Abstract
Outbreaks of coral diseases are one of the greatest threats to reef corals in the Caribbean, yet the mechanisms that lead to coral diseases are still largely unknown. Here we examined the spatial-temporal dynamics of white-pox disease on Acropora palmata coral colonies of known genotypes. We took a Bayesian approach, using Integrated Nested Laplace Approximation algorithms, to examine which covariates influenced the presence of white-pox disease over seven years. We showed that colony size, genetic susceptibility of the coral host, and high-water temperatures were the primary tested variables that were positively associated with the presence of white-pox disease on A. palmata colonies. Our study also showed that neither distance from previously diseased individuals, nor colony location, influenced the dynamics of white-pox disease. These results suggest that white-pox disease was most likely a consequence of anomalously high water temperatures that selectively compromised the oldest colonies and the most susceptible coral genotypes.
Introduction
Infectious diseases are a major cause of coral decline worldwide, and are one of the main reasons that two Caribbean corals, Acropora palmata (Lamarck, 1816) and A. cervicornis (Lamarck, 1816), are now listed as threatened under the US Endangered Species Act [1]. Although outbreaks of coral disease have occurred since at least the 1970s in the Caribbean [2], researchers are still trying to determine which coral diseases are infectious, and whether the infectious diseases are also contagious [3]. Understanding whether coral diseases are contagious or not can help elucidate the mechanisms that drive disease activity on contemporary reefs.
An infectious disease is generally caused by micro-organisms such as bacteria, protozoans, fungi, or viruses, which enter organisms, survive, multiply [4], and cause negative physiological changes within the infected organisms. A contagious disease is one that is communicable by contact with, or through, some secretion from the infected individual [4]. Several studies have determined that many coral diseases are caused by microbial agents. In fact, much of the research within the past several decades has focused on identifying putative pathogens [5], [6], [7], [8], [9]. Still, it is unclear whether most coral diseases are indeed contagious. Determining whether a disease is contagious will provide critical information that is necessary to reduce disease outbreaks. For example, if a disease outbreak is caused by a novel pathogen that passes from individual to individual (i.e., it is contagious) then controlling the pathogenic source will reduce disease impacts. However, if a disease outbreak is primarily a result of an environmental stress on the population, then steps need to be taken to reduce that stress.
Over the last 20 years white-pox disease has caused considerable coral mortality on Caribbean reefs [6]. White-pox disease was first documented in 1996 on Eastern Dry Rocks Reef off Key West, Florida [10], and was considered to be exclusive to A. palmata [6]. Subsequent studies showed that white-pox disease affected A. palmata populations throughout the Caribbean [11]. Indeed, white-pox disease was reportedly responsible for approximately an 85% decline in A. palmata, between 1996 and 1998, on reefs throughout the Florida Keys [6].
White-pox disease is believed to be caused by an infectious agent. In 2002 Patterson and colleagues satisfied Koch's postulates and linked white-pox disease with the bacteria Serratia marcescens (Bizio, 1823) [6]. S marcescens is a gram-negative motile bacterium that is commonly found within the gut of many vertebrates, including humans, although it can also exist as a free-living microbe in soil and in seawater [12]. Yet S. marcescens, the putative pathogen, was not consistently found in corals showing signs of white-pox disease in the Florida Keys [13], nor were the bacteria found in diseased samples from St. John, US Virgin Islands [14]. Additionally, Lesser and Jarrett did not detect S. marcescens in either colonies of A. palmata that showed signs of white-pox disease, or in healthy-appearing colonies of A. palmata in the Bahamas [15]. S. marcescens was, however, found within the tissue of 'healthy' appearing coral colonies in St. John [14]. These conflicting results indicate that S. marcescens might not be the only causative agent of white-pox disease, or the bacteria might be only pathogenic under certain environmental conditions.
Previous studies have suggested that white-pox disease is also contagious. Field surveys from reefs in the Florida Keys showed colonies with white-pox disease were clustered, which suggests that the disease is contagious [6]. However, the spatial analyses of these surveys did not account for the naturally clustered distribution of coral colonies of A. palmata within their sites. Furthermore, colony fragmentation within populations is a common mode of asexual reproductionaaAz within corals [16]. Since asexual fragmentation is the most dominant mode of reproduction in A. palmata, clones on reefs are close, often adjacent, and therefore nearest neighbors are frequently the same genotype [17]. For example, in the Florida Keys, USA, patch reefs contain several colonies of A. palmata, but most colonies are of the same genotype [18]. Of the twenty A. palmata colonies collected on both Horseshoe Reef and Little Grecian Reef by Baums and colleagues, only one genotype was detected on each reef [18]. On the land [19] and in the oceans [20] susceptibility to disease varies among genotypes. Therefore, without knowing the distribution of the coral genotypes, the resultant clustering patterns, particularly in places such as the Florida Keys where cloning is high, may be a reflection of the genotypic susceptibility of clones, rather than a reflection of the spatial pattern of a contagious disease.
In addition to determining whether a disease is contagious or not, understanding the environmental conditions that foster disease outbreaks is critical. Two of the environmental factors that are known to influence the dynamics of coral disease are water temperature and irradiance [21], [22], [23], [24]. Temperature anomalies have been positively associated with outbreaks of white syndrome in the Great Barrier Reef [25]. Furthermore, coral bleaching caused by high water temperatures increases the likelihood of disease activity in the Caribbean as well as in the Pacific Ocean [10], [26], [27], and most likely also reduces the innate immune system of corals [28], [29], [47]. Elevated levels of irradiance also increase the severity of some coral diseases [30] and can lead to compromised coral hosts [31].
Another factor that may influence disease susceptibility is colony size. A large colony has a larger surface area than a small colony, which could translate to a higher ‘target’ area for pathogenic infections. Additionally, the size of a coral colony may be an indication of colony age, although fragmentation events can create small colonies that may be very old [19], [32]. Large colonies, however, are also likely to be long-lived individuals and could possibly suffer from senescence [33]. The prevalence of white-pox disease tends to increase with colony size, but whether an increase in prevalence is a consequence of an increased ‘target’ area for pathogens, or the result of senescence is currently unknown [34]. Determining whether white-pox disease is contagious (i.e., influenced by spatial location in relation to other infected individuals) will provide insight into whether ‘target’ area or senescence causes large colonies to be more susceptible to disease infection than small colonies.
We examined the dynamics of white-pox disease on 69 A. palmata colonies in the US Virgin Islands (USVI) over seven years. The goals of this study were to: (i) use a space-time Bayesian model to determine whether spatial and temporal patterns of white-pox disease were indicative of a contagious disease that was potentially transmitted to nearest neighbors, and (ii) test a suite of covariates that might influence disease activity. We were particularly interested in the occurrence of reinfections of particular genotypes, whether colony size played a role in infection, and to what extent irradiance and water temperature affected the prevalence of white-pox disease.
Materials and Methods
Field surveys
Haulover Bay is located on the northeast side of St. John, US Virgin Islands. This bay supports a fringing reef adjacent to the shoreline. The reef is populated with isolated colonies of A. palmata, between 1 and 3 m depth. In February 2003, every colony of A. palmata within the west side of Haulover Bay was identified, photographed, and tagged, for a total of 69 individual colonies. Each colony was also given a Global Positioning System (GPS) waypoint. These colonies were then monitored every month for the next 7 years, from February 2003 to December 2009, for the presence or absence of white-pox disease [34] (Table S1). A previous study examined the genotypes of 48 of the 69 corals found in Haulover Bay [35]. Out of the 48 coral colonies analyzed, 43 colonies were genetically distinct [35]. Therefore, in the present study, any detection of spatial clustering patterns of white-pox disease within Haulover Bay, St. John (USVI) would be a result of contagious disease transmission, rather than a result of genetic susceptibility of clones.
Environmental parameters
Water temperature data was collected using a Hobo Temperature Pro v2 data logger, attached to the substrate, which recorded temperature every 10 minutes. Temperature data used in the model were the average water temperature recorded for the 30 days prior to the field survey. Approximations of solar insolation (300-5000 nm), measured as kW m−2 day−1, were obtained for Haulover Bay from the trigonometric polynomial approximations, which calculated average monthly values on a 1°×1° coarse grid [36]. The equation was formulated from data made available by the National Aeronautics and Space Administration (NASA) Langley Research Center (LaRC) Atmospheric Science Data Center Surface meteorological and Solar Energy (SSE) 6.0 web portal supported by the NASA LaRC Prediction of Worldwide Energy Resource (POWER) Project (http://eosweb.larc.nasa.gov/sse/).
The model
We took a Bayesian space-time modeling approach adapted from Cameletti and colleagues to analyze the presence or absence of white-pox disease on the monitored 69 colonies of A. palmata [37] (Text S1). We let y(si,t) represent the realization of the spatio-temporal binomial process Y(·,·), which denotes the presence or absence of white-pox disease at colony i = 1,…,d, located at si and day t = 1,…,T. We assumed that y(si,t) = z(si,t) β + ξ(si,t) + ε(si,t)
where z(si,t) is [z1(si,t),…zp(si,t)] that represents the vector of p covariates for colony location si at time t. β is (β1,…,βp), the coefficient vector. ξ(si,t) is the realization of the state process, which is the unobserved level of disease occurrence that is assumed to be a spatio-temporal Gaussian field that changes over time with first order autoregressive dynamics. ε(si,t) is the measurement error defined by a Gaussian white-noise process (∼ N (0, σ2ε). We used the specified model with a binomial response variable [37]. The output of the model provides the mean, the standard deviation, the 2.5% and 97.5% quantiles, and the mode for the correlation coefficients of each covariate. Significant values are those with 2.5% and 97.5% quantile ranges that do not span zero. Positive and negative values depict the direction of the association.
Our approach used a Gaussian Markov Random Field (GMRF) function, which is a spatial process that models the spatial dependence of data observed on geographic regions [38]. The GMRF computational properties were enhanced by using Integrated Nested Laplace Approximations (INLA) [39] for Bayesian inference. INLA is a computationally effective algorithm that produces fast and accurate approximations of posterior distributions [37]. All analyses were conducted using R version 3.0.1 [40] and the INLA package (www.r-inla.org; see Text S1).
For the last decade, Markov Chain Monte Carlo (MCMC) techniques have been used in Bayesian analysis to predict the posterior marginal distribution. We note that INLA is a recent alternative to MCMC techniques in spatial-temporal modelling to predict the posterior marginal distribution. INLA techniques combine Gaussian Field with Matérn covariance functions to produce GMRFs by using stochastic partial differential equations (SPDE). This process speeds up the estimates and accuracy, and INLA does not have the same convergence problems as MCMC techniques. The SPDE approach also uses a finite element representation to define the Matérn field by triangulation of the domain. This approach is appropriate for our data, which were taken at irregular discrete locations on a coral reef (Fig 1).
Figure 1. Density plots of colonies of Acropora palmata at Haulover Bay in St. John, United States Virgin Islands (USVI).
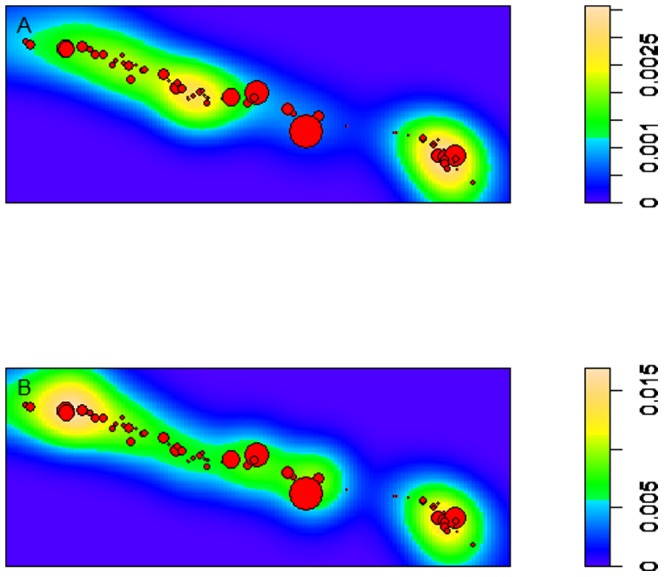
Density plot in A) is based on the expected number of random points per unit area (i.e. colony density), where warm colors (yellow) denote dense areas of individual colonies, whereas cool colors indicate locations sparse of coral colonies. The density plot in B) represents the intensity of white-pox disease reoccurrence within the spatial plane of annual disease activity during the 7-year study period, from February 2003 to December 2009. The axes of the spatial area are 220 m by 560 m. The red dots represent the locations of individual coral colonies. The size of the red dots represents initial colony size recorded in February 2003. Density plots were created using the density function of the R package ‘spatstat’.
Eight covariates were tested to determine whether they had a significant association with the presence or absence of white-pox disease on individual colonies through time (1). These covariates included: the spatial location of each colony measured as (i) the easting, and (ii) the northing locations (as georeferenced Universal Transverse Mercator (UTM) units), (iii) colony size (in cm3), (iv) the number of previous incidences of white-pox disease for each colony (we note that, although some colonies may have had a long history of disease before our study commenced, we started every colony at zero at the commencement of our study), (v) the distance to the nearest neighboring colony, (vi) the distance from a previously infected colony, (vii) water temperature, and (viii) solar insolation. Because water temperature and solar insolation vary on scales larger than the size of Haulover Bay [41], these covariate values were the same for all colonies within each time step, but varied for each time step. To illustrate spatial patterns in coral colony density and intensity of disease activity, a kernel smoothed intensity function was applied and plotted to the point pattern spatial data using the spatstat package in R [42].
Results
Three of the tested covariates significantly influenced the presence or absence of disease on individual coral colonies. Colony size, number of previous infections, and water temperature all showed a significant positive association with white-pox disease presence (Table 1). There was no significant effect on disease activity of colony location variables (easting or northing), or distance to the closest colony. Additionally, the distance to a previously infected colony did not affect disease presence either. The mean correlation coefficients indicate that previous incidences of disease had the strongest correlation (0.93), followed by water temperature (0.43), and colony size (0.30). The density plot of disease incidences over the 7-year period showed that areas of high disease activity, particularly in Haulover Bay's northwest region, did not coincide with high densities of coral colonies (Fig 1A, 1B). In addition, the level of solar insolation did not affect the activity of white-pox disease.
Table 1. Posterior estimates of the covariate coefficient vector β of the linear model (Eq. 1) testing the effects of multiple covariates on the presence of white-pox disease on coral colonies of Acropora palmata monitored monthly from February 2003 to December 2009.
| Fixed effects | Mean | Standard deviation | 2.5% quantile | 50% quantile | 97.5% quantile | Mode |
| Intercept | −3.29 | 0.25 | −3.79 | −3.29 | −2.83 | −3.28 |
| Northing | 0.29 | 0.83 | −1.32 | 0.29 | 1.89 | 0.28 |
| Easting | 0.44 | 0.81 | −1.14 | 0.43 | 2.05 | 0.42 |
| Colony size | 0.30 | 0.09 | 0.12 | 0.30 | 0.47 | 0.30 |
| Previous incidences | 0.93 | 0.13 | 0.68 | 0.93 | 1.17 | 0.94 |
| Distance | −0.13 | 0.14 | −0.41 | −0.12 | 0.14 | −0.12 |
| Previous distance | −0.07 | 0.06 | −0.19 | −0.07 | 0.04 | −0.07 |
| Water temperature | 0.43 | 0.07 | 0.29 | 0.43 | 0.58 | 0.43 |
| Solar insolation | 0.02 | 0.07 | −0.12 | 0.02 | 0.16 | 0.01 |
The 8 covariates were: (i) easting; (ii) northing; (iii) colony size; (iv) previous incidences of disease; (v) distance to nearest neighbor; (vi) distance to previously infected colony; (vii) water temperature; and (viii) solar insolation. Significant covariates are those with correlation coefficients that do not cross 0 within the 2.5 and 97.5% quantile. Rows that are bold indicate significant differences. Positive and negative values represent the directional relationship between the covariate and disease presence.
Discussion
The results of the model indicated that the spatial location of a particular coral colony did not significantly influence the probability of a colony manifesting white-pox disease. Additionally, the distance to nearest neighbors, and the distance to colonies that were previously infected with white-pox disease also had no significant influence on disease presence or absence. If white-pox disease is in fact contagious, then the colonies neighboring the infected colonies should be at a greater risk of obtaining the disease than more distant colonies, and spatial clustering would have occurred. The results did not show a neighborhood effect. Therefore, white-pox disease is most likely not a contagious disease in situ, showing no form of dependency on colony density.
Colony size significantly influenced the activity of white-pox disease. Compared with small-sized corals, large-sized colonies were more likely to show signs of white-pox disease. These results may be a reflection of an infectious disease that is a function of the available ‘target’ area, or a result of colony senescence [34]. Several other coral diseases, including ulcerative white-spot disease on massive Porites spp. and white-plague disease [43], [44], are more common on large-sized colonies, but the direct mechanism is still unclear. Since the distance from a previously infected colony did not influence white-pox disease occurrence, at t+1 (i.e., in the following month), then the positive association between colony size and disease activity was not likely to be a consequence of simply a larger target area. Our results instead suggest that large colonies are more likely to be susceptible to white-pox because they are older, with potentially reduced defense mechanisms because of senescence, than small colonies.
The number of previous infections that each A. palmata colony had experienced also significantly affected whether disease occurred on colonies within a given time step. Therefore, the more disease incidences a colony had experienced, the more likely that disease would again appear. There are several possibilities that may explain why the number of previous infections may have affected the probability of reinfection. One possibility may be that once a colony is infected with a pathogen, the infectious agent may reside within the organism and an environmental cue may reinitiate the manifestation of disease signs. Colonies that are initially infected may also become more susceptible over time. An alternative explanation is that colonies that acquire disease may be genetically more predisposed to the particular infectious agent that causes white-pox disease on A. palmata. Indeed, the identified pathogen, S. marcescens, has been found within healthy colonies more often than in diseased colonies at Haulover Bay, St. John (USVI) [14]. These results suggest that S. marcescens may be a regular component of the A. palmata microbiome within Haulover Bay, and that some coral genotypes might be more innately susceptible to the pathogenicity of this infectious agent than other coral genotypes, especially under stressful environmental conditions.
Previous studies have shown that some coral genotypes are resistant to disease infections [45], but their percentages were low (∼6%). Susceptibility, most likely, follows a continuum, where few individuals are resistant and few individuals are highly susceptible to disease. Although more experimental work is needed, our results suggest that there are several genotypes of A. palmata within Haulover Bay that are highly susceptible to white-pox disease. This information, combined with previous studies that showed that the putative pathogen is a common component of both diseased and non-diseased A. palmata colonies in Haulover Bay [14], indicate that the manifestation of white-pox disease is most likely a consequence of genetic susceptibility to environmental stress, rather than a consequence of repeated, novel infections.
Environmental conditions that are known to influence the prevalence of white-pox disease include high water temperature [21]. The present study showed that high temperatures were highly correlated with, and most likely influenced by, white-pox disease on A. palmata at Haulover Bay. Solar insolation, however, was not a significant covariate of disease (Table 1). The positive association between white-pox disease and temperature has been previously documented within Haulover Bay [35], on neighboring reefs in St. John [21], and in the Florida Keys [6]. White-pox disease has a seasonal cycle; prevalence tends to increase during months of high sea-surface temperature [35]. Several studies suggest that the positive association between disease prevalence on corals and water temperature is most likely linked to host susceptibility, rather than to pathogenic virulence [21], [46]. Indeed, high water temperature causes stress to coral colonies, often making them more susceptible to disease infection [25], [28], [47], [48], [49].
Our study showed that the presence of white-pox disease on A. palmata was a combination of high water temperatures and the genetic susceptibility of the host. Furthermore, white-pox disease did not appear to be contagious in situ. The pathogens that cause these specific signs of disease are, therefore, likely to be a common component of the coral's microbiome, but they only elicit signs of disease when found on susceptible coral hosts, and only when environmental conditions favor disease activity. Our spatial analysis showed that colony location had no influence on the presence or absence of white-pox disease. Therefore, future resilience of A. palmata to white-pox disease relies on (i) the survivability of specific coral genomes and (ii) the proliferation of disease resistant corals under strong selective pressure by the environment.
Supporting Information
Disease and environmental data used within the Bayesian space-time model.
(CSV)
R code for the Bayesian space-time model, adapted from Camelleti and colleagues [35].
(DOCX)
Acknowledgments
We would like to thank Caroline Rogers and the US Geological Survey for providing this extensive data set. Also, thank you to those who helped to collect data in the field including T. Spitzack, A. Bright, R. Brewer, and J. Herlan, National Park Service Biologists C. Stengel, S. Caseau, and J. Hopkins, also volunteers K. Vahling, C Kauffman, H. Smart, C. Beckowitz, P. Nieves, and J. Perry. We thank Sandra van Woesik and two anonymous reviewers for editorial comments.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
Data collected for this project was supported by funds from the U.S. Geological Survey Natural Resource Preservation Program and the National Oceanic and Atmospheric Administration Coral Reef Ecosystem Studies Program, and by in-kind contributions from the National Park Service (Virgin Islands National Park). Support for E. Muller was provided by a Mote Marine Laboratory Postdoctoral Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hogarth WT (2006) Endangered and threatened species: final listing determinations for elkhorn coral and staghorn coral. Fed Regist 71: 26852–26861. [Google Scholar]
- 2. Gladfelter WB (1982) White-band disease in Acropora palmata: Implications for the structure and growth of shallow reefs. Bull Mar Sci 32: 639–643. [Google Scholar]
- 3. Muller EM, van Woesik R (2012) Caribbean coral diseases: primary transmission or secondary infection? Glob Chang Biol 18: 3529–3535. [Google Scholar]
- 4.Stedman TL (1976) Stedman's Medical Dictionary. Williams and Wilkin Company, Baltimore. 1678 p. [Google Scholar]
- 5. Smith GW, Ives LD, Nagelkerken IA, Ritchie KB (1996) Caribbean sea-fan mortalities. Nature 383: 487. [Google Scholar]
- 6. Patterson KL, Porter JW, Ritchie KB, Polson SW, Mueller E, et al. (2002) The etiology of white pox a lethal disease of the Caribbean elkhorn coral, Acropora palmata . Proc Natl Acad Sci USA 99: 8725–8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denner EBM, Smith GW, Busse HJ, Schumann P, Nartz T, et al. (2003) Aurantimonas coralicida gen. nov., sp. nov., the causative agent of white plague type II on Caribbean scleractinian corals. Int J Syst Evol Microbiol 53: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 8. Cervino JM, Hayed R, Goreau TJ, Smith GW (2004) Zooxanthellae regulation in yellow blotch/band and other coral diseases contrasted with temperature related bleaching: In situ destruction vs expulsion. Symbiosis 37: 63–85. [Google Scholar]
- 9. Gil-Agudelo DL, Smith GW, Weil E (2006) The white band disease type II pathogen in Puerto Rico. Rev Biol Trop 54: 59–67.18457175 [Google Scholar]
- 10. Holden C (1996) Coral disease hot spot in the Florida Keys. Science 274: 2017. [Google Scholar]
- 11.Sutherland KP, Ritchie KB (2004) White pox disease of the Caribbean elkhorn coral, Acropora palmata. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer, Berlin. pp. 289–300. [Google Scholar]
- 12.Grimont PA, Grimont F (1994) Genus VIII Serratia Bizio, 1823. In: Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST, editors. Bergey's manual of determinative bacteriology, vol 4. Williams and Wilkins, Baltimore. pp. 477–484. [Google Scholar]
- 13. Sutherland KP, Porter JW, Turner JW, Thomas BJ, Looney EE, et al. (2010) Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata . Environ Microbiol 12: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 14. Polson SW, Higgins JL, Woodley CM (2009) PCR-based assay for detection of four coral pathogens. Proc 11th Int Coral Reef Symp 1: 251–255. [Google Scholar]
- 15. Lesser MP, Jarett JK (2014) Culture-dependent and culture-independent analyses reveal no prokaryotic community shifts or recovery of Serratia marcescens in Acropora palmata with white pox disease. FEMS Microbiol Ecol 88: 457–467. [DOI] [PubMed] [Google Scholar]
- 16. Roth L, Muller EM, van Woesik R (2013) Tracking Acropora fragmentation and population structure through thermal-stress events. Ecol Modell 263: 223–232. [Google Scholar]
- 17. Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7: 207–226. [Google Scholar]
- 18. Baums IB, Miller MW, Hellberg ME (2006) Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata . Ecol Monogr 76: 503–519. [Google Scholar]
- 19. Hammond-Kosack KE, Jones JDG (1997) Plant disease resistance genes. Annu Rev Plant Physiol Plant Mol Biol 48: 575–607. [DOI] [PubMed] [Google Scholar]
- 20. Vollmer SV, Kline DI (2008) Natural disease resistance in threatened staghorn corals. PLoS ONE 3: e3718 10.1371/journal.pone.0003718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muller EM, Rogers CS, Spitzack AS, van Woesik R (2008) Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US Virgin Islands. Coral Reefs 27: 191–195. [Google Scholar]
- 22. Harvell CD, Altizer S, Cattadori IM, Harrington L, Weil E (2009) Climate change and wildlife diseases: when does the host matter the most? Ecology 90: 912–920. [DOI] [PubMed] [Google Scholar]
- 23. Muller EM, van Woesik R (2010) Black-band disease dynamics: prevalence, incidence, and acclimatization of light. J Exp Mar Biol Ecol 397: 52–57. [Google Scholar]
- 24. Muller EM, van Woesik R (2009) Shading reduces coral-disease progression. Coral Reefs 28: 757–760. [Google Scholar]
- 25. Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, et al. (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol 5(6): e124 10.1371/journal.pbio.0050124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jones RJ, Bowyer J, Hoegh-Guldberg O, Blackall LL (2004) Dynamics of a temperature-related coral disease outbreak. Mar Ecol Prog Ser 281: 63–77. [Google Scholar]
- 27. Miller J, Muller E, Rogers C, Atkinson A, Whelan K, et al. (2009) Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands (USVI). Coral Reefs 28: 925–937. [Google Scholar]
- 28. Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322: 1–14. [Google Scholar]
- 29. Reed K, Muller EM, van Woesik R (2010) Coral immunology and resistance to disease. Dis Aquat Org 90: 85–92. [DOI] [PubMed] [Google Scholar]
- 30. Boyett HV, Bourne DG, Willis BL (2007) Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar Biol 151: 1711–1720. [Google Scholar]
- 31. Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H (2004) Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef building corals. Plant Cell Physiol 45: 251–255. [DOI] [PubMed] [Google Scholar]
- 32. Hughes TP (1984) Population dynamics based on individual size rather than age: a general model with a reef coral example. Am Nat 123: 778–795. [Google Scholar]
- 33. Irikawa A, Casareto BE, Suzuki Y, Aagostini S, Hidaka M, et al. (2011) Growth anomalies on Acropora cytherea corals. Mar Pollut Bull 62: 455–460. [DOI] [PubMed] [Google Scholar]
- 34. Muller EM, Rogers CS, van Woesik R (2013) Early signs of recovery of Acropora palmata in St. John, US Virgin Islands. Mar Biol 16: 359–365. [Google Scholar]
- 35. Rogers CS, Muller EM (2012) Bleaching, disease and recovery in the threatened scleractinian coral, Acropora palmata, in St. John US Virgin Islands: 2003–2010. Coral Reefs 31: 807–819. [Google Scholar]
- 36. van Woesik R, Lachamoise F, Koksal S (2006) Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol Lett 9: 390–398. [DOI] [PubMed] [Google Scholar]
- 37.Cameletti M, Lindgren F, Simpson D, Rue H (2012) Spatio-temporal modeling of particulate matter concentration through the SPDE approach. AStA Advances in Statistical Analysis, 97 (2). pp. 109–131. ISSN 1863–8171.
- 38.Rue H, Held L (2005) Gaussian Markov Random Fields: Theory and Applications, Chapman & Hall.
- 39. Rue H, Martino S, Chopin N (2009) Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J R Stat Soc Series B Stat Methodol 71: 319–392. [Google Scholar]
- 40.R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org/. Accessed 2014 Oct 14.
- 41. Wagner D, Mielbrecht E, van Woesik R (2008) Application of landscape ecology to spatial variance of water quality parameters along the Florida Keys reef tract. Bull Mar Sci 83: 553–569. [Google Scholar]
- 42.Baddeley A, Turner R (2005) Spatstat: an R package for analyzing spatial point patterns. J Stat Softw 12: 1-42. ISSN: 1548–7660. Available: http://www.jstatsoft.org. Accessed 2014 Oct 14.
- 43. Nugues MM (2002) Impact of a coral disease outbreak on coral communities in St. Lucia: What and how much has been lost? Mar Ecol Prog Ser 229: 61–71. [Google Scholar]
- 44. Raymundo LJH, Harvell CD, Reynolds TL (2003) Porites ulcerative white spot disease: description, prevalence, and host range of a new coral disease affecting Indo-Pacific reefs. Dis Aquat Org 56: 95–104. [DOI] [PubMed] [Google Scholar]
- 45. Kline DI, Vollmer SV (2011) White band disease (type I) of endangered Caribbean Acroporid corals is caused by pathogenic bacteria. Sci Rep 1: 7 10.1038/srep00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol 346: 36–44. [Google Scholar]
- 47. Harvell CD, Mitchell CE, Ward J, Altizer S, Dobson AP, et al. (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296: 2158–2162. [DOI] [PubMed] [Google Scholar]
- 48. Mydlarz LD, McGinty ES, Harvell CD (2010) What are the physiological and immunological responses of coral to climate warming and disease? J Exp Biol 213: 934–945. [DOI] [PubMed] [Google Scholar]
- 49. Mydlarz LD, Couch CS, Weil E, Smith G, Harvell CD (2009) Immune defences of healthy, bleached and diseased Montastraea faveolata during a natural bleaching event. Dis Aquat Org 87: 67–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disease and environmental data used within the Bayesian space-time model.
(CSV)
R code for the Bayesian space-time model, adapted from Camelleti and colleagues [35].
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.


