Abstract
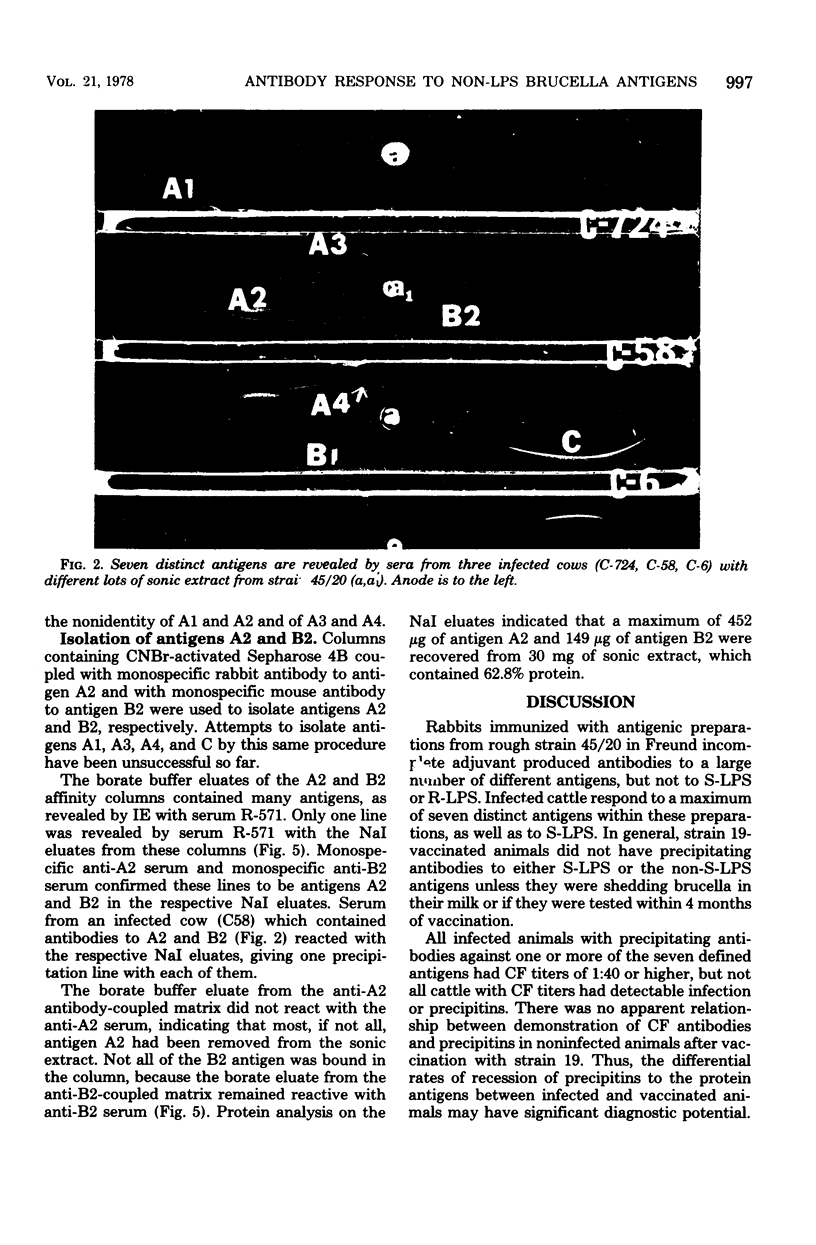
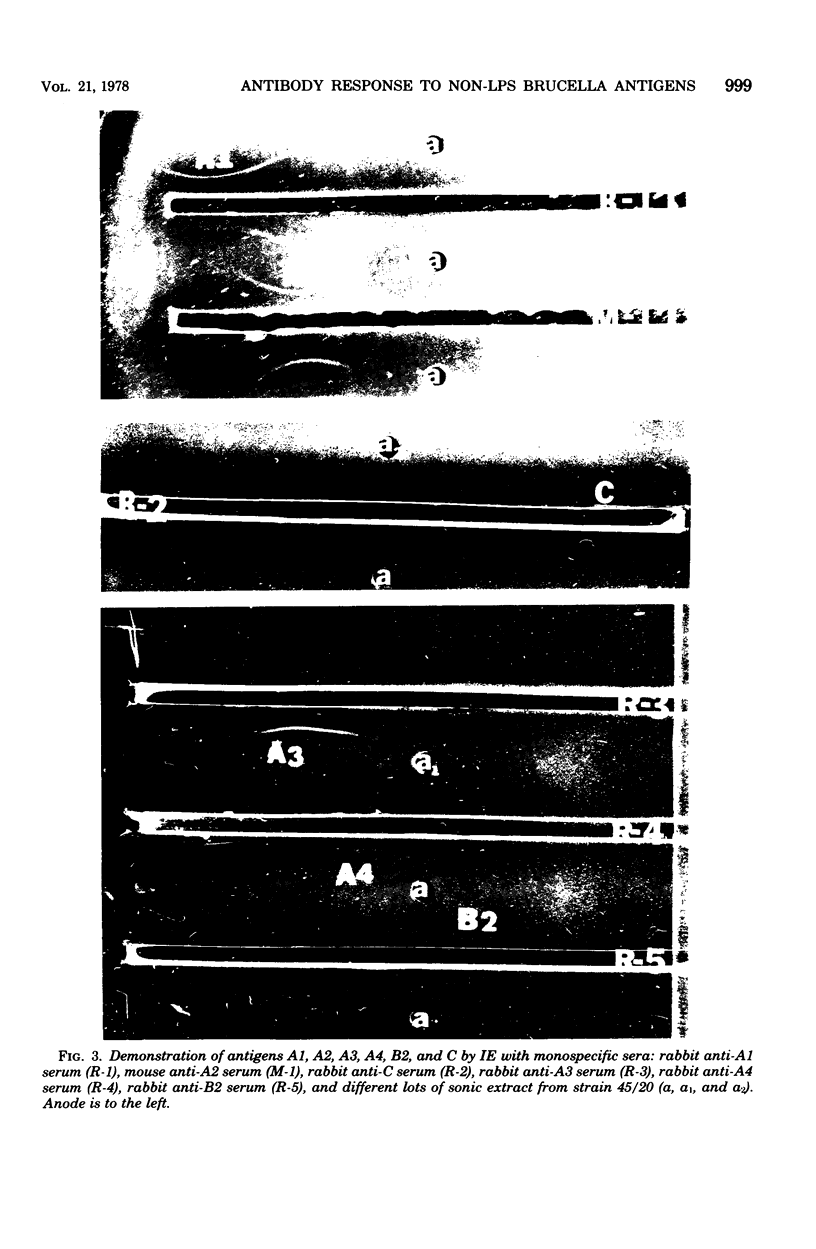
The smooth lipopolysaccharide complex of the outer surface of smooth Brucella abortus cells is believed to be the antigenic component involved in serological tests routinely used for the diagnosis of brucellosis. Sera from cattle vaccinated or infected with B. abortus generally contain antibody directed toward the smooth lipopolysaccharide complex. The brucella organism contains a large number of other antigenically distinct components. The biological significance of some of these antigens has been demonstrated by showing that sera from infected cattle have precipitins to these components. These sera revealed up to seven distinct lines in immunoelectrophoresis with a protein-rich antigen mixture prepared from rough strain B. abortus 45/20, whereas sera from strain 19-vaccinated cattle did not reveal these lines at 4 or more months after vaccination. Monospecific antisera were prepared against six antigens in this mixture, and the purification of two of them by antibody affinity chromatography is described.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUCE W., JONES L. M. The use of gel diffusion precipitin plates in the study of Brucella. Bull World Health Organ. 1958;19(1):187–196. [PMC free article] [PubMed] [Google Scholar]
- Corbel M. J. Evaluation of an immunodiffusion test for the detection of antibodies to Brucella abortus in bovine serum. J Med Microbiol. 1973 Feb;6(1):67–76. doi: 10.1099/00222615-6-1-67. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Deig E. F. Efficient production of large volumes of immune ascitic fluid from mice. Arch Gesamte Virusforsch. 1974;45(1-2):155–156. doi: 10.1007/BF01240555. [DOI] [PubMed] [Google Scholar]
- Diaz R., Chordi A. Immunoelectrophoretic analysis of Brucella suis. Experientia. 1966 Dec 15;22(12):825–826. doi: 10.1007/BF01897440. [DOI] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Leong D., Wilson J. B. Surface antigens of smooth brucellae. J Bacteriol. 1968 Oct;96(4):893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Wilson J. B. Antigenic relationship of Brucella ovis and Brucella melitensis. J Bacteriol. 1967 Apr;93(4):1262–1268. doi: 10.1128/jb.93.4.1262-1268.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dáz R., Maravi-Poma E., Rivero A. Comparison of counter-immunoelectrophoresis with other serological tests in the diagnosis of human brucellosis. Bull World Health Organ. 1976;53(4):417–424. [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Baughn R. E., McGhee J. R. Some physical, chemical, and taxonomic features of the soluble antigens of the Brucellae. J Infect Dis. 1970 May;121(5):522–527. doi: 10.1093/infdis/121.5.522. [DOI] [PubMed] [Google Scholar]
- Hebert G. A. Ammonium sulfate fractionation of sera: mouse, hamster, guinea pig, monkey, chimpanzee, swine, chicken, and cattle. Appl Microbiol. 1974 Feb;27(2):389–393. doi: 10.1128/am.27.2.389-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert G. A., Pelham P. L., Pittman B. Determination of the optimal ammonium sulfate concentration for the fractionation of rabbit, sheep, horse, and goat antisera. Appl Microbiol. 1973 Jan;25(1):26–36. doi: 10.1128/am.25.1.26-36.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. M., Diaz R., Taylor A. G. Characterization of allergens prepared from smooth and rough strains of Brucella melitensis. Br J Exp Pathol. 1973 Oct;54(5):492–508. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Myers D. M., Jones L. M., Varela-Diaz V. M. Studies of antigens for complement fixation and gel diffusion tests in the diagnosis of infections caused by Brucella ovis and other Brucella. Appl Microbiol. 1972 May;23(5):894–902. doi: 10.1128/am.23.5.894-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P. A preliminary report on the efficacy of adult cattle vaccination using Strain 19 in selected dairy herds in Florida. Proc Annu Meet U S Anim Health Assoc. 1976;(80):91–106. [PubMed] [Google Scholar]
- OLITZKI A. L. [Differences between antigenic specificity of nonsoluble particles and soluble extracts prepared from Brucellae by disintegration]. Proc Soc Exp Biol Med. 1959 Jun;101(2):388–390. doi: 10.3181/00379727-101-24952. [DOI] [PubMed] [Google Scholar]
- Parikh I., March S., Cuatercasas P. Topics in the methodology of substitution reactions with agarose. Methods Enzymol. 1974;34:77–102. doi: 10.1016/s0076-6879(74)34009-8. [DOI] [PubMed] [Google Scholar]
- Stemshorn B., Nielsen K. The bovine immune response to Brucella abortus I. A water soluble antigen precipitated by sera of some naturally infected cattle. Can J Comp Med. 1977 Apr;41(2):152–159. [PMC free article] [PubMed] [Google Scholar]







