Abstract
Background
Anaemia reduces cognitive potential in school children, retards their growth and predisposes them to other diseases. As there is a paucity of data on the current burden of P. falciparum, S. mansoni and soil transmitted helminths (STH) infections and their correlation with schoolchildren’s anemia in the Democratic Republic of Congo (DRC), we collect these data.
Methods
This study reports baseline data collected from a randomized controlled trial investigating the impact of IPT with SP and SP-PQ on anemia and malaria morbidity in Congolese schoolchildren (Trial registration: NCT01722539; PACTR201211000449323). S. mansoni and STH infections were assessed using kato-katz technique. Malaria infection and hemoglobin concentration were assessed using Blood smear and Hemocontrol device, respectively.
Results
A total of 616 primary schoolchildren from 4 to 13 years old were enrolled in the study. The prevalence of Plasmodium spp. infection was 18.5% (95%CI:15.6–21.9). Amongst those infected, 24 (21%), 40 (35.1%), 40 (35.1%), 10 (8.8%), had light, moderate, heavy, very high malaria parasite density, respectively. Above 9 years of age (p = 0.02), male and history of fever (p = 0.04) were both associated with malaria infection. The overall prevalence of S. mansoni infection was 6.4% (95%CI:4.4–9.1). Girls were associated with S. mansoni infection (p = 0.04). T. trichiura was the most prevalent STH infection (26.3%), followed by A. lumbricoides (20.1%). Co-infection with malaria-S. mansoni and malaria-STH was, respectively, 1.5% (CI95%:0.7–3.3) and 6.4% (CI95% 4.4–9.1). The prevalence of anemia was found to be 41.6% (95%CI:37.7–45.6) and anemia was strongly related with Plasmodium ssp infection (aOR:4.1; CI95%:2.6–6.5;p<0.001) and S. mansoni infection (aOR:3.3;CI95%:1.4–7.8;p<0.01).
Conclusion
Malaria and S. mansoni infection were strongly associated with high prevalence of anemia in schoolchildren. Therefore, specific school-based interventions, such as intermittent preventive treatment or prophylaxis, LLITN distribution, anthelminthic mass treatment and micronutrient supplementation are needed to improve school children’s health.
Introduction
Malaria, Schistosomiasis and Soil Transmitted Helminth (STH) infections compose a considerable disease burden in schoolchildren in developing countries. These parasitic infections show a similar geographic distribution and polyparasitism of P.falciparum, schistosomiasis and STH infections have been reported from various epidemiological settings in Africa [1]–[5].
In endemic malaria areas, the prolonged carriage of P.falciparum triggers the development of acquired immunity that controls blood-stage parasitaemia, thereby reducing clinical symptoms and life-threatening complications in older children and adults [6]. Asymptomatic Plasmodium infections, if untreated, persist and maintain malaria-induced inflammation that is commonly associated with iron deficiency anaemia (IDA) due to impaired intestinal iron absorption, impaired iron release from hepatocytes, and impairment of the recycling of iron derived from phagocytosis of parasitized red blood cells [7]. It has also been suggested that high levels of tumor necrosis factor (TNF) induces marked dyserythropoietic changes in the red cell precursors and increased erythrophagocytosis [8]–[10]. In Low Income Countries (LICs), more than half of the school-aged population suffers from anaemia and in Sub Saharan Africa, approximately 85 million school-aged children are affected. Anaemia reduces their cognitive potential, retards their growth, and predisposes them to other diseases [11]. Moreover, malaria accounts for about for 13–50% of all annual school absenteeism and consequently impairs educational achievements of children [12]. Schistosomiasis and STH also inflict significant adverse effects on health such as anemia, stunting, protein-calorie malnutrition, fatigue, and poor cognitive development. During Schistosomiasis, anemia results from four processes: iron deficiency due to extra-corporal blood loss; splenic sequestration; autoimmune hemolysis and inflammatory anemia [13].
Findings on the interaction of malaria and helminth infections on the health of children are controversial. Some studies have shown a protective effect of helminths on symptomatic malaria. Severe worm burden seems to suppress malaria symptoms [14], [15]. On the other hand, others have highlighted an increased severity and incidence of malaria during helminths co-infection [16], [17]. In addition an additive effect of P. falciparum, Schistosomiasis and/or STH infection on childhood anemia has been reported [18], [19].
The Democratic Republic of Congo (DRC) is at present one of the most affected countries by malaria [20]. Although high, the exact burden of malaria as well as other parasitic diseases is unclear partly because of lack of reliable surveillance system. Moreover, only 54.1% of the patients presenting fever and seeking health care were truly infected with malaria [21]. In fact, the DRC and Nigeria contribute to about 24% of non-malarial fever reported worldwide [21]. This highlights the urgent need of conducting studies to provide accurate data on the current burden of these diseases in DRC and others settings.
While a hand full of studies are dedicated to the groups most vulnerable for malaria such as children under the age of five and pregnant women, very little is known about the ongoing burden of asymptomatic P. falciparum, Schistosomiasis and STH infections, or their relationship with anemia in primary schoolchildren. The aim of this study was therefore to determine the prevalence of asymptomatic malaria, Schistosomiasis and STH infections in apparently healthy children attending schools in a semirural area in Kinshasa, DRC, as well as their relationship with anemia.
Materials and Methods
Study area
Kinshasa is one of the biggest cities in Africa and divided into 25 administrative zones or municipalities that cover about 9,965 km2. According to the national health policy, 25 Health Zones (HZs) roughly cover the 25 administrative zones. These HZs are also divided into Health Areas (HAs). Kimbanseke is the most densely populated peripheral municipality of Kinshasa housing 946, 372 inhabitants in 2004 [22]. The study was conducted in the Mokali HA. It is the most populated HA of Kimbanseke HZ, with an estimated 27,455 inhabitants. The participants were recruited at EP Boyambi and EP Likabo, two primary schools nearest to the regional health centre. Both schools were built by the Catholic Church and each school has 14 teachers and 12 classes. The esxpected number of schoolchildren was 650 per school at the beginning of the year.
Study design
This study analyses cross-sectional, baseline data from an open label, randomised, controlled trial enrolling asymptomatic school children and investigating the impact of intermittent preventive treatment (IPT) with Sulfadoxine-Pyrimethamine (SP) and SP plus Piperaquine (PQ) on anemia and malaria morbidity in Congolese schoolchildren (Trial registration: NCT01722539; PACTR201211000449323 ). The trial design and protocol are described in detail elsewhere [23].
Briefly, asymptomatic primary school children were recruited from the 10th to the 24th November 2012. Children whose parents did not provide written informed consent or presenting fever, muscle aches or other symptoms suggestive for malaria at the time of enrolment were excluded. Children participating in another clinical trial were also excluded from the study. A structured questionnaire was used to obtain information on age, river contact, history of fever, diarrhea and abdominal pain.
Laboratory analysis
Malaria diagnostic
Finger-prick blood specimens were taken for analysis of Plasmodium infection using microscopic examination for malaria parasites. Giemsa-stained thick blood smears (TBS) were used for microscopy. Blood slides were examined using light microscopy at 1000 × magnification. Hundred microscopic fields were examined in the thick smear before concluding that a blood slide was negative. All slides were read twice by experienced microscopists. If the discrepancy was greater than 15%, a third reader was used to confirm diagnosis. The parasite density per microliter of blood was calculated using the following formula: (Number of trophozoites x 8000)/Number of leucocytes. Parasite counts were utilized to classify the intensity of Plasmodium spp. infection into light, moderate, heavy, or very heavy infections respectively as followed: 1–499 parasites/µL, 500–1,999 parasites/µL, 2,000–9,999 parasites/µL and ≥10,000 parasites/µL.
Stool collection and parasite determination
Fecal samples were collected and taken to the laboratory of Parasitology of the University of Kinshasa for analysis using the Kato-Katz technique [24]. Slides were examined microscopically in a systematic manner 24 hours after preparation; S. mansoni and/or helminth eggs were counted and the number obtained was multiplied by the factor 24 in order to get the number of eggs per gram of feces (epg). Egg counts were utilized to classify infection intensities into light, moderate, or heavy infections respectively as followed: for S. mansoni 1–99 epg, 100–399 epg, ≥400 epg; A. lumbricoides, 1–4,999 epg, 5,000–49,999 epg and ≥50,000 epg; and for T. trichiura, 1–999 epg, 1,000–9,999 epg and ≥10,000 epg [25].
Urine analysis
Urine samples were analysed using the centrifugation method as described by Okanla [26]. Briefly, the samples were left to stand on the bench for about 30 min. Thereafter, the urine in each sample was drawn off leaving the last 10 mL in the bottle. The content of each bottle was shaken to suspend the sediment and was transferred into a 20 mL centrifuge tube. The tubes were centrifuged at 1 000 r/min for 5 min. The supernatant was discarded and the residue was put on a clean glass slide and examined under 10× objective lens of the microscope. The intensity of infection was estimated according to the number of eggs per 10 mL urine.
Measuring hemoglobin concentration
Hemoglobin (Hb) levels were determined using a HemoControl device (EKF Diagnostics, Germany). Anemia was defined by a hemoglobin concentration <11, <11.5 and <12 g/dL, respectively for schoolchildren of less than 5 years, 5–11.9 years and 12–14.9 years old. Anemia was classified as severe anaemia Hb <7 g/dl, moderate anaemia Hb: 7–9.9 g/dl and mild anaemia Hb: 10–11.4 g/dl. [27].
Measuring malnutrition
Weight and height were measured in each child. With these measurements we calculate the following indicators using the WHO Anthroplus software: a) height-for-age Z-score (HAZ) to assess stunting in children 6 to 240 months old; b) weight-for-age Z-score (WAZ) to assess underweight in children of 6 to 120 months of age; and c) BMI-for-age Z-score (BAZ) to assess thinness in children of 6 to 60 months of age [28].
Statistical analysis
The sample size was estimated on the basis of the expected additional impact on anemia of the intervention arm versus the comparator [23]. Data were entered and stored in Epi info7. Frequencies were used to assess the prevalence of asymptomatic malaria and anaemia in school children. Differences in mean values for continuous variables (e.g., HAZ, WAZ, BAZ, Hb, parasite densities) were assessed using the student t-test analysis. One-way ANOVA was used to analyze differences in Hb concentration mean of the study population by intestinal infection status and by infection intensity (negative, light and moderate-to-heavy) of each parasite species. Odds ratios (ORs), 95% confidence intervals (CIs) were calculated and p<0.05 values were considered to be statistically significant. Multivariate logistic regression models were constructed to identify factors associated with anemia in schoolchildren. Statistical analyses were done using SPSS statistical program, version 22 (SPSS, Chicago, IL, USA).
Ethical considerations
The investigators agreed to conduct the present study in full agreement with the principles of the Declaration of Helsinki’ and subsequent relevant amendments. The study was approved by the Ethical Committees of the University of Antwerp, Belgium and of the School of Public Health, University of Kinshasa, DRCongo. Approval was also obtained from the Ministry of Health of DRCongo. Prior to the start of the project, special permission was obtained from the DRCongo Ministry of Education and the local health and education authorities of Biyela. A series of meetings were also held in the participating schools to explain the nature and purpose of the trial. Written informed consent (IC) was obtained from each parents or legal guardians of all children prior enrolment. An oral assent was obtained, as well, from children who were 12-years-old or older. All participants were administered albendazole (400 mg single oral dose) and praziquantel (40 mg/kg) against helminth infections. Children suffering from clinical malaria were treated according to national policy.
Results
Characteristics of schoolchildren
In total, 989 children aged between 4 and 13 years (median: 8 years and IQR:7.5–9.5 years) were assessed for eligibility in November 2012 of which 616 (62.3%) met the inclusion criteria and whose parents consented. A high frequency of refusal was found 324 (32.8%), with no real differences between schools in terms of percentage of parents refusing (Table 1). Children whose parents did not consent were similar to those included in regard to age, sex and class (data not shown). Forty nine, 49 children (4.9%) were excluded at baseline for the following raisons including: fever, history of multiple transfusions and weight <14 Kgs. Included children in the two primary schools were broadly similar in regard to age, sex, anthropometric indices, bed net use, contact with river, and symptoms histories characteristics. Anemia and parasitic infections were also similar between schools (Table 1). The mean height was 121 cm (SD:11.7) and the mean weight was 24.5 kg (SD 5.6). The most commonly reported symptoms were history of fever (31.0%;CI95%:27.4–34.9), abdominal pain (26.8%;CI95% 23.4–30.5), diarrhea (15.3% CI95% 12.6– 18.4), and bloody feces (11.2% CI95% 8.9–14.0). Eleven percent of the children reported a history of blood transfusion (11.5%;CI95% 9.2–14.4). Two hundred seventy seven school children (45%; IQR:41–49) were using the river as source of water to bathe or to wash their clothes.
Table 1. General characteristics of children attending two primary schools in Mokali heath area, in Kinshasa, 2012.
| Variables | Boyambi School N = 558 | % (95% CI) | Likabo School N = 431 | % (95% CI) | P value | All N = 989 | % 95% CI | |
| Number of children (from class 1–5, %) | 558 | 56.4 (53.3–59.5) | 431 | 43.6 (40.5–46.7) | 989 | – | ||
| Refused/non response (%) | 168 | 30.1 (26.4–34.1) | 156 | 36.2 (31.5–40.8) | 324 | 32.8 (28.3–36.7) | ||
| Excluded children (%) | 29 | 5.2 (3.6–7.5) | 20 | 4.6 (2.9–7.2) | 49 | 4.95 (3.7–6.6) | ||
| Excluded for fever (%) | 20 | 3.6 (2.3–5.6) | 14 | 3.3 (1.9–5.5) | 34 | 3.4 (2.4–4.8) | ||
| Excluded for multiple transfusion (%) | 6 | 1.1 (0.44–2.5) | 4 | 0.93 (0.30–2.5) | 10 | 1.0 (0.51–1.9) | ||
| Excluded for Weight <14 Kgs (%) | 3 | 0.54 (0.14–1.7) | 2 | 0.46 (0.08–1.9) | 5 | 0.51 (0.19–1.3) | ||
| Number of included children (%) | 360 | 58.4 (54.4–62.4) | 256 | 41.6 (37.7–45.6) | 616 | 62.3 (57.3–67.7) | ||
| Female(%) | 170 | 47.2 (42–52.5) | 128 | 50 (43.7–56.3) | 298 | 48.4 (44.4–52.4) | ||
| Median Age (IQR, years) | 8 (7–9) | – | 7 (7–8) | – | 0.97 | 8 (7.5–9.5) | – | |
| Mean weight (SD, Kgs) | 24.56 (5.9) | – | 24.51 (5.2) | – | 0.91 | 24.53 (5.6) | – | |
| Mean Height(SD, Cm) | 125.9 (12.7) | – | 126.5 (10.1) | – | 0.53 | 126.2 (11.7) | – | |
| Mean Hb (SD, g/dl) | 11.54 (1.2) | – | 11.64 (1.3) | – | 0.32 | 11.6 (1.2) | – | |
| Anthropometric indicators of malnutrition | ||||||||
| WHZ <−2 Z-score | 5 | 1.4 (0.51–3.4) | 1 | 0,39 (0.01 −2.2) | 6 | 0.97 (0.4 −2.2) | ||
| WAZ <−2 Z-score | 5 | 1.4(0.51 −3.4) | 1 | 0.39 (0.01 −2.2) | 6 | 0.97 (0.4 −2.2) | ||
| HAZ <−2 Z-score | 1%) | 0.28 (0.01 −1.8) | 0 | - | 1 | 0.16 (0.01–1.1) | ||
| Anaemia (%) | 157 | 43.6 (38.5 −48.9) | 100 | 39.1 (33.1–45.3) | 257 | 41.7 (37.8–45.7) | ||
| Severe anaemia (Hb <7 g/dl; %) | 0 | - | 1 | 1 (0.03–5.5) | 1 | 0.4 (0.01–2.2) | ||
| Moderate anaemia (Hb: 7–9.99 g/dl;%) | 38 | 24.2 (17.7–31.7) | 24 | 24 (16 −33.6) | 62 | 24.1 (19–29.8) | ||
| Mild anaemia (Hb:10–11.49 g/dl; %) | 119 | 75.8 (68.3–82.3) | 75 | 75 (65.3–3.1) | 194 | 75.5 (69.8–80.6) | ||
| Symptom histories | ||||||||
| History of fever(%) | 100 | 27.8 (23.3–32.8) | 91 | 35.5 (29.7–41.8) | 191 | 31 (27.4–34.9) | ||
| History of diarrhea (%) | 59 | 16.4 (12.8–20.7) | 35 | 13.7 (9.7 −18.5) | 94 | 15.3 (12.6 −18.4) | ||
| History of abdominal pain (%) | 92 | 25.6 (21.2–30.5) | 73 | 28.5 (23.1–34.5) | 165 | 26.8 (23.4–30.5) | ||
| History of bloody feces | 33 | 9.2 (6.5 −12.8) | 36 | 14.1 (10.1–18.9) | 69 | 11.2 (8.9–14) | ||
| History of transfusion | 47 | 13.1 (9.8 −17.1) | 24 | 9.4 (6.1–13.6) | 71 | 11.5 (9.2–14.4) | ||
| History of cough (%) | 70 | 19.4 (15.6 −24) | 72 | 28.1 (22.7–34.1) | 142 | 23.1 (19.8 −26.6) | ||
| History of itch (%) | 51 | 14.2 (10.8–18.3) | 52 | 20.3 (15.6–25.8) | 103 | 16.7 (13.9–20) | ||
| Parasitic infections | ||||||||
| Malaria infection (%) | 77 | 21.4 (17.3 −26.1) | 37 | 14.5 (10.4–19.4) | 119 | 18.5 (15.6 −21.9) | ||
| Schistosoma mansoni infection (%) N = 457 | 21 | 7.2 (4.5–10.8) | 8 | 4.9 (2.1–9.4) | 29 | 6.4 (4.4–9.1) | ||
| STH infection (%) N = 457 | 96 | 32.8 (27.4 −38.5) | 54 | 32.9 (25.8–40.7) | 150 | 32.8 (28.6 −37.4) | ||
| Other characteristics | ||||||||
| Contact with river (%) | 175 | 48.6 (43.4 −53.9) | 102 | 39.8 (33.8–46.1) | 277 | 45 (41–49) | ||
| Bednet ownership (%) | 60 | 17.1 (13.4–21.6) | 58 | 24.2 (19–30.2) | 118 | 20.3 (16.9 −23.6) | ||
| Bednet use (%) | 37 | 61.7 (48.2 −73.9) | 44 | 75.9 (62.8 −86.1) | 81 | 68.6 (59.5–76.9) | ||
EP: Ecole primaire; 95% CI: 95% confidence interval; Hb : haemoglobin.
Excluded symptomatic children
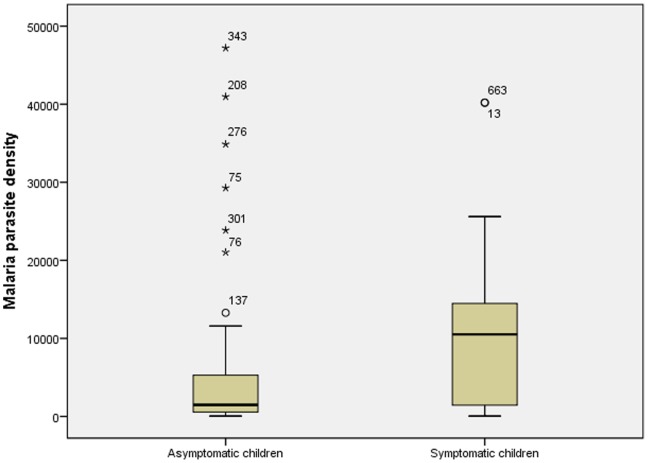
A total of 34 (3.4%;CI95%: 2.4–4.8) children presented fever with a mean temperature of 38.6 (SD 1.2). Blood smear, hemoglobin concentration and stool examination were performed, before they were treated according to the national policy. The prevalence of malaria parasitaemia amongst febrile children was 41.2% (CI95%:24.7–59.3). The median malaria parasite density was 9840/µL (CI95%:4660–14480). No S. mansoni infection was diagnosed in symptomatic children (Table 2). However, the frequencies of STH infections were similar in both symptomatic and asymptomatic children (Table 3). The prevalence of anemia in this group was found to be 47.1% (CI95% :29.8–64.9) (Table 2).
Table 2. General characteristics of symptomatic children, excluded from the trial.
| Characteristic | N 34 | % | (95%CI) |
| Mean Temperature (SD, °C) | 38.6 (1.2) | ||
| Median age (IQR, years) | 8 (7–9)- | – | – |
| Mean height (SD, cm) | 134 (10.1) | – | – |
| Mean weight (SD, kgs) | 26.0 (4.84) | – | – |
| Mean Hb (SD, g/dl) | 11.44 (0.99) | – | – |
| Symptoms | |||
| Fever | 34 | 100% | – |
| History of diarrhea | 3 | 8.8% | (1.9–23.7) |
| History of abdominal pain | 8 | 23.5% | 10.8–41.2 |
| History of bloody feces | 3 | 8.8% | 1.9–23.7 |
| History of transfusion | 3 | 8.8% | 1.9–23.7 |
| History of cough | 7 | 20.6% | 8.7–37.9 |
| History of itch | 7 | 20.6% | 8.7–37.9 |
| Other characteristics | |||
| Contact with river | 11 | 32.4% | 17.4–50.5 |
| Ownership of bed net | 4 | 18.2% | 5.2–40.3 |
| Use of bed net | 4 | 18.2% | 5.2–40.3 |
| Anthropometric indicators of malnutrition | |||
| WHZ <−2 Z-score | 1 | 2.9% | 0.07–15.3 |
| WAZ <−2 Z-score | 1 | 2.9% | 0.07–15.3 |
| HAZ <−2 Z-score | 0 | – | – |
| Anaemia (%) | 16 | 47.1% | 29.8–64.9 |
| Severe anaemia (Hb <7 g/dl; %) | – | – | – |
| Moderate anaemia (Hb: 7–9.99 g/dl;%) | 1 | 6.3% | 0.16–30.2 |
| Mild anaemia (Hb:10–11.49 g/dl; %) | 15 | 93.8% | 69.8–99.8 |
Table 3. Frequencies of parasitic infections, in children attending two primary schools in Mokali heath semi-rural area, in Kinshasa, 2012.
| Asymptomatic children (N = 616) | Symptomatic children (N = 34) | ||||||||
| N | % | CI95% | PD; Median, IQR | N | % | CI95% | PD; Median, IQR | P value | |
| Parasitic infections | |||||||||
| Plasmodium spp . infection (TBS) (N = 616a/34b) | 114 | 18.5 | 15.6–21.9 | 1478 (557–5279) | 14 | 41.2 | 24.7–59.3 | 9840 (4660–14480) | 0.01 |
| Light parasitaemia 1–499 parasites/µL | 24 | 21 | 14–29.7 | 3 | 21.4 | 4.7–50.8 | |||
| Moderate parasitaemia 500–1,999 n/µL | 40 | 35.1 | 26.4–44.6 | 1 | 7.1 | 0.2–33.9 | |||
| Heavy parasitaemia 2,000–9,999 n/µL | 40 | 35.1 | 26.4–44.6 | 3 | 21.4 | 4.7–50.8 | |||
| Very heavy parasitaemia ≥10,000 n/µL | 10 | 8.8 | 4.3−15.5 | 7 | 50.0 | 23–77 | |||
| S. mansoni infection (Kato-Katz) (N = 457a/30b) | 29 | 6.4 | 4.4–9.1 | 48 (24–76) | 0 | – | – | – | – |
| Light parasite load 1–99 | 24 | 82.8 | 64.2–94.2 | – | |||||
| Moderate parasite load 100–399 | 3 | 10.3 | 2.2–27.4 | – | |||||
| High parasite load ≥400 | 2 | 6.9 | 0.9–22.8 | – | |||||
| Soil Transmitted Helminth infections | 150 | 32.8 | 28.6–37.4 | 7 | 23.3 | 12.1–38.4 | |||
| A. lumbricoides infection (Kato-Katz) | 92 | 20.1 | 16.6−24.2 | 708 (300–2448) | 3 | 10.0 | 2.1–26.5 | 1448 (48–2640) | 0.1 |
| Light parasite load 1–4999 epg | 82 | 89.1 | 80.9–94.7 | 3 | 100 | – | |||
| Moderate/heavy parasite load ≥5000 epg | 10 | 10.9 | 5.3–19.1 | 0 | _ | ||||
| T. trichiura infection (Kato-Katz) | 120 | 26.2 | 22.3–30.6 | 72 (58–2424) | 4 | 13.3 | 3.8–30.7 | 96 (72–96) | 0.2 |
| Light parasite load 1–999 epg | 117 | 97.5 | 92.9–99.5 | 4 | 100 | - | |||
| Moderate/heavy parasite load ≥1000 epg | 3 | 2.5 | 0.5−7.1 | 0 | |||||
| E. vermicularis (Kato-Katz) | 1 | 0.2 | 0.01–1.4 | 24 (24–24) | 0 | ||||
| Co-infections (malaria - S. mansoni ) | 7 | 1.5 | 0.7–3.3 | 0 | |||||
| Co-infections (malaria - STH) | 29 | 6.4 | 4.4–9.1 | 2 | 6.7 | 0.72–19.7 | |||
| Co-infection of at least two parasite species | 79 | 17.3 | 15.3–20.8 | 2 | 6.7 | 0.72–19.7 | |||
PD: Parasite Density; a: N° of asymptomatic children; b: N° of symptomatic children, TBS: Tick Blood Smear.
Asymptomatic malaria infection
The prevalence rate of Plasmodium spp. infection was 18.5% (CI95%:15.6–21.9) and was found to be significantly lower than in symptomatic children. P. falciparum was the most prevalent species (98.2%;CI95%:93.8–99.8). P.malariae was diagnosed in two children. In those with parasitemia, the median parasite density was 1478/µl (IQR:557–5279) and was also significantly lower than 9840 parasites/µL found in symptomatic children (p = 0.01) (Table 3.) (Fig. 1). The intensity of infection was found to be 24 (21%) with light, 40 (35.1%) moderate, 40 (35.1%) heavy, and 10 (8.8%) presenting very heavy parasite density (Table 1). Schoolchildren younger than 9 years old (aOR:0.47 CI95% 0.26– 0.86; p = 0.02), and girls (aOR:0.57;CI95%:0.37–0.86; p<0.01) were less likely to have malaria infection. History of fever was an independent risk factor for malaria infection (aOR:1.6 CI95% 1.0–2.4;p = 0.03) (Table 4). No significant difference in parasite density could be found between the children with or without history of fever (p = 0.09).
Figure 1. Difference of parasite density median between symptomatic and asymptomatic children.
Table 4. Predictors for asymptomatic P. falciparum infection, Schistosoma mansoni infection and Soil Transmitted Helminth infections in children attending two primary schools in Mokali heath semi-rural area, in Kinshasa, 2012.
| Plasmodium spp. Infection | S. mansoni infection | STH infections | ||||||||
| Characteristic | ORa (95% CI) | P value | ORb (95% CI ) | P value | ORc (95% CI ) | P value | ||||
| Age | 4–9 | 0.47 (0.26–0.86) | 0.01* | – | – | – | 0.52 | 0.29–0.97 | 0.04* | |
| 10–13 | 1 | – | 1 | |||||||
| Sex | Female | 0.57 | 0.37–0.86 | <0.01* | 2.4 | 1.1–5.4 | 0.04* | 0.62 | 0.41–0.92 | 0.02* |
| Male | 1 | 1 | 1 | |||||||
| History of fever | Yes | 1.6 | 1.0–2.4 | 0.04* | – | – | – | NA | ||
| No | 1 | – | ||||||||
| Contact with river | Mango river | NA | 2.7 | 1.2–6.0 | 0.02* | NA | ||||
| Other river | 1.0 | 0.5–2.1 | 0.9 | |||||||
| No | 1 | |||||||||
NA - Not applicable, OR- odds ratio, CI- Confidence Interval.
Adjusted for: bed net use, S. mansoni and STH infections.
Adjusted for: age, history of abdominal pain and diarrhea.
Adjusted for: history of abdominal pain and diarrhea.
*- significant at p<0.05.
S. mansoni infection
The overall prevalence of S. mansoni infection was 6.4% (CI95%:4.4–9.1) and the median parasite density was 48 eggs/g (IQR:24–76)(Table 3). The likelihood of having S. mansoni infection was high in children bathing in Mango river (aOR:2.7;CI95%:1.2–6.0;p = 0.02) and in girls (aOR: 2.3 CI95% 1.0–5.0; P = 0.04). Other factors such as history of abdominal pain, diarrhea, bloody feces before enrolment did not demonstrate any association with S. mansoni infection (Table 4).
STH infections
The prevalence of STH infections was estimated to be 32.8% (CI95%:28.6–37.4). Only two of the three majorSTH were found: Trichuris trichiura (26.3%) and Ascaris lumbricoides (20.1%). Hookworms were not found. However one case of a non-STH, Enterobius vermicularis was diagnosed. Girls (aOR:0.62;CI95%:0.41–0.92;p = 0.02) and younger children (aOR:0.52;CI95% 0.29–0.97;p = 0.04) had a significant lower risk for STH infection (Table 4). Factors such as history of abdominal pain and diarrhea were not associated to STH infection (p = 0.9) (data not shown).
S. haematobium infection
No infection with S. haematobium were found in the children’s urine.
Co-infections
Co-infection with malaria and a helminth was common though we did not observe any S. mansoni-STH co-infection. The prevalence rate of malaria-S. mansoni and malaria-STH co-infections was respectively 1.5% (CI95%:0.7–3.3) and 6.4% (CI95% 4.4–9.1) (fig. 2). There was no association found between helminth status and malaria infection (p = 0.3) or schistosomiasis and malaria infection (p = 0.7) (data not shown). Co-infection with at least two parasite species (Malaria-S. mansoni, Malaria- STH or between two STH species) was found in 79 participants (17,3%;CI95%:15.3–20.8).
Figure 2. Venn diagram showing Malaria – S. mansoni and malaria – STH infections co-infections.
Nutritional status
The nutritional status of most children was within healthy parameters but a few cases of stunting (HAZ<−2) 0.16%, thinness (BAZ<−2) 0.65% and underweight (WAZ<2) 0.97% were observed (Table 1).
Anemia
The mean hemoglobin concentration was 11.6 g/dL (SD±1.4) and was found to be lower in children with asymptomatic malaria infection compared to uninfected children (p<0.001) (table 5). Hb concentration was not lower in children with Schistosomiasis (p = 0.06) or STH infection (p = 0.12). Children with co-infections had a 1.2 g/dl lower Hb (p = 0.01) when infected with malaria-S.mansoni, and a 0.66 g/dl Hb lower when co-infected with STH (p = 0.02) than all not co-infected (Table 5). Other factors such as malnutrition, history of fever, history of transfusion and the intensity of parasitic infections were not correlated to the Hb concentration.
Table 5. Mean Hb, anaemia prevalence and predictors for anaemia in asymptomatic children attending two primary schools in Mokali heath semi- rural area, in Kinshasa, 2012.
| Variables | N (%) | Total (N) | Mean Hb g/dL (SD) | P value | Anemia | cOR | P value | aOR | P value | |
| Age | 4–9 | 556 (90.3%) | 616 | 11.56 (1.25) | 0.2 | 249(44.8%) | 1.0 | 0.1 | ||
| 10–13 | 60 (9.7%) | 11.77 (1.16) | 27(45.0%) | 1 | – | - | ||||
| Sex | Female | 298 (48.4%) | 616 | 11.63 (1.18) | 0.4 | 130(43.6%) | 0.91 | 0.6 | – | |
| Male | 318 (51.6%) | 11.53 (1.29) | 146(45.9%) | 1 | ||||||
| Malaria infection | Yes | 114 (18.5%) | 616 | 10.78 (1.44) | <0.001* | 81(71.0%) | 3.8 | <0.001* | 4.5 (2.6–7.7) | <0.001* |
| No | 502 (81.5%) | 11.76 (1.12) | 195(38.8%) | 1 | ||||||
| S. mansoni infection | Yes | 29 (6.4%) | 457 | 11.15 (1.12) | 0.06 | 20(69.0%) | 2.9 | 0.01* | 2.5 (1.0–6.0) | 0.01* |
| No | 428 (93.6%) | 11.60 (1.24) | 185(43.22%) | 1 | ||||||
| T. Trichiura Infect. | Yes | 120 (26.3%) | 457 | 11.50 (1.22) | 0.4 | 54 (45.0%) | 1.0 | 0.9 | – | – |
| No | 337 (73.7%) | 11.60 (1.25) | 151 (44.8%) | 1 | ||||||
| A. Lumbricoides Infect. | Yes | 92 (20.2%) | 457 | 11.45 (1.13) | 0.3 | 47 (51.1%) | 1.4 | 0.2 | – | |
| No | 365 (79.8%) | 11.60 (1.26) | 158 (43.2%) | 1 | ||||||
| Intestinal infection status | Mixed | 57 (12.5%) | 457 | 11.26 (1.18) | 0.12 | 34(59.6%) | 1.9 | 0.03* | – | – |
| Mono | 122 (26.7%) | 11.63 (1.17) | 49(40.1%) | 0.76 | 0.2 | – | ||||
| No | 278 (60.8%) | 11.61 (1.27) | 122(43.9%) | 1 | ||||||
| History of fever | Yes | 191 (31.0%) | 616 | 11.47 (1.26) | 0.3 | 87 (45.5%) | 1.3 | 1.2 | – | – |
| No | 425 (69.0%) | 11.63 (1.23) | 169 (39.7%) | 1 | ||||||
| History of transfusion | Yes | 71 (11.5%) | 616 | 11.61 (1.41) | 0.8 | 31 (43.7%) | 1.1 | 0.7 | – | – |
| No | 545 (88.5%) | 11.57 (1.22) | 225 (41.3%) | 1 | ||||||
| Ownership and use of Bednet | Yes and used | 81 (13.1%) | 589 | 11.50 (1.22) | 0.8 | 36 (44.4%) | 1.1 | 0.7 | – | – |
| Yes and not used | 37 (6.0%) | 11.65 (1.23) | 14 (37.8) | 0.83 | 0.6 | – | – | |||
| No | 471 (76.5%) | 11.58 (1.26) | 199 (42.3%) | 1 | ||||||
| Malaria parasite density | Very heavy | 10 (8.8%) | 114 | 10.79 (1.85) | 0.7 | NA | ||||
| Heavy | 40 (35.1%) | 10.94 (1.27) | ||||||||
| Moderate | 40 (35.1%) | 10.59 (1.48) | ||||||||
| Low | 24 (21%) | 10.84 (0.83) | ||||||||
| S. mansoni infection density | Heavy | 2 (6.9%) | 29 | 11.20 (1.01) | 0.3 | NA | ||||
| Moderate | 3 (10.3%) | 11.16 (0.80) | ||||||||
| Low | 24 (82.8%) | 11.14 (1.21) | ||||||||
| Co-infection (Malaria- SCH) | Yes | 7(1.5%) | 457 | 10.37 (0.93) | 0.01* | NA | ||||
| No | 450 (98.5%) | 11.59 (1.23) | ||||||||
| Co-infection (Malaria –Helminth) | Yes | 29 (6.3%) | 457 | 10.96 (1.48) | 0.02* | NA | ||||
| No | 428 (93.7%) | 11.61 (1.25) |
N - Number, SD- Standard Deviation, c OR- crude Odds- Ratio, aOR- adjusted Odds-Ratio, NA- Not Applicable, SCH-Schistosomiasis.
*- significant at p<0.05.
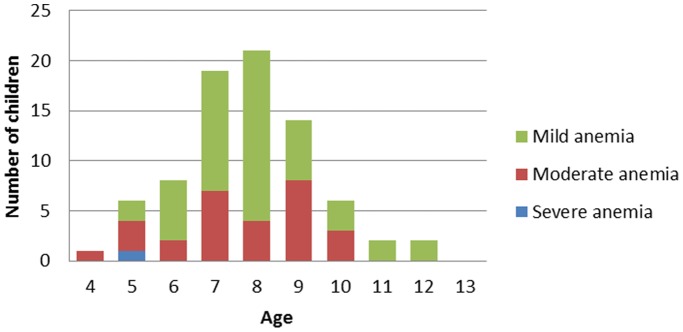
The prevalence of anemia was estimated to be 41.6% (CI95%:37.7–45.6) with no difference between sexes (p = 0.6). It was more common in P.falciparum-infected children (71.0%) than in uninfected children (38.8%) (p<0.001) (Table 4). Of these anemic schoolchildren, 193 (31.3%), 62 (10.1%), 1 (0.2%), had respectively, mild, moderate and severe anemia (table 5). In this study, mild anemia occurred more frequently in children younger than 9 years with a peak in 8 year olds (figure 3). No significant differences were found in the malaria and S. mansoni parasite density in the children presenting mild, moderate or severe anemia (data not shown). The multivariate regression model shows that anemia was associated with asymptomatic malaria infection (aOR:4.5;CI95%:2.6–7.7;p<0.001) and S. mansoni infection (aOR:2.5;CI95%: 1.0–6.0; p<0.01) independently (Table 4). No interaction between S. mansoni and malaria infection was observed.
Figure 3. Distribution of anaemia in malaria infected children according to age in Kinshasa.
Discussion
In the Mokali Health Area, a semi-rural area of Kinshasa located in the Health Zone of Kimbanseke, the prevalence of asymptomatic malaria infection in schoolchildren was found to be 18.5%. Similar observations were made in 1981–1983 in Kinshasa, and 2000 in Kimbanseke [29]. In this study, the increased malaria risk for older children was unexpected (Table 4). The prevalence of asexual stages of P. falciparum in endemic areas is supposed to decrease significantly with age, because children would gradually developed some degree of immunity against the malaria parasite, as a result of repeated infections [30]. However, this observation was also reported in the Kikimi Health Zone also located in Kimbanseke zone [29]. In a study conducted in Brazzaville, a higher malaria prevalence in older children was attributed to the increased use of antimalarial drugs, particularly in early childhood [31]. There was a significant association between history of fever around the time of the enrolment and malaria parasitemia, and this agrees with a study conducted in Nigeria [32]. On the other hand, this study revealed a prevalence of symptomatic children of 3.4%, with 41.2% having a positive tick blood smear. This rate of symptomatic children at school was high and unexpected. These results suggests that malaria in school age children, thought usually asymptomatic, can result into mild and somewhat well tolerated symptoms compared to under five years children. Symptomatic children had a significantly higher malaria parasite density compared to those asymptomatic. These findings underline the complexity of the clinical presentation of P. falciparum infection in endemic areas.
Like malaria, STH were highly prevalent in the study population (32.8%). This could be the result of poor sanitary conditions in the Health Area of Mokali. This study recorded a prevalence of 26.2% for T. trichiura having the highest prevalence, followed by A. lumbricoïdes (20.1%). These values are significantly lower than 90% and 83.3% respectively for A. lumbricoïdes and T. trichiura reported by Vandepitte in 1960 in Kinshasa [33]. The prevalence of these two parasites declined and was found to be respectively 57% and 11% in 1980 [34]. These drastic changes in prevalence could be explained by the education and increase awareness [35]. The prevalence found in this study showed a further decrease of A. lumbricoides infection, however improved sanitary, access to adequate water supply and access to health care should further decrease the prevalence of STH infections.
This study also estimated the prevalence of S. mansoni infection to be 6.4%. This prevalence is significantly lower compared to 89.3% reported in 2012 in Kasansa Health Zone, another endemic setting for S. mansoni in DRC [36]. Girls were more likely to be infected than boys. This is probably because they are traditionally responsible for water related household chores in poor countries [37], therefore being more frequently in contact with contaminated water. Children who regularly bathed in the Mango river were significantly more likely to be infected than those who did not. These findings emphasize the need for extensive malacological studies in this area to identify the intermediate host species specifically in Mango river. Reported history of bloody feces, diarrhea and abdominal pain were not related to S. mansoni infection. Similar observation was found in Yemeni in California [38]. This could be due to the low parasite load observed in the study population (more than 80% having light parasite load). Most of the infected children were probably in the chronic phase of the disease. Therefore, they presented a low grade of acute symptoms although anemia was significantly associated with infection.
Co-infection with P. falciparum and S. mansoni occurred at very low levels (1.5%). This is consistent with findings from Kenya in 2008–09 and Ethiopia 2008–09 and Uganda 2006 [39]. However, P. falciparum and STH co-infections were more frequent (6.4%). No association was found between malaria infection and S. mansoni infection neither between malaria infection and STH infection. This is in total agreement with previously reported data from Tanzania in 2010 [40].
On the other hand, the prevalence of anemia in primary schoolchildren was found to be 41.6%. This was lower than 67% observed in Kasansa, DRC in 2012 [36]. The likelihood of having anemia was about 4 times more in malaria infected schoolchildren. Mean hemoglobin concentration was significantly lower in malaria infected children compared to uninfected children with an incremental Hb level of 0.98 g/dL. The present study as many others conducted in others settings across Africa [41], [42], demonstrated the major role played by malaria in the occurrence of anemia in schoolchildren in sub-Saharan Africa. In disagreement with other findings [43], S. mansoni infection was also found to be an independent risk factor for anemia in schoolchildren. No interaction was found between asymptomatic malaria infection and S. mansoni in regard to anaemia.
The study has a number of limitations. First, given the high rate of refusal (32.8%), which may lead to a selection bias, the reported data may not be representative of the schools surveyed. However, given that children whose parents did not consent were similar to those included in regard to age, sex and class, we have no reason to suspect that children in these two groups differed greatly in regard to other characteristics not assessed. This high proportion of refusal may indirectly suggest a negative perception of IPT or other malaria intervention in schoolchildren by the community. This underlines the urgent need to assess the perception and potential social and cultural barriers that can prevent an efficient implementation of malaria control strategies in schoolchildren. Second, asymptomatic malaria infection is mostly characterized by low grade parasitemia [44]. Conventional microscopy, the laboratory method used in the present study, is not sensitive enough to detect low-grade, asymptomatic infections. Therefore, a highly sensitive PCR-based diagnosis, which is between 2.7-fold and 8.6-fold more sensitive than conventional microscopy in detecting malaria parasites in apparently health children [45], [46], would provide a more accurate picture of asymptomatic plasmodium spp carriage. Finally, no hookworms were found in Mokali’s schoolchildren. This could be at least partly explained by the fact that, the Kato-Katz slides were examined after 24 hours, which situation may lead to overclearance of hookworm eggs by glycerol.
Conclusion
This study demonstrated that P. falciparum infection was highly prevalent in schoolchildren of Biyela Health Zone, and along with S. mansoni infection, they contribute to a great extent to the occurrence of anemia. These results highlight the important role of school-based interventions, which may include: deworming, micronutrients and intermittent preventive treatment for malaria for the control of anemia among African schoolchildren.
Acknowledgments
The authors thank all schoolchildren who participated in this study and the staff of the two primairy schools (EP BOYAMBI and EP LIKABO). We also thank Axel Ibalanky, Henri Kabongo, Ernest Makengo, Bruno Nsilulu, Deddy Mukundi, Sylvie Mussamba, Ornella Kipasa, Nono Ndosi, Francia Mbombo, Daniel Pama and Victor Okenge, for assisting in data collection and sample collection and analysis.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financed by the VLIR UOS project (ZRDC2012 70447), University of Antwerp (JPV), and Fonds Wetenschappelijk Onderzoek (FWO) project G.0978.11N. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2001) Schistosomiasis and soil-transmitted infections. 54th World Health Assembly, agenda item 13.3 resolution WHA 54.19.
- 2. Petney TN, Andrews RH (1998) Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int J Parasitol 28: 377–93. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis: first report of the joint WHO expert committees. WHO Technical Report Series. Geneva: World Health Organization. [PubMed]
- 4. Buck AA, Anderson RI, MacRae AA (1978) Epidemiology of poly-parasitism I. Occurrence, frequency and distribution of multiple infections in rural communities in Chad, Peru, Afghanistan and Zaire. Trop Med Parasitol 29: 61–70. [PubMed] [Google Scholar]
- 5. Henning L, Schellenberg D, Smith T, Henning D, Alonso P, et al. (2004) A prospective study of Plasmodium falciparum multiplicity of infection and morbidity in Tanzanian children. Trans R Soc Trop Med Hyg 98: 687–94. [DOI] [PubMed] [Google Scholar]
- 6. Smith T, Felger I, Tanner M, Beck HP (1999) Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg 93 Suppl 1 59–64. [DOI] [PubMed] [Google Scholar]
- 7. Verhoef H (2010) Asymptomatic malaria in the etiology of iron deficiency anemia: a malariologist's viewpoint. Am J Clin Nutr 92: 1285–6. [DOI] [PubMed] [Google Scholar]
- 8.White NJ, Ho M (1992). The pathophysiology of malaria. In: Advances in Parasitology. Baker, J.R., Muller, (editors). 3rd edition. New York: Academic Press 84–175.
- 9. Faquin WC, Schneider TJ, Goldberg MA (1992) Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood 79: 1987–94. [PubMed] [Google Scholar]
- 10. Jelkmann W, Pagel H, Wolff M, Fandrey J (1992) Monokines inhibiting erythropoietin production in human hepatoma cultures and in isolated perfused rat kidneys. Life Sci 50: 301–8. [DOI] [PubMed] [Google Scholar]
- 11. Hotez PJ, Kamath A (2009) Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 25: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bundy DA, Lwin S, Osika JS, McLaughlin J, Pannenborg CO (2000) What should schools do about malaria?. Parasitol Today 16: 181–2. [DOI] [PubMed] [Google Scholar]
- 13. Friedman JF, Kanzaria HK, McGarvey ST (2005) Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends in Parasitology 21: 386–392. [DOI] [PubMed] [Google Scholar]
- 14. Lemaitre M, Watier L, Briand V, Garcia A, Le Hesran JY, et al. (2014) Co-infection with Plasmodium falciparum and Schistosoma haematobium: Additional Evidence of the Protective Effect of Schistosomiasis on Malaria in Senegalese Children. Am J Trop Med Hyg 90: 329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brutus L, Watier L, Hanitrasoamampionona V, Razanatsoarilala H, Cot M (2007) Confirmation of the protective effect of Ascaris lumbricoides on Plasmodium falciparum infection: results of a randomized trial in Madagascar. Am J Trop Med Hyg 77: 1091–5. [PubMed] [Google Scholar]
- 16. Hesran J, Akiana J, Ndiaye EI, Dia M, Senghor P, et al. (2004) Severe malarial attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans R Soc Trop Med Hyg 98: 397–399. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro AE, Tukahebwa EM, Kasten J, Clarke SE, Magnussen P, et al. (2005) Epidemiology of helminths infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg 99: 18–24. [DOI] [PubMed] [Google Scholar]
- 18. Midzi N, Mtapuri-Zinyowera S, Mapingure MP (2010) Consequences of polyparasitism on anaemia among primary school children in Zimbabwe. Acta Trop 115: 103–11. [DOI] [PubMed] [Google Scholar]
- 19. Ezeamama EE, McGarvey ST, Acosta LP (2008) The synergistic effect of concomitant schistosomiasis, hookworm, and trichuris infections on children’s anemia burden. PLoS Negl Trop Dis 2: e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. World Malaria Report 2008. Who website. Available: http://www.who.int/malaria/publications/atoz/9789241563697/en/. Accessed 2014 October 14.
- 21. Gething PW, Kirui VC, Alegana VA, Okiro EA, Noor AM, et al. (2010) Estimating the Number of Paediatric Fevers Associated with Malaria Infection Presenting to Africa’s Public Health Sector in 2007. PLoS Medicine 7 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nzuzi FL (2008) Kinshasa: Ville et Environnement, Paris, L’Harmattan.
- 23. Doua JY, Matangila JR, Lutumba P, Van geertruyden JP (2013) Intermittent preventive treatment: efficacy and safety of sulfadoxine-pyrimethamine and sulfadoxine-pyrimethamine plus piperaquine regimens in schoolchildren of the Democratic Republic of Congo: a study protocol for a randomized controlled trial. Trials 24: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo 14: 397–400. [PubMed] [Google Scholar]
- 25.WHO (2002) Prevention and control of schistosomiasis and soil transmitted helminthiasis. Geneva, Switzerland: WHO Expert Committee. [PubMed]
- 26. Okanla EO (1991) Schistosomiasis: influence of parental occupation and rural or urban dwelling on prevalence. Nig J Pure Appl Sci 6: 154–159. [Google Scholar]
- 27.Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. WHO website. Available: http://www.who.int/vmnis/anaemia/prevalence/en/. Accessed 2014 October 14.
- 28. de Onis M, Habicht JP (1996) Anthropometric reference data for international use: Recommendations from a WHO Expert Committee. Am J Clin Nutr 64: 650–658. [DOI] [PubMed] [Google Scholar]
- 29.Kazadi W, Sexton J, Makengo B, Bompela WO, Matezo W (2004) Malaria in primary school children and infants in Kinshasa, Democratic Republic of the Congo: Surveys from the 1980S and 2000. Am. J. Trop. Med. Hyg (Suppl 2): 97–102. [PubMed]
- 30. Bruce MC, Donnelly CA, Packer M, Lagog M, Gibson N, et al. (2000) Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology 121: 247–256. [DOI] [PubMed] [Google Scholar]
- 31. Trape JF (1987b) Malaria and urbanization in Central Africa: the example of Brazzaville. Part IV: Parasitological and serological surveys in urban and surrounding rural areas. Trans R SocTrop Med Hyg 81 Suppl 2 27–33. [DOI] [PubMed] [Google Scholar]
- 32. Gbadegesin RA, Sodeinde O, Adeyemo AA, Ademowo OG (1997) Body temperature is a poor predictor of malaria parasitaemia in children with acute diarrhoea. Ann Trop Paediatr 1: 89–94. [DOI] [PubMed] [Google Scholar]
- 33.Vandepitte J (1988) Helminthologie médicale. Université de Kinshasa, 122 pages.
- 34. Jancloes MF, Cornet P (1980) Epidemiological control of intestinal nematodoses in a rural area of Zaïre. Revue épidémiologique. Santé Publique 28: 89–103. [PubMed] [Google Scholar]
- 35. Asaolu SO, Ofoezie IE (2003) The role of health education and sanitation in the control of helminth infection. Acta Trop 86: 283–294. [DOI] [PubMed] [Google Scholar]
- 36.Kabongo M (2012) Impact of Schistosomiaisis in Kasansa Health Zone in Democratic Republic of Congo. Public Health Thesis paper 220.
- 37.The World's Women (2010) Trends and Statistics. United Nations New York.
- 38. Warren KS, Mahmoud AA, Cummings P, Murphy DJ, Houser HB (1974) Schistosomiasis mansoni in Yemeni in California: duration of infection, presence of disease, therapeutic management. Am J Trop Med Hyg 23: 902–9. [DOI] [PubMed] [Google Scholar]
- 39. Brooker SJ, Pullan RL, Gitonga CW, Ashton RA, Kolaczinski JH, et al. (2012) Plasmodium-helminth coinfection and its sources of heterogeneity across East Africa. J Infect Dis 205 841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mazigoi HD, Kidenya BR, Ambrose EE, Zinga M, Waihenya R (2010) Association of intestinal helminths and P. falciparum infections in co-infected school children in northwest Tanzania. Tanzan J Health Res 12: 299–301. [DOI] [PubMed] [Google Scholar]
- 41. Magalhaes RJ, Clements AC (2011) Mapping the risk of anaemia in preschoolage children: the contribution of malnutrition, malaria, and helminth infections in West Africa. PLoS Med 8: 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA (2007) Child development: risk factors for adverse outcomes in developing countries. Lancet 369: 145–157. [DOI] [PubMed] [Google Scholar]
- 43. Sturrock RF, Kariuki HC, Thiongo FW, Gachare JW, Omondi BG, et al. (1996) Schistosomiasis mansoni in Kenya: relationship between infection and anaemia in schoolchildren at the community level. Trans R Soc Trop Med Hyg 90: 48–54. [DOI] [PubMed] [Google Scholar]
- 44. Roucher C, Rogier C, Dieye-Ba F, Sokhna C, Tall A, et al. (2012) Changing malaria epidemiology and diagnostic criteria for Plasmodium falciparum clinical malaria. PLoS One 7: e46188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang B, Han SS, Cho C, Han JH, Cheng Y, et al. (2014) Comparison of Microscopy, Nested-PCR, and Real-Time-PCR Assays Using High-Throughput Screening of Pooled Samples for Diagnosis of Malaria in Asymptomatic Carriers from Areas of Endemicity in Myanmar. J Clin Microbiol 6 1838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roper C, Elhassen IM, Hviid L (1996) Detection of very low level Plasmodium falciparum infections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. Am J Trop Med Hyg 54: 325–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.