Abstract
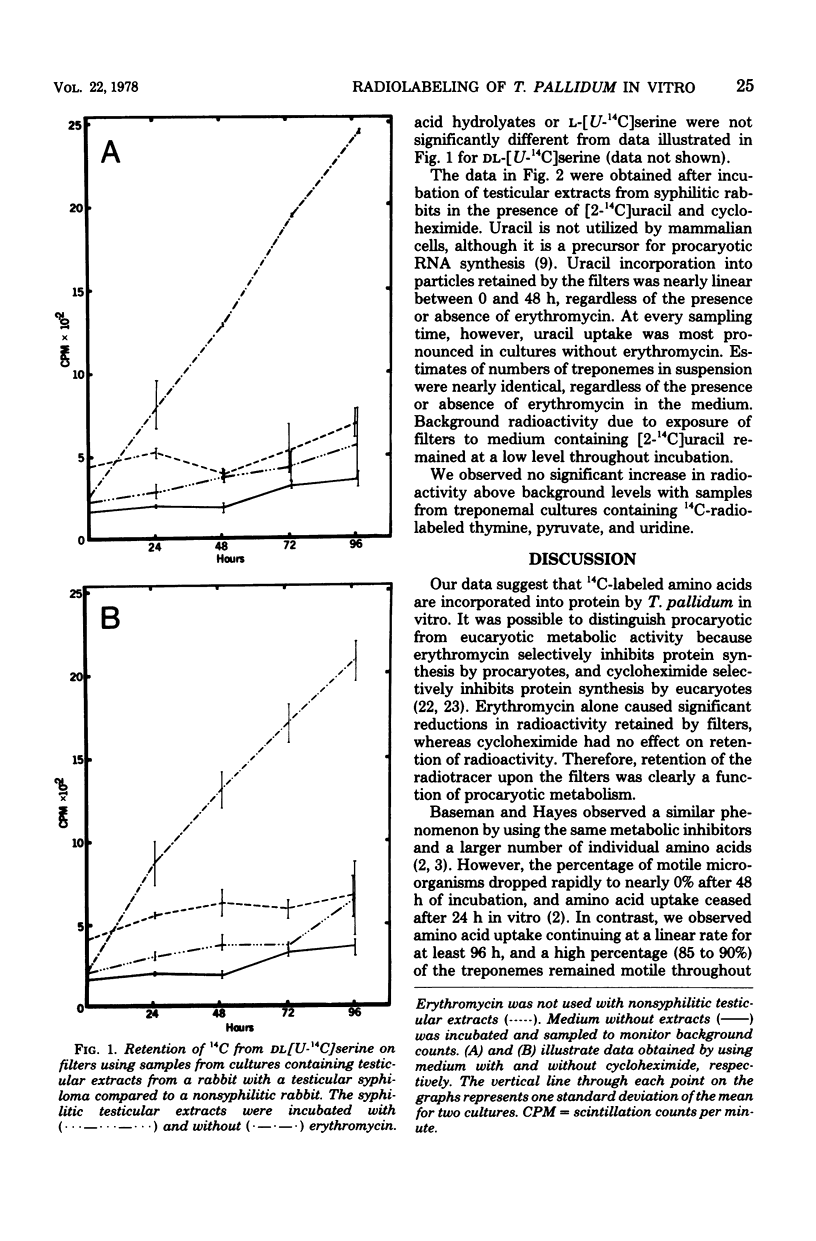
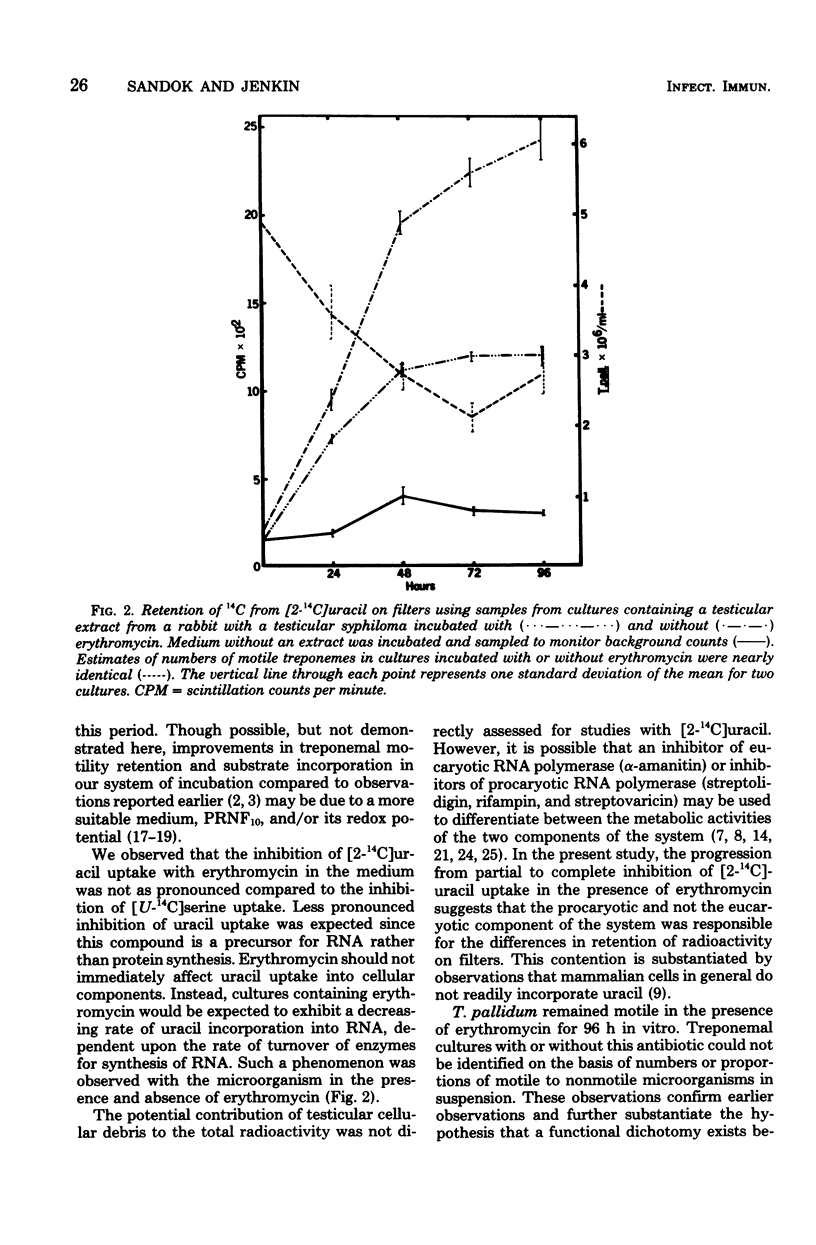
We observed uptake of [U-14C]serine, U-14C-labeled amino acid hydrolysates, and [2-14C]uracil by virulent Treponema pallidum in vitro for at least 96 h. No uptake of [2-14C]thymine, [1-14C]pyruvate, [U-14C]pyruvate, and [2-14C]uridine was detected. Treponemal protein and RNA biosynthetic activity was identified by erythromycin inhibition of amino acid and uracil uptake. Radioactivity due to uptake of radiolabeled amino acids by residual testicular cells in the cultures remained at background levels regardless of the presence or absence of cycloheximide. Accumulation of the radiolabeled substrates by T. pallidum proceeded at a linear rate for 48 to 96 h during incubation in vitro. The longevity of substrate uptake using the system of incubation described will facilitate future studies on the metabolism of the microorganism to help determine essential growth factors and environmental conditions for multiplication of T. pallidum in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Johnson R. C., Peterson D. Metabolism of Common Substrates by the Reiter Strain of Treponema pallidum. Infect Immun. 1971 Jun;3(6):727–734. doi: 10.1128/iai.3.6.727-734.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Anabolic potential of virulent Treponema pallidum. Infect Immun. 1977 Dec;18(3):857–859. doi: 10.1128/iai.18.3.857-859.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Protein synthesis by Treponema pallidum extracted from infected rabbit tissue. Infect Immun. 1974 Dec;10(6):1350–1355. doi: 10.1128/iai.10.6.1350-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Hayes N. C. Virulent Treponema pallidum: aerobe or anaerobe. Infect Immun. 1976 Mar;13(3):704–711. doi: 10.1128/iai.13.3.704-711.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. In vivo differentiation of yeast cytoplasmic and mitochondrial protein synthesis with antibiotics. Biochem Biophys Res Commun. 1966 Oct 5;25(1):8–13. doi: 10.1016/0006-291x(66)90631-0. [DOI] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incefy G. S., Rifkind A. B., Kappas A. Inhibition of delta-aminolevulinate synthetase induction by alpha-amanitin in avian liver cell cultures. Biochim Biophys Acta. 1974 Sep 13;361(3):331–344. doi: 10.1016/0005-2787(74)90376-1. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Specific action of alpha-amanitin on mammalian RNA polymerase protein. Nature. 1970 Jan 3;225(5227):60–62. doi: 10.1038/225060b0. [DOI] [PubMed] [Google Scholar]
- Lim D. V., James A. N., Williams R. P. Radiolabeling of and macromolecular syntheses in Neisseria gonorrhoeae types 1 and 4. Appl Environ Microbiol. 1977 Feb;33(2):328–333. doi: 10.1128/aem.33.2.328-333.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane A. W., Lamb A. J., Christodoulou C., Lukins H. B. The biogenesis of mitochondria, VI. Biochemical basis of the resistance of Saccharomyces cerevisiae toward antibiotics which specifically inhibit mitochondrial protein synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1288–1293. doi: 10.1073/pnas.59.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews H. M., Jenkin H. M., Crilly K., Sandok P. L. Effects of fatty acids on motility retention by Treponema pallidum in vitro. Infect Immun. 1978 Mar;19(3):814–821. doi: 10.1128/iai.19.3.814-821.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S., Yamazaki H., Nitta K., Umezawa H. Inhibition of DNA-dependent RNA polymerase reaction of Escherichia coli by an antimicrobial antibiotic, streptovaricin. Biochim Biophys Acta. 1968 Apr 22;157(2):322–332. doi: 10.1016/0005-2787(68)90086-5. [DOI] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Ribosomal ribonucleic acid synthesis by virulent Treponema pallidum. Infect Immun. 1978 Mar;19(3):854–860. doi: 10.1128/iai.19.3.854-860.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M., Graves S. R., Knight S. T. Retention of motility of Treponema pallidum (Nichols virulent strain) in an anaerobic cell culture system and in a cell-free system. J Clin Microbiol. 1976 Jan;3(1):72–74. doi: 10.1128/jcm.3.1.72-74.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M., Matthews H. M., Roberts M. S. Unsustained multiplication of treponema pallidum (nichols virulent strain) in vitro in the presence of oxygen. Infect Immun. 1978 Feb;19(2):421–429. doi: 10.1128/iai.19.2.421-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Knight S. T., Jenkin H. M. Examination of various cell culture techniques for co-incubation of virulent Treponema pallidum (Nichols I strain) under anaerobic conditions. J Clin Microbiol. 1976 Oct;4(4):360–371. doi: 10.1128/jcm.4.4.360-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller N. L., Cox C. D. Catabolism of glucose and fatty acids by virulent Treponema pallidum. Infect Immun. 1977 Apr;16(1):60–68. doi: 10.1128/iai.16.1.60-68.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Stokes G. V. Cycloheximide-resistant glycosylation in L cells infected with Chlamydia psittaci. Infect Immun. 1974 Mar;9(3):497–499. doi: 10.1128/iai.9.3.497-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Otaka T., Kaji A. Further studies on the mechanism of erythromycin action. Biochim Biophys Acta. 1973 Nov 26;331(1):128–140. doi: 10.1016/0005-2787(73)90425-5. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Friis R. R., Moulder J. W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973 Feb;127(2):155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox R. R., Guthe T. Treponema pallidum. A bibliographical review of the morphology, culture and survival of T. pallidum and associated organisms. Bull World Health Organ. 1966;35:1–169. [PMC free article] [PubMed] [Google Scholar]



