ABSTRACT
In sheep and goats, exposure of seasonally anestrous females to males or their fleece/hair activates the gonadotropin-releasing hormone (GnRH) pulse generator leading to pulsatile luteinizing hormone (LH) secretion. Pheromones emitted by sexually mature males are thought to play a prominent role in this male effect. In the present study, we first aimed to clarify whether the male goat pheromone is effective in ewes. Seasonally anestrous St. Croix ewes were exposed to hair extracts derived from either intact or castrated (control) male Shiba goats. The male goat-hair extract significantly increased LH secretion compared to the control, suggesting that an interspecies action of the male pheromone occurs between sheep and goats. Using the male goat-hair extract as the pheromone source, we then aimed to clarify the neural pathway involved in the signal transduction of the male pheromone. Ewes were exposed to either the goat-hair extract or the control and sacrificed 2 hr after the exposure. Expression of c-Fos, a marker of neuronal activation, was immunohistochemically examined. The male goat-hair extract significantly increased the c-Fos expression compared to the control in regions of the vomeronasal system, such as the accessory olfactory bulb and medial amygdala, and the arcuate nucleus. The main olfactory bulb did not exhibit any significant increase in the c-Fos expression by the male goat-hair extract. This result suggests that the neural signal of the male pheromone is conveyed to the GnRH pulse generator through the activated regions in ewes.
Keywords: goat, male effect, neural pathway, pheromone, sheep
In seasonal breeders, reproductive status during the non-breeding period is characterized by the infrequent discharge of gonadotropins from the pituitary, gonadal quiescence and behavioral anestrus. Interestingly, the introduction of a mature male into a flock of seasonally anestrous females eventually induces an ovulation in sheep and goats [47, 48]; a phenomenon called the male effect [4, 9, 12, 29]. Although several factors, such as visual cues and learning, seem to be involved [9, 11, 12], it is generally accepted that the olfactory signal, the pheromone, plays a dominant role in the male effect, since the effect is seen when the presence of the male is replaced by its fleece/hairs [6, 24] or their extracts [6, 14, 25].
The gonadotropin-releasing hormone (GnRH) pulse generator in the hypothalamus governs pulsatile GnRH secretion and thereby regulates pulsatile luteinizing hormone (LH) secretion from the pituitary [20, 23, 28]. Because the initial endocrine event following the reception of the male pheromone in the anestrous female is an abrupt increase in the frequency of pulsatile LH secretion [12, 29, 30], it has been generally accepted that the central target of the male pheromone is the GnRH pulse generator [9, 29, 39]. It has been demonstrated that the exposure to male goat hairs promptly stimulates the GnRH pulse generator in female goats [15, 17, 32, 33] using an electrophysiological technique that monitors neural activity of the neural substrate [31, 35]. This is a striking contrast to other mammalian pheromones, for which the neural mechanisms underlying pheromone actions are scarcely known. Thus, the male effect would serve as a unique model to investigate the neural pathways participating in the pheromone signal transduction.
Neural regions involved in the signal transduction pathways of pheromonal cues have been investigated by analyzing the expression of c-Fos, a marker protein for neuronal activation [16], in several species. In rodents, it has been demonstrated that the exposure of the vaginal fluid of the female golden hamster [10] or the urine of the female rat [3] to conspecific male animals increased the number of c-Fos-immunoreactive (-ir) cells in regions of the vomeronasal system, such as the accessory olfactory bulb (AOB), medial amygdala (MeA) and bed nucleus of the stria terminalis (BNST), but had little effect on the expression of c-Fos-ir cells in the main olfactory system including the main olfactory bulb (MOB), posterior lateral cortical amygdala (PLCo) and piriform cortex (PYR). On the other hand, Gelez and Fabre-Nys [13] have shown that the presence of a ram significantly increased the number of c-Fos-ir cells in several regions of anestrous ewes, which include the MOB, PLCo, PYR and dentate gyrus as well as the AOB, posterior medial cortical amygdala (PMCo) and ventromedial hypothalamic nucleus (VMH). This result suggests that both the main olfactory and vomeronasal systems participate in the central mechanism of the male effect in the ewe. However, it should be noted that the ewe might have received a variety of stimuli from the ram, such as visual and auditory signals and odor of the urine, in addition to the male pheromone. Therefore, those c-Fos-ir cells may represent neural substrates that process not only the male pheromone but also other signals relating social interactions between sexes or individuals in sheep.
Pheromones are defined as chemicals that act within conspecifics [19]. However, Over et al. [42] demonstrated that a mixture of male goat hairs of the Alpine, Saanen and Bunte deutsche Edelziege breeds significantly increased LH pulse frequency in ewes of the Merino d’Arles and Ile-de France breeds during the non-breeding season. If this is a universal phenomenon between sheep and goats, the biological impacts, except for pheromone activity, of male goat hairs on ewes would be lower compared to the presence of a ram or its fleece and can therefore be used to investigate the neural pathway involved in the stimulation of the GnRH pulse generator by the male pheromone in ewes.
In the present study, we investigated whether the interspecies action of the male pheromone occurs between St. Croix ewes and male Shiba goats, a combination of the sheep and goat breeds different from that in the previous study [42]. Since it has been demonstrated that an acidic fraction of the male goat hair extract exhibits pheromone activity in female goats [6, 14, 42], this partially purified fraction was used as the pheromone source to reduce unwanted stimuli to a greater extent. After confirming the interspecies action of the male pheromone in our experimental model, we then investigated the neural pathway participated in the signal transduction of the male pheromone using the goat extract to stimulate brain regions of the ewe.
MATERIALS AND METHODS
Animals: Eight mature St. Croix ewes (Body weight, 50–60 kg) maintained in the Animal Resource Science Center of the University of Tokyo were used. They were isolated from rams for at least 3 months and were kept in a pen under natural photoperiod. They were fed with a soybean meal and roll bale silage and had free access to water and supplemental minerals. Experiments were conducted from June to July, the non-breeding season of this species. Prior to the experiment, the ewes were habituated to a manipulation of the sample exposure by inserting the muzzle into an empty plastic cup for a few min at least once a day for one week. All experiments were approved by the Board for the Care and Use of Animals of the Graduate School of Agricultural and Life Sciences of the University of Tokyo, Tokyo, Japan.
Preparation of the pheromone source: Adult male Shiba goats were used for the pheromone source. Hairs were taken from the parietal and shoulder regions of either sexually intact or castrated male goats [18]. Hairs collected from 4–6 goats were combined in each preparation and subjected to the following fractionation. First, lipid components of the hair were extracted by supercritical CO2 fluid extraction and were dissolved in diethyl ether. Then, they were separated into acidic, neutral and basic fractions by the counter-current distribution method, using sodium hydroxide and hydrochloric acid. The acidic fraction was divided into small aliquots corresponding to 2 mg of the lipid component and stored at −20°C until used.
Prior to the start of the experiment in the ewes, male pheromone activity in each hair preparation was examined in goats by the electrophysiological method previously described [15, 17, 32, 33]. The result confirmed that the preparation from the intact male goats exhibited the male pheromone activity while that from the castrated goats did not. The former was termed “goat pheromone”, while the latter was termed “control” in the present study.
Pheromone exposure: Immediately before the exposure, the preparation was absorbed on a small piece of wiping paper, and the paper was allowed to dry for a few min. It was put between two plastic cups, of which the bottom of the inner cup was replaced with a mesh [17]. Upon exposure, the ewe’s muzzle was inserted into the cup and kept there for 2 min. This manipulation was repeated 3 times with a 10-min interval between the start of each exposure.
Experiment 1: Effects of the goat pheromone on LH secretion in ewes: On the day of the experiment, a catheter was inserted in the jugular vein of the ewes. Blood samples were collected through the catheter every 10 min for 4 hr. Two hr after the start of blood sampling, the ewes were subjected to the sample exposure. Four ewes were exposed to the goat pheromone, and the remaining 4 ewes were exposed to the control. With one-hr interval period, the experimental protocol was repeated, adopting a crossover design. Blood samples were centrifuged at 3,000 rpm for 15 min, and plasma was stored at −20°C until the determination of LH concentrations.
Experiment 2: Effects of the goat pheromone on c-Fos expression in the central nervous system of ewes: Two weeks after Experiment 1, the ewes were randomly divided into the pheromone (n=4) and control (n=3) groups, and exposed to the goat pheromone or control, respectively, in the same manner as in Experiment 1. One ewe was not included in this experiment due to a health problem. Two hr after the first 2-min exposure, the ewes were sacrificed by an overdose of pentobarbital (Dainippon Pharmaceutical Corporation, Osaka, Japan, 300 mg/kg), and the head of the ewe was perfused bilaterally through the carotid arteries with 5 l of 10 mM phosphate buffered saline (PBS) containing 3,000 U heparin/l and 0.7% sodium nitrite, followed by 5 l of 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The brain was removed, and tissue blocks containing the olfactory bulb, amygdala and hypothalamus were immersed in the same fixative overnight at 4°C and then in 20% sucrose in 0.1 M PB at 4°C until they sank.
LH Assays: Plasma LH concentrations were measured in duplicate by a double-antibody radioimmunoassay using rabbit anti-ovine LH serum (YM #18) [37] and expressed in terms of the ovine LH standard (NIDDK-oLH-I-4). Goat anti-rabbit immunoglobulin serum (1PGKR001, Shibayagi, Shibukawa, Japan) was used as a secondary antibody. The least detectable LH concentration was 0.098 ng/ml for 200-µl plasma samples, and the intra-and inter-assay coefficients of variation were 5.1% at 3.82 ng/ml and 6.5% at 3.55 ng/ml, respectively.
Tissue preparation: The block containing the olfactory bulb was cut sagittally, while the other blocks were cut frontally. Serial sections (50-µm-thick) were cut on a freezing microtome and kept in the cryoprotectant solution [51] at −20°C. First, every sixth section of each brain structure was stained with 0.2% cresyl violet to allow histological identification of the brain areas by referring to atlases of the Shiba goat [52] and rat [43].
Immunohistochemistry for c-Fos was carried out in seven areas of the main olfactory system: the mitral and granule cell layers of the MOB, anterior and posterior parts of PYR, basal amygdala (BA), anterior cortical amygdala (ACo) and PLCo; eight areas of the vomeronasal olfactory system: the mitral/tufted and granule cell layers of the AOB, MeA, PMCo, anterior and posterior parts of BNST, medial preoptic area (MPOA) and VMH; and other five areas of the limbic system and hypothalamus; the central amygdala (CeA), vascular organ of the lamina terminalis (OVLT), paraventricular nucleus (PVN), supraoptic nucleus (SON) and arcuate nucleus (ARC). Sections were carefully matched across ewes according to the morphological structures and were selected so that three representative sections from the medial, median and lateral portions of each layer were included for the AOB and MOB or three representative ones from anterior, middle and posterior portions were included for the other brain areas.
Immunohistochemistry: Sections of the pheromone group and those of the control group were always stained in parallel. Free-floating sections were rinsed with PBS containing 0.5% Triton X-100 (PBST) and treated with 3% H2O2 in methanol for 15 min. After extensive washing with PBST, they were successively incubated with 5% normal goat serum (Vector Laboratories, Burlingame, CA, U.S.A.) in PBST containing 2% Block Ace (Dainippon Pharmaceutical Corporation) for 1 hr and then with a rabbit polyclonal antibody against the Fos protein (Ab-5, Oncogene Science Products, Cambridge, MA, U.S.A., diluted 1:10,000 in PBST containing 0.5% Block Ace and 2% normal goat serum) for 48 hr at 4°C. Following three 15-min rinses with PBST, sections were incubated with a goat anti-rabbit IgG (Vector Laboratories, diluted 1:400 in PBST containing 0.5% Block Ace and 2% normal goat serum) for 3 hr and with an avidin-biotin complex solution (Vector Laboratories, elite kit, diluted 1:50 in PBST) for 1 hr. Each step was followed by three 15-min washes with PBST. After the last wash, sections were immersed in 50 mM Tris-HCl, pH 7.6, then reacted with chromogen solution consisting of 0.04% 3,3′-diaminobenzidine (Sigma Chemical, St. Louis, MO, U.S.A.), 0.03% H2O2, 0.5% nickel ammonium sulfate and 0.01% cobalt chloride in 50 mM Tris-HCl for 8 min. The reaction was terminated by several washes with 50 mM Tris-HCl. All reactions were performed at room temperature unless otherwise stated. The sections were mounted on gelatinized glass slides, dried and then briefly counterstained with 0.1% cresyl violet to delimit the nucleus. They were dehydrated and cover-slipped.
Use of the anti-c-Fos antibody preabsorbed with human c-Fos peptide (Oncogene) or omission of the secondary antibody during the immunohistochemical procedure resulted in the complete elimination of the positive signal (data not shown).
Count of c-Fos-ir cells: The number of c-Fos-ir cells was counted in each area on the right side of the brain. Sections were photographed using an Eclipse E800M microscope (Nikon, Tokyo, Japan) with a FDX-35 camera (Nikon), and a square was drawn on photomicrographs at the center of each area. For the MOB, three squares were drawn at the center of the dorsal, rostral and ventral parts of each layer. The size of the square (the exact size on the tissue section) was as follows: the mitral (0.03 mm2) and granule (0.03 mm2) cell layers of the MOB, the anterior (1 mm2) and posterior (1 mm2) parts of PYR, BA (1 mm2), ACo (1 mm2), PLCo (1 mm2), the mitral/tufted (0.03 mm2) and granule (0.03 mm2) cell layers of the AOB, MeA (1 mm2), PMCo (1 mm2), the anterior (0.4 mm2) and posterior (0.3 mm2) parts of BNST, MPOA (1.3 mm2), VMH (1 mm2), CeA (1 mm2), OVLT (0.05 mm2), PVN (0.3 mm2), SON (0.3 mm2) and ARC (0.7 mm2). A person who knew neither the experimental group nor the brain area counted the number of clearly stained c-Fos positive signals in the square. For the MOB, the number in three squares was totaled. In each area, the number of positive signals was divided by the size of the square and presented as the count per mm2.
Data analysis: To evaluate the effect of the goat pheromone on the LH secretion in the ewe, the mean value of LH concentrations at each time point was calculated in the pheromone and control treatments, and they were analyzed statistically by Student’s t-tests. A statistical comparison of the number of Fos-ir neurons between the pheromone and control groups was performed by Mann-Whitney U-test. The levels of significance were P<0.05. All values are expressed as mean ± SEM.
RESULTS
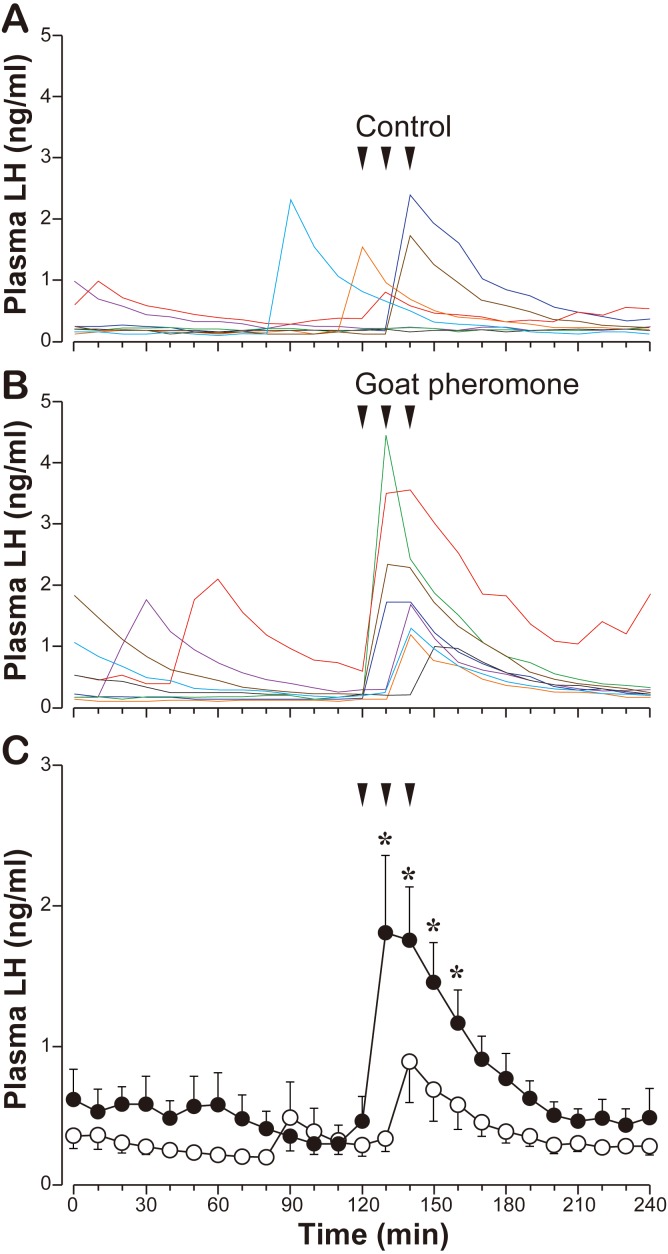
Experiment 1: Effects of the goat pheromone on LH secretion in ewes: Individual profiles of the LH response to the goat pheromone and control, as well as mean group values during the 4-hr sampling period, are shown in Fig. 1. During the pre-treatment period (0–120 min), temporal increases in LH concentrations were observed in the both treatments, indicating that spontaneous pulsatile LH secretion occurred, although its frequency seemed to be low. In the control group, LH secretion increased slightly after the first exposure in one ewe and with a relatively high magnitude after the second exposure in two ewes (Fig. 1A). The remaining five ewes did not show any apparent changes in LH secretion after the exposure. In the goat pheromone group, LH secretion increased after the first exposure in 4 ewes and after the second exposure in 3 ewes (Fig. 1B). In the ewes that responded to the first exposure, it appeared that the second exposure also stimulated LH secretion in 3 ewes, because elevated LH concentrations were observed at two successive time points (130 and 140 min). Statistical analysis of the group values revealed that the goat pheromone significantly increased LH secretion compared with the control (P<0.05) in the seasonally anestrous ewes (Fig. 1C).
Fig. 1.
Effects of the goat pheromone exposure on LH secretion in seasonally anestrous ewes. A, individual profiles (n=8) of LH concentrations in the control (the hair extract of castrated male goats) exposure. B, individual profiles (n=8) of LH concentrations in the goat pheromone (the hair extract of intact male goats) exposure. Lines with the same color in A and B indicate the results of the same individual. C, the mean ± SEM of LH concentrations in each treatment. Open circles, the control exposure. Solid circles, the goat pheromone exposure. Arrows indicate timing of exposure. *, significantly different (P<0.05, Student’s t-tests) compared to the corresponding control value.
Experiment 2: Effects of the goat pheromone on c-Fos expression in the central nervous system of ewes: The effect of the goat pheromone on cellular activation was analyzed in 20 brain areas schematically shown in Fig. 2, including those in the olfactory bulb (Fig. 2A), the hypothalamus (Fig. 2B–2E) and the amygdaloid complex (Fig. 2C and 2D). Representative photomicrographs of a pair of sections of the control- and goat pheromone-treated ewes are shown in Fig. 3. The c-Fos positive signals were characterized as small round or oval products in the cell nuclei. The mean numbers of c-Fos-ir cells in the areas examined are summarized in Table 1.
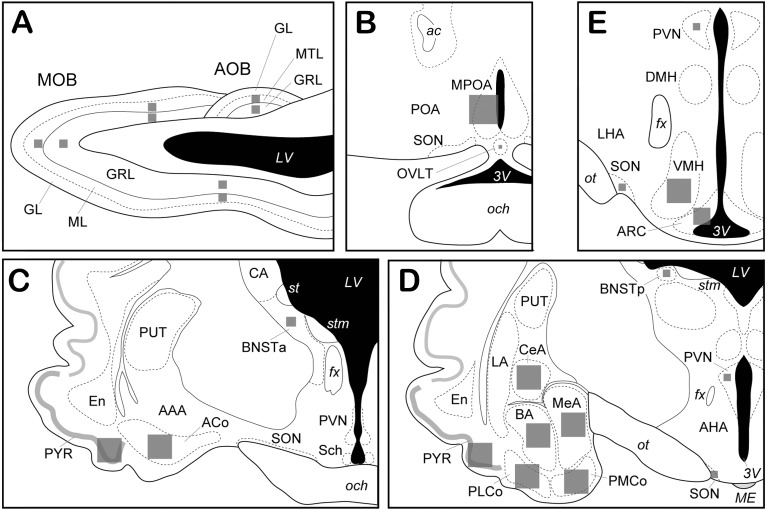
Fig. 2.
Schematic illustrations of brain regions. A, a sagittal view of the olfactory bulb. B–E, coronal views of the hypothalamus and the amygdaloid complex (the rostro-caudal order). Gray squares schematically show the areas in which the number of c-Fos positive cells was counted in each brain region. AAA, anterior amygdaloid area; ACo, anterior cortical amygdala; AHA, anterior hypothalamic area; AOB, accessory olfactory bulb; ARC, arcuate nucleus; BA, basal amygdala, BNSTa, anterior part of the bed nucleus of the stria terminalis; BNSTp, posterior part of the bed nucleus of the stria terminalis; CA, caudate nucleus; CeA, central amygdala; DMH, dorsomedial hypothalamic nucleus; En, endopiriform nucleus; LA, lateral amygdala; LHA, lateral hypothalamic area; MeA, medial amygdala; MOB, main olfactory bulb; MPOA, medial preoptic area; OVLT, vascular organ of the lamina terminalis; PMCo, posterior medial cortical amygdala; PLCo, posterior lateral cortical amygdala; POA, preoptic area; PUT, putamen; PVN, paraventricular nucleus of the hypothalamus; PYR, piriform cortex; Sch, suprachiasmatic nucleus; SON, supraoptic nucleus; VMH, ventromedial hypothalamic nucleus, GL, glomerular layer; GRL, granule cell layer; ML, mitral cell layer; MTL, mitral/tufted cell layer; ac, anterior commissure; fx, fornix; och, optic chiasm; ot, optic tract; st, stria terminalis; stm, stria medullaris of the thalamus; LV, lateral ventricle; ME, median eminence; 3V, third ventricle.
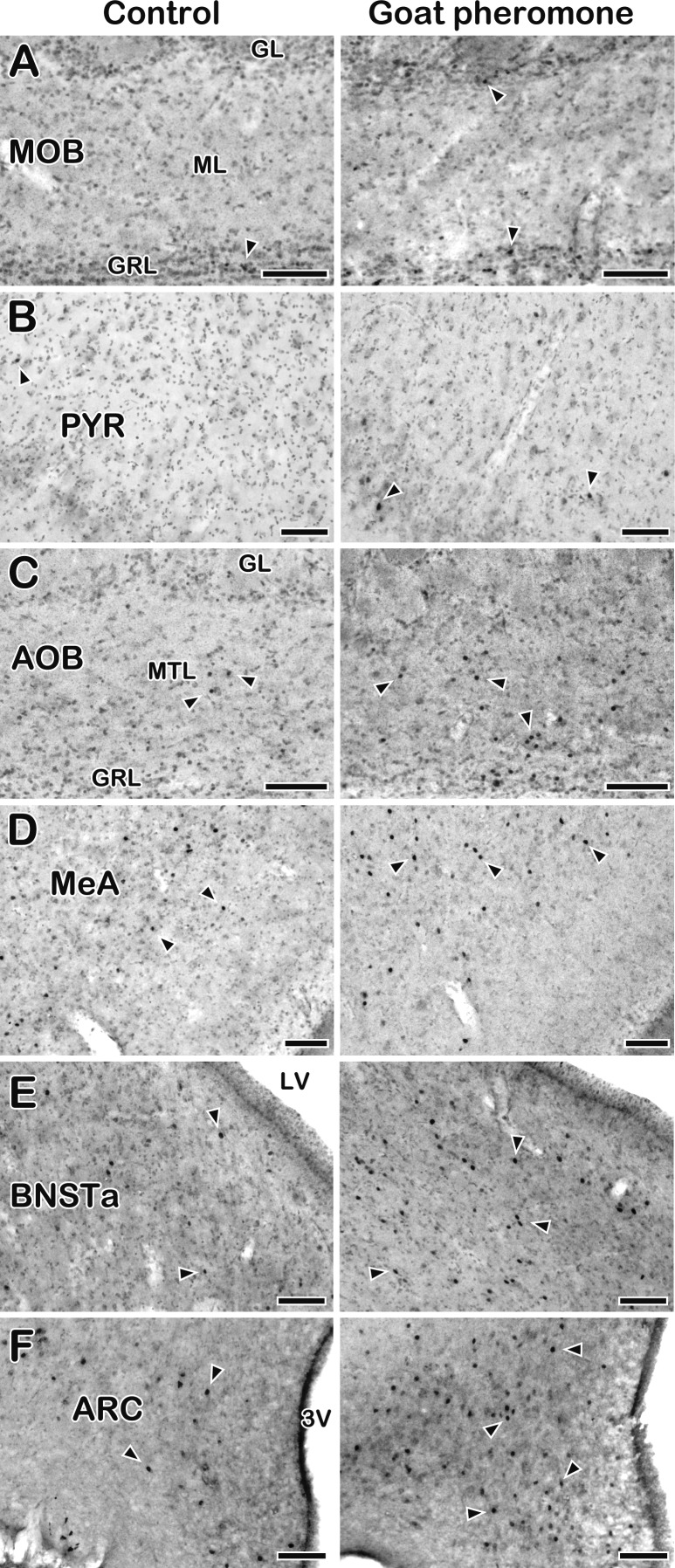
Fig. 3.
Photomicrographs of c-Fos-ir cells in several brain regions in seasonally anestrous ewes exposed to the control (left column) or goat pheromone (right column). A, main olfactory bulb (MOB). B, piriform cortex (PYR). C, accessory olfactory bulb (AOB). D, medial amygdala (MeA). E, anterior part of the bed nucleus of the stria terminalis (BNSTa). F, arcuate nucleus (ARC). Sections were briefly counter-stained for Nissl. Arrowheads indicate representative c-Fos-ir materials. GL, glomerular layer; GRL, granule cell layer; ML, mitral cell layer; MTL, mitral/tufted cell layer, LV, lateral ventricle; 3V, third ventricle. Scale bars, 100 µm.
Table 1. Effects of the goat pheromone exposure on the c-Fos expression in ewes.
| Brain regions | Treatment | ||
|---|---|---|---|
| Control | Goat Pheromone | ||
| (n=3)a) | (n=4)a) | ||
| Main olfactory system | |||
| Main Olfactory bulb (MOB) | |||
| Mitral cell layer (ML) | 951.7 ± 127.9 | 1,160.3 ± 99.0 | |
| >Granule cell layer (GRL) | 426.9 ± 82.1 | 475.0 ± 49.1 | |
| Piriform cortex (PYR) | |||
| anterior part | 192.3 ± 22.4 | 147.3 ± 24.0 | |
| posterior part | 99.3 ± 16.4 | 149.8 ± 19.7* | |
| Amygdala | |||
| Basal amygdala (BA) | 82.3 ± 33.8 | 105.3 ± 10.6 | |
| Anterior cortical amygdala (ACo) | 79.7 ± 26.6 | 102.8 ± 21.1 | |
| Posterior lateral cortical amygdala (PLCo) | 112.0 ± 34.4 | 114.8 ± 28.3 | |
| Vomeronasal olfactory system | |||
| Accessory olfactory bulb (AOB) | |||
| Mitral/Tufted cell layer (MTL) | 14.0 ± 4.2 | 41.0 ± 12.2* | |
| Granule cell layer (GRL) | 14.0 ± 4.9 | 52.5 ± 14.1* | |
| Amygdala | |||
| Medial amygdala (MeA) | 113.7 ± 19.7 | 194.3 ± 15.3* | |
| Posterior medial cortical amygdala (PMCo) | 56.0 ± 14.1 | 150.5 ± 40.2* | |
| Bed nucleus of the stria terminals | |||
| anterior part | 61.3 ± 11.8 | 112.3 ± 13.5* | |
| posterior part | 72.7 ± 23.7 | 59.5 ± 12.5 | |
| Medial preoptic area (MPOA) | 64.0 ± 27.9 | 256.5 ± 120.8* | |
| Vascular organ of the lamina terminals (OVLT) | 14.7 ± 2.7 | 33.0 ± 4.7* | |
| Ventromedial hypothalamic nucleus (VMH) | 142.0 ± 41.1 | 255.8 ± 58.0 | |
| Limbic system | |||
| Central amygdala (CeA) | 103.3 ± 19.5 | 125.5 ± 10.1 | |
| Hypothalamus | |||
| Arcuate nucleus (ARC) | 189.7 ± 28.3 | 403.3 ± 82.4* | |
| Paraventricular nucleus (PVN) | 110.7 ± 38.5 | 197.5 ± 72.1 | |
| Supraoptic nucleus (SON) | 55.3 ± 8.4 | 84.3 ± 16.3 | |
a) The number of ewes. Data are expressed as the mean ± SEM of the density of Fos-ir cells (the number of Fos-ir cells per mm2) in each brain region. *, significantly different (P<0.05, Mann-Whitney U test) compared to the corresponding control value.
Although c-Fos positive signals were observed to be scattered throughout the mitral and granule cell layers of the MOB (Fig. 3A), the numbers of those signals in the goat pheromone group were comparable to those in the control group. Similarly, goat pheromone exposure had no effect on the c-Fos expression in the other areas of the main olfactory system, except in the PYR. The number of c-Fos-ir cells was slightly, but significantly, higher in the posterior portion of the PYR (Fig. 3B). In contrast to the main olfactory system, goat pheromone exposure affected c-Fos expression in a majority of the areas of the vomeronasal system. The number of c-Fos-ir cells in the goat pheromone group was significantly higher than that in the control group in the mitral/tufted and granule cell layers of the AOB (Fig. 3C), MeA (Fig. 3D), PMCo, anterior portion of the BNST (Fig. 3E), MPOA and OVLT. In the VMH, although the number of c-Fos-ir cells tended to be higher in the goat pheromone group than the control group, the difference was not statistically significant. In the hypothalamus, a significant increase in c-Fos immunoreactivity caused by goat pheromone exposure was seen in the ARC (Fig. 3F), but not in the PVN and SON.
DISCUSSION
The present study demonstrated that exposure to the mature male goat hair extract significantly increased LH secretion and activated a majority of the areas in the vomeronasal olfactory system and the ARC in seasonally anestrous ewes. The results suggest that the brain areas activated by the goat hair extract are involved in the signal transduction of the male effect pheromone.
Although spontaneous LH pulses were occasionally observed during the 4-hr period in both goat pheromone and control groups, pulsatile increases in LH secretion occurred coincidentally in seven of eight ewes and the mean LH concentrations were significantly higher in the ewes exposed to the goat pheromone than the control ewes (Fig. 1). The result clearly indicates that the goat pheromone stimulates LH secretion in the seasonally anestrous ewes, confirming the results of a previous study [42]. The consistent result between the two studies, using a completely different combination of sheep and goat breeds, suggests that buckhairs stimulate LH secretion in ewes, regardless of the breed of each species. Likewise, it has been demonstrated that exposure of the female Shiba goat to ram (the Corriedale breed) wool stimulates the GnRH pulse generator and pulsatile LH secretion [17]. Moreover, the putative pheromone receptor (V1R) homologue genes are remarkably conserved between sheep and goats [36]. These lines of evidence imply that very similar, if not identical, molecules are released from male sheep and goats, and represent the pheromone activity in the females of both species, although the biological significance of such interspecies action of the male pheromone remains unclear.
We demonstrated that exposure to the goat pheromone prominently activated the vomeronasal system, but had few effects on the main olfactory system, though it did affect the PYR in the ewes (Table 1), which is consistent with the findings in rodents [3, 10]. The present results are also partially comparable, in terms of the activation of the vomeronasal system, to previous studies in sheep [8, 13], in which the ram was introduced to the ewe’s pen. However, there are several discrepancies between the present results and those of previous studies, regardless of the fact that both the male goat hair extract and presence of the ram similarly facilitated pulsatile LH secretion in seasonally anestrous ewes.
The ram introduction activated the mitral and granule layers of the MOB and the basal and cortical amygdala [13], whereas the goat pheromone exposure did not. This might be due to the ram-related odors that are detected by the olfactory epithelium and activate the main olfactory system. Although the male goat hair extract used in the present study may also contain several odorous substances, their amounts are presumed to be remarkably small. Therefore, the present analytical method could not detect the significant effect in the main olfactory system, except in the PYR. On the other hand, the activation of the vomeronasal system was similar in both studies, implicating that the male pheromone signal is processed exclusively through the vomeronasal system. However, this is unlikely, since it has been reported that ewes subjected to the destruction of the vomeronasal organ or the section of the vomeronasal nerve still exhibited LH response to the ram [7]. It is, therefore, plausible to propose that the male pheromone of sheep and goats is a mixture of several substances, one detected and processed through the vomeronasal system and the other through the main olfactory system. This hypothesis is supported by the fact that all goat V1Rs identified till date are expressed in both the vomeronasal organ and olfactory epithelium [36]. It has also been demonstrated that the neutral fraction and the acidic fraction of buckhair extracts retain the pheromone activity [6]. Furthermore, 4-ethyloctanal—which has recently been shown to potently stimulate the GnRH pulse generator and is thus considered as a likely male pheromone candidate in goats—is a chemical included in the neutral components of male goat headspace volatiles [32]. An interesting issue to be examined in future studies is whether the main olfactory system of ewes (or female goats) is activated by exposure to the neutral fraction of buckhair extracts or 4-ethyloctanal.
The present study found that exposure of ewes to the goat pheromone activates the ARC (Table 1), which was not demonstrated by the ram introduction [13]. It is unclear whether this inconsistency is due to the difference in the stimuli, because another study reported an increase in c-Fos expression in the ARC following the ram introduction [8]. The activation of the ARC is of particular significance. Several lines of evidence suggest that a population of kisspeptin neurons in the ARC participates in the male effect. First, kisspeptin neurons in the ARC have been proposed to be an intrinsic source of neural activity of the GnRH pulse generator [26, 34, 40, 44] that is the central target of the male pheromone [9, 29, 39]. Second, ARC kisspeptin neurons receive direct efferent projections from the MeA in goats [45]. The MeA is considered to be a key neural substrate that relays pheromone signals to target nuclei in the hypothalamus [2, 21, 27] and was activated by the goat pheromone (Table 1) and the ram introduction [13]. Third, the c-Fos expression in ARC kisspeptin neurons of ewes was significantly increased by the ram introduction [8]. Fourth, electrodes positioned close vicinity of ARC kisspeptin neurons [38, 50] showed that exposure of female goats to male goat hairs [32, 33, 45] or 4-ethyoctanal [32] immediately evoked GnRH pulse generator activity. Fifth, administration of an antagonist for kisspeptin abrogated the LH response to the ram introduction in ewes [8], and exposure to male goat hairs failed to induce GnRH pulse generator activity when female goats were pretreated with an antagonist for neurokinin B [45], a substance co-expressed in ARC kisspeptin neurons that plays a pivotal role in GnRH pulse generation [40, 50]. Thus, it is plausible to postulate that the signal of the male pheromone is transmitted via the MeA to ARC kisspeptin neurons and thereby induces the GnRH/LH pulse [39]. Although the chemical identity of the c-Fos-ir cells was not examined in the present study, these c-Fos-ir cells (Fig. 3F) may at least in part be kisspeptin neurons.
The VMH receives massive projections from the MeA [22, 45, 46, 49] and is thought to be one of key hypothalamic nuclei for the pheromone signal processing [2, 21]. In concert, the previous studies demonstrated that the ram introduction activated the VMH [8, 13]. On the other hand, the number of c-Fos-ir cells in the VMH was not significantly different between the goat pheromone and control exposure in the present study (Table 1). These results suggest that the olfactory signals emitted by the ram consist of at least two kinds of signal; one transmitted to the VMH and the other to the GnRH pulse generator located outside the VMH (perhaps in the ARC, as discussed above) of ewes. The male goat hair extract appears to lack the former. The activation of the VMH by the ram introduction may represent other aspects of the male effect than the stimulation of GnRH/LH secretion, such as the modulation of sexual behavior in the female [1, 5, 41].
In conclusion, by employing a different combination of the goat and sheep breeds from the previous study [42], we demonstrated in this study that exposure of seasonally anestrous ewes to the male goat hair extract stimulates LH secretion, implicating that the interspecies action of the male pheromone is a universal phenomenon in these animals. The present study also showed that the exposure of the buckhair extract activated several specific areas, including the AOB, MeA, and ARC, but not the nuclei in the main olfactory system except in the PYR. The results suggest that the male pheromone signal can be transmitted solely through the vomeronasal system to the GnRH pulse generator, which may be located at the ARC, although the participation of the main olfactory system in this pathway is highly likely.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (S) (21228006) from the Japan Society for the Promotion of Science. The authors grateful to Dr. JunYou Li and Mr. Ryuji Sako in Graduate School of Agricultural and Life Sciences, the University of Tokyo for their animal management and support of the experiment. We also wish to thank Ms. Y. Sakairi in Animal Physiology Research Unit of National Institute of Agrobiological Sciences for her valuable technical assistance throughout the study.
REFERENCES
- 1.Blache D., Fabre-Nys C. J., Venier G.1991. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res. 546: 241–249. doi: 10.1016/0006-8993(91)91488-M [DOI] [PubMed] [Google Scholar]
- 2.Brennan P. A.2001. The vomeronasal system. Cell. Mol. Life Sci. 58: 546–555. doi: 10.1007/PL00000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bressler S. C., Baum M. J.1996. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience 71: 1063–1072. doi: 10.1016/0306-4522(95)00493-9 [DOI] [PubMed] [Google Scholar]
- 4.Chemineau P.1987. Possibilities for using bucks to stimulate ovarian and oestrous cycles in anovulatory goats— a review. Livest. Prod. Sci. 17: 135–147. doi: 10.1016/0301-6226(87)90059-5 [DOI] [Google Scholar]
- 5.Clark A. S., Pfeifle J. K., Edwards D. A.1981. Ventromedial hypothalamic damage and sexual proceptivity in female rats. Physiol. Behav. 27: 597–602. doi: 10.1016/0031-9384(81)90228-6 [DOI] [PubMed] [Google Scholar]
- 6.Claus R., Over R., Dehnhard M.1990. Effect of male odour on LH secretion and the induction of ovulation in seasonally anoestrous goats. Anim. Reprod. Sci. 22: 27–38. doi: 10.1016/0378-4320(90)90035-E [DOI] [Google Scholar]
- 7.Cohen-Tannoudji J., Lavenet C., Locatelli A., Tillet Y., Signoret J. P.1989. Non-involvement of the accessory olfactory system in the LH response of anoestrous ewes to male odour. J. Reprod. Fertil. 86: 135–144. doi: 10.1530/jrf.0.0860135 [DOI] [PubMed] [Google Scholar]
- 8.De Bond J. A., Li Q., Millar R. P., Clarke I. J., Smith J. T.2013. Kisspeptin signaling is required for the luteinizing hormone response in anestrous ewes following the introduction of males. PLoS ONE 8: e57972. doi: 10.1371/journal.pone.0057972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgadillo J. A., Gelez H., Ungerfeld R., Hawken P. A. R., Martin G. B.2009. The ‘male effect’ in sheep and goats–Revisiting the dogmas. Behav. Brain Res. 200: 304–314. doi: 10.1016/j.bbr.2009.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Fewell G. D., Meredith M.1994. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J. Neurosci. 14: 3643–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelez H., Archer E., Chesneau D., Campan R., Fabre-Nys C.2004. Importance of learning in the response of ewes to male odor. Chem. Senses 29: 555–563. doi: 10.1093/chemse/bjh054 [DOI] [PubMed] [Google Scholar]
- 12.Gelez H., Fabre-Nys C.2004. The “male effect” in sheep and goats: a review of the respective roles of the two olfactory systems. Horm. Behav. 46: 257–271. doi: 10.1016/j.yhbeh.2004.05.002 [DOI] [PubMed] [Google Scholar]
- 13.Gelez H., Fabre-Nys C.2006. Neural pathways involved in the endocrine response of anestrous ewes to the male or its odor. Neuroscience 140: 791–800. doi: 10.1016/j.neuroscience.2006.02.066 [DOI] [PubMed] [Google Scholar]
- 14.Hamada T.1995. Central mechanisms of reproductive pheromone. Thesis, the University of Tokyo (in Japanese).
- 15.Hamada T., Nakajima M., Takeuchi Y., Mori Y.1996. Pheromone-induced stimulation of hypothalamic gonadotropin-releasing hormone pulse generator in ovariectomized, estrogen-primed goats. Neuroendocrinology 64: 313–319. doi: 10.1159/000127134 [DOI] [PubMed] [Google Scholar]
- 16.Hoffman G. E., Lyo D.2002. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? J. Neuroendocrinol. 14: 259–268. doi: 10.1046/j.1365-2826.2002.00775.x [DOI] [PubMed] [Google Scholar]
- 17.Ichimaru T., Mogi K., Ohkura S., Mori Y., Okamura H.2008. Exposure to ram wool stimulates gonadotropin-releasing hormone pulse generator activity in the female goat. Anim. Reprod. Sci. 106: 361–368. doi: 10.1016/j.anireprosci.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 18.Iwata E., Wakabayashi Y., Kakuma Y., Kikusui T., Takeuchi Y., Mori Y.2000. Testosterone-dependent primer pheromone production in the sebaceous gland of male goat. Biol. Reprod. 62: 806–810. doi: 10.1095/biolreprod62.3.806 [DOI] [PubMed] [Google Scholar]
- 19.Karlson P., Luscher M.1959. Pheromones’: a new term for a class of biologically active substances. Nature 183: 55–56. doi: 10.1038/183055a0 [DOI] [PubMed] [Google Scholar]
- 20.Karsch F, J. 1984. The hypothalamus and anterior pituitary gland. pp. 1–20. In: Reproduction in Mammals, Vol. 3, Hormonal Control of Reproduction 2nd ed. (Austin, C. R. and Short, R. V. eds.), Cambridge University Press, Cambridge. [Google Scholar]
- 21.Keveme E. B.1983. Pheromonal influences on the endocrine regulation of reproduction. Trends Neurosci. 6: 381–384. doi: 10.1016/0166-2236(83)90170-4 [DOI] [Google Scholar]
- 22.Kevetter G. A., Winans S. S.1981. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J. Comp. Neurol. 197: 81–98. doi: 10.1002/cne.901970107 [DOI] [PubMed] [Google Scholar]
- 23.Knobil E.1981. Patterns of hypophysiotropic signals and gonadotropin secretion in the rhesus monkey. Biol. Reprod. 24: 44–49. doi: 10.1095/biolreprod24.1.44 [DOI] [PubMed] [Google Scholar]
- 24.Knight T. W., Lynch P. R.1980a Source of ram pheromones that stimulate ovulation in the ewe. Anim. Reprod. Sci. 3: 133–136. doi: 10.1016/0378-4320(80)90040-8 [DOI] [Google Scholar]
- 25.Knight T. W., Lynch P. R.1980b The pheromone from rams that stimulates ovulation in the ewe. Proc. Aust. Soc. Anim. Prod. 13: 74–76. [Google Scholar]
- 26.Lehman M. N., Coolen L. M., Goodman R. L.2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151: 3479–3489. doi: 10.1210/en.2010-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C. S., Kaba H., Saito H., Seto K.1990. Neural mechanisms underlying the action of primer pheromones in mice. Neuroscience 36: 773–778. doi: 10.1016/0306-4522(90)90019-Z [DOI] [PubMed] [Google Scholar]
- 28.Lincoln D. W., Fraser H. M., Lincoln G. A., Martin G. B., McNeilly A. S.1985. Hypothalamic pulse generators. Recent Prog. Horm. Res. 41: 369–419. [DOI] [PubMed] [Google Scholar]
- 29.Martin G. B., Oldham C. M., Cogni È. Y., Pearce D. T.1986. The physiological responses of anovulatory ewes to the introduction of rams— A review. Livest. Prod. Sci. 15: 219–247. doi: 10.1016/0301-6226(86)90031-X [DOI] [Google Scholar]
- 30.Martin G. B., Scaramuzzi R. J.1983. The induction of oestrus and ovulation in seasonally anovular ewes by exposure to rams. J. Steroid Biochem. 19: 869–875. doi: 10.1016/0022-4731(83)90026-2 [DOI] [PubMed] [Google Scholar]
- 31.Mori Y., Nishihara M., Tanaka T., Shimizu T., Takeuchi Y., Hoshino K.1991. Chronic recording of electrophysiological manifestation of the hypothalamic gonadotropin-releasing hormone pulse generator in the goat. Neuroendocrinology 53: 392–395. doi: 10.1159/000125746 [DOI] [PubMed] [Google Scholar]
- 32.Murata K., Tamogami S., Itou M., Ohkubo Y., Wakabayashi Y., Watanabe H., Okamura H., Takeuchi Y., Mori Y.2014. Identification of an olfactory signal molecule that activates the central regulator of reproduction in goats. Curr. Biol. 24: 681–686. doi: 10.1016/j.cub.2014.01.073 [DOI] [PubMed] [Google Scholar]
- 33.Murata K., Wakabayashi Y., Sakamoto K., Tanaka T., Takeuchi Y., Mori Y., Okamura H.2011. Effects of brief exposure of male pheromone on multiple-unit activity at close proximity to kisspeptin neurons in the goat arcuate nucleus. J. Reprod. Dev. 57: 197–202. doi: 10.1262/jrd.10-070E [DOI] [PubMed] [Google Scholar]
- 34.Navarro V. M., Gottsch M. L., Chavkin C., Okamura H., Clifton D. K., Steiner R. A.2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29: 11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishihara M., Takeuchi Y., Tanaka T., Mori Y.1999. Electrophysiological correlates of pulsatile and surge gonadotrophin secretion. Rev. Reprod. 4: 110–116. doi: 10.1530/ror.0.0040110 [DOI] [PubMed] [Google Scholar]
- 36.Ohara H., Nikaido M., Date-Ito A., Mogi K., Okamura H., Okada N., Takeuchi Y., Mori Y., Hagino-Yamagishi K.2009. Conserved repertoire of orthologous vomeronasal type 1 receptor genes in ruminant species. BMC Evol. Biol. 9: 233. doi: 10.1186/1471-2148-9-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkura S., Ichimaru T., Itoh F., Matsuyama S., Okamura H.2004. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology 145: 3239–3246. doi: 10.1210/en.2003-1516 [DOI] [PubMed] [Google Scholar]
- 38.Ohkura S., Takase K., Matsuyama S., Mogi K., Ichimaru T., Wakabayashi Y., Uenoyama Y., Mori Y., Steiner R. A., Tsukamura H., Maeda K.I., Okamura H.2009. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J. Neuroendocrinol. 21: 813–821. doi: 10.1111/j.1365-2826.2009.01909.x [DOI] [PubMed] [Google Scholar]
- 39.Okamura H., Murata K., Sakamoto K., Wakabayashi Y., Ohkura S., Takeuchi Y., Mori Y.2010. Male effect pheromone tickles the gonadotrophin-releasing hormone pulse generator. J. Neuroendocrinol. 22: 825–832. doi: 10.1111/j.1365-2826.2010.02037.x [DOI] [PubMed] [Google Scholar]
- 40.Okamura H., Tsukamura H., Ohkura S., Uenoyama Y., Wakabayashi Y., Maeda K.2013. Kisspeptin and GnRH pulse generation. pp. 297–323. In: Kisspeptin Signaling in Reproductive Biology, Advances in Experimental Medicine and Biology (Kauffman, A. S. and Smith, J. T. eds.), Springer, New York. [DOI] [PubMed] [Google Scholar]
- 41.Oomura Y., Aou S., Koyama Y., Fujita I., Yoshimatsu H.1988. Central control of sexual behavior. Brain Res. Bull. 20: 863–870. doi: 10.1016/0361-9230(88)90103-7 [DOI] [PubMed] [Google Scholar]
- 42.Over R., Cohen-Tannoudji J., Dehnhard M., Claus R., Signoret J. P.1990. Effect of pheromones from male goats on LH-secretion in anoestrous ewes. Physiol. Behav. 48: 665–668. doi: 10.1016/0031-9384(90)90208-L [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G., Watson C.1998. The rat brain in stereotaxic coordinates. Academic Press, London. [DOI] [PubMed] [Google Scholar]
- 44.Rance N. E., Krajewski S. J., Smith M. A., Cholanian M., Dacks P. A.2010. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 1364: 116–128. doi: 10.1016/j.brainres.2010.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakamoto K., Wakabayashi Y., Yamamura T., Tanaka T., Takeuchi Y., Mori Y., Okamura H.2013. A population of kisspeptin/neurokinin B neurons in the arcuate nucleus may be the central target of the male effect phenomenon in goats. PLoS ONE 8: e81017. doi: 10.1371/journal.pone.0081017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saper C. B., Swanson L. W., Cowan W. M.1976. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J. Comp. Neurol. 169: 409–442. doi: 10.1002/cne.901690403 [DOI] [PubMed] [Google Scholar]
- 47.Schinckel P. G.1954. The effect of the presence of the ram on the ovarian activity of the ewe. Aust. J. Agric. Res. 5: 465–469. doi: 10.1071/AR9540465 [DOI] [Google Scholar]
- 48.Shelton M.1960. Influence of a male goat on the initiation of estrous cycling and ovulation of Angora does. J. Anim. Sci. 19: 368–375. [Google Scholar]
- 49.Stoddard-Apter S. L., MacDonnell M. F.1980. Septal and amygdalar efferents to the hypothalamus which facilitate hypothalamically elicited intraspecific aggression and associated hissing in the cat. An autoradiographic study. Brain Res. 193: 19–32. doi: 10.1016/0006-8993(80)90942-7 [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi Y., Nakada T., Murata K., Ohkura S., Mogi K., Navarro V. M., Clifton D. K., Mori Y., Tsukamura H., Maeda K. I., Steiner R. A., Okamura H.2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 30: 3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson R. E., Jr, Wiegand S. J., Clough R. W., Hoffman G. E.1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159. doi: 10.1016/0196-9781(86)90076-8 [DOI] [PubMed] [Google Scholar]
- 52.Zuccolilli G. O., Hayashi S., Mori Y.1995. Hypothalamic structures of the goat on stereotaxic coordinates. J. Vet. Med. Sci. 57: 459–467. doi: 10.1292/jvms.57.459 [DOI] [PubMed] [Google Scholar]