cheng M, Fan U, Johannes K, Anderson MS. Antigenic targets in Aire-mediated ovarian autoimmunity. J Immunol. 2011;186 Meeting Abstract Supplement:44.41. [Google Scholar]
- Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. j Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong ZB, Nelson LM. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology. 1999;140:3720–3726. doi: 10.1210/endo.140.8.6911. [DOI] [PubMed] [Google Scholar]
- Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26:267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- Alimohammadi M, Björklund P, Hallgren Å, Pöntynen N, Szinnai G, Shikama N, et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358:1018–1028. doi: 10.1056/NEJMoa0706487. [DOI] [PubMed] [Google Scholar]
- Hou Y, DeVoss J, Dao V, Kwek S, Simko JP, McNeel DG, et al. An aberrant prostate antigen-specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans. J Clin Invest. 2009;119:2031–2041. doi: 10.1172/JCI38332. [DOI] [PMC free article] [PubMed] [Google Scholar]