ABSTRACT
Resveratrol has a neuroprotective effect against cerebral ischemia. The objective of this study was to identify proteins that are differentially expressed in the cerebral cortex of vehicle- and resveratrol-treated animals during ischemic injury. Focal cerebral ischemia was induced as middle cerebral artery occlusion (MCAO) in male rats. Rats were treated with vehicle or resveratrol before MCAO, and cerebral cortex was collected 24 hr after MCAO. Cerebral cortex proteins were identified by two-dimensional gel electrophoresis and mass spectrometry. Several proteins were identified as differentially expressed between vehicle- and resveratrol-treated animals. Among these proteins, expression of peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I and ubiquitin carboxy terminal hydrolase L1 was decreased in the vehicle-treated group, whereas resveratrol attenuated the injury-induced decrease in expression of these proteins. However, expression of collapsing response mediator protein 2 was increased in the vehicle-treated group, whereas resveratrol prevented the injury-induced increase in the expression of this protein. These findings suggest that resveratrol modulates the expression of various proteins that associated with oxidative stress and energy metabolism in focal cerebral ischemia.
Keywords: ischemia, proteomics, rat, resveratrol
Cerebral ischemia is one of the leading causes of morbidity and mortality in clinical cases. Cerebral ischemia induces the generation of reactive oxygen species and the release of calcium, ultimately leading to neuronal cells death and severe neurologic impairment [25, 30]. Middle cerebral artery occlusion (MCAO) has been accepted as a general animal model of focal cerebral ischemia [19].
Resveratrol (3,5,4’-trihydroxystilbene) is naturally present in medical plants [4]. Resveratrol has biological activities including anti-inflammatory and anti-oxidative effects [15, 33]. Resveratrol has neuroprotective properties in both cerebral ischemia and neurodegenerative disease, such as Alzheimer’s disease and Parkinson’s disease [14, 22, 29]. Resveratrol decreases infarct volume and improves neurologic scores in a focal cerebral ischemia model [10]. Although the neuroprotective properties of resveratrol have been described, its neuroprotective mechanism is unclear. Our previous study demonstrated that cerebral ischemia induces the up- and down-regulation of specific protein that mediates neuronal cells death [17]. We think that resveratrol treatment modulates various proteins in cerebral ischemic condition. The purpose of this study was to investigate differentially expressed protein by resveratrol treatment in cerebral ischemia. The comprehensive analysis by two-dimensional electrophoresis (2-DE) can provide information for differentially expressed proteins between the two groups [23]. Thus, we used two-dimensional gel electrophoresis and mass spectrometry to identify specific proteins that change by resveratrol in ischemic brain injury.
MATERIALS AND METHODS
Animals and treatment: Adult male Sprague–Dawley rats (225–250 g, n=36) were purchased from Samtako Animal Breeding Center (Osan, Korea) and were randomly divided into a sham-operated group, vehicle-treated group and resveratrol-treated group (n=12 per group). All procedures for animal use were approved by the Institutional Animal Care and Use Committee of Gyeongsang National University (GNU-130723-R0050). Experimental animals were housed at 18–22°C under a 12 hr light/12 hr dark cycle and had free access to a pellet diet and tap water. Resveratrol (30 mg/kg, Sigma, St. Louis, MO, U.S.A.) was dissolved in 2% dimethyl sulfoxide (DMSO) as vehicle and was injected intraperitoneally as described previously [19]. Resveratrol or vehicle was injected immediately after middle cerebral artery occlusion (MCAO).
Middle cerebral artery occlusion: Rats were anesthetized with sodium pentobarbital (100 mg/kg), and MCAO was carried out as described previously [21]. Briefly, the right common carotid artery, external carotid and internal carotid were exposed through a midline cut. A 4/0 nylon filament with a heated rounded tip was inserted from the external carotid artery into the internal carotid artery and advanced until the rounded tip occluded the origin of the middle cerebral artery. Sham-operated animals were subjected to the same procedure, except for insertion of the filament. At 24 hr after the onset of permanent occlusion, animals were decapitated, and the right cerebral cortex was isolated.
2-Dimensional gel electrophoresis: Proteomic analysis was carried out as our previously described method [32]. Proteins were extracted from the right cerebral cortex by homogenization in buffer solution (8 M urea, 4% CHAPS, ampholytes and 40 mM Tris–HCl) followed by centrifugation at 16,000 g. Protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA, U.S.A.) according to the manufacturer’s protocol. Immobilized pH gradients (IPG, pH 4–7 and pH 6–9, 17 cm, Bio-Rad) were incubated in rehydration buffer (8 M urea, 2% CHAPS, 20 mM DTT, 0.5% IPG buffer and bromophenol blue) for 13 hr at room temperature. Assayed protein samples were loaded on IPG strips (pH 4–7 and 6–9), and isoelectric focusing (IEF) was performed as follows: 200 V (1 hr), 500 V (1 hr), 1,000 V to 8,000 V (30 min) and 8,000 V (5 hr) using an IPG phore unit (GE Healthcare, Uppsala, Sweden). Strips were incubated with equilibration buffer (6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, 50 mM Tris-HCl and bromophenol blue) and loaded on gradient gels (7.5–17.5%), followed by second-dimension electrophoresis using Protein-II XI electrophoresis equipment (Bio-Rad). Settings were 5 mA per gel for 2 hr followed by 10 mA per gel at 10°C.
Silver staining, image analysis and protein identification: Silver staining was performed as follows: fixation (12% acetic acid and 50% methanol) for 2 hr, washing with 50% ethanol and then treatment with 0.2% sodium thiosulfate. Gels were washed with deionized water and stained in silver solution (0.2% silver nitrate). Gels were developed in 0.2% sodium carbonate solution, and gel images were collected using an Agfar ARCUS 1200™ scanner (Agfar-Gevaert, Mortsel, Belgium). PDQuest 2-D analysis software (Bio-Rad) was used to analyze differences in protein spots among the different groups. Differentially expressed protein spots were excised and destained. Gel particles were digested in trypsin-containing buffer, and the extracted peptides were analyzed using a Voyager-DETM STR biospectrometry workstation (Applied Biosystem, Forster City, CA, U.S.A.) for peptide mass fingerprinting. Database searches were carried out using MS-Fit and ProFound software. SWISS-PROT and NCBI were used as protein sequence databases.
Western blot analysis: Western blot analysis was carried out as our previously described method [13, 32]. Right cerebral cortex was dissolved in lysis buffer (1 M Tris–HCl, 5 M sodium chloride, 0.5% sodium deoxycholate, 10% sodium dodecyl sulfate, 1% sodium azide and 10% NP-40). Protein concentration was determined using a bicinchoninic acid (BCA) kit (Pierce, Rockford, IL, U.S.A.) according to the manufacturer’s protocol. Equal volumes of protein (30 µg per sample) were electrophoresed on 10% SDS-PAGE gels, and the proteins were transferred to poly-vinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, U.S.A.). To minimize nonspecific binding, membranes were blocked with skim milk for 1 hr at room temperature. PVDF membranes were washed in Tris-buffered saline containing 0.1% Tween-20 (TBST) and then incubated with antibodies against the following proteins: peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I, ubiquitin carboxy terminal hydrolase L1, collapsing response mediator protein 2 and actin (diluted 1:1,000, Cell Signaling Technology, Beverly, MA, U.S.A.). Membranes were sequentially reacted with secondary antibody (1:5,000, Pierce). ECL Western blot analysis system (Amersham Pharmacia Biotech, Piscataway, NJ, U.S.A.) was used for detection according to the manufacturer’s protocol. The intensity analysis was carried out using SigmaGel 1.0 (Jandel Scientific, San Rafael, CA, U.S.A.) and SigmaPlot 4.0 (SPSS Inc., Point Richmond, CA, U.S.A.).
Reverse transverse-PCR amplification: Total RNA from right cerebral cortices was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.A.) following the manufacturer’s protocol. For reverse transcription, we used Superscript III reverse transcriptase from Invitrogen following the manufacturer’s manuals. The primer sequences are represented in Table 1. The amplification PCR reaction consisted of an initial denaturation at 94°C for 5 min, followed by 35 cycles from 94°C for 30 sec, annealing at 54°C for 30 sec and an extension at 72°C for 1 min and a final extension for 10 min at 72°C. RT-PCR products were separated on a 1% agarose gel and visualized under UV light. The intensity analysis of RT-PCR products was carried out using SigmaGel 1.0 (Jandel Scientific) and SigmaPlot 4.0 (SPSS Inc.).
Table 1. Sequence of the primers used for PCR amplification.
| Gene | Primer sequences (F, Forward; R, Reverse) | Product size (bp) | |
|---|---|---|---|
| Peroxiredoxin-5 | F: | 5′-GGAGTCCCTGGGGCATTTAC-3′ | 392 |
| R: | 5′-GACATTCTGGTCAGGGCCTC-3′ | ||
| NAD (+)-dependent isocitrate dehydrogenase | F: | 5′-AAAAATCCATGGCGGTTCTGTG-3′ | 404 |
| R: | 5′-GGTCCCCATAGGCGTGTCG-3′ | ||
| Apolipoprotein A-I | F: | 5′-TGTTGGTCGCCTACAGGAAC-3′ | 223 |
| R: | 5′- TCGCGTTTTTGTGAAGCTCG-3′ | ||
| Ubiquitin carboxyl terminal hydrolase isozyme L1 (UCH-L1) | F: | 5′-CTAGGGCTGGAGGAGGAGAC-3′ | 296 |
| R: | 5′-TTGTCCCCTGAAGAGAGAGC-3′ | ||
| Collapsing response mediator protein 2 (CRMP-2) | F: | 5′-TGGTTTCAGCTTGTCTGGTG-3′ | 454 |
| R: | 5′ -TGACAGGAAGGTGCTGACTG-3′ | ||
| β-actin | F: | 5′-GGGTCAGAAGGACTCCTACG-3′ | 238 |
| R: | 5′- TTTCACTGCGGCTGATGTAG-3′ | ||
Data analysis: All data are expressed as means ± SEM. The results for each group were compared by one-way analysis of variance (ANOVA) followed by Student’s t-test. The difference for comparison was considered significant at P<0.05.
RESULTS
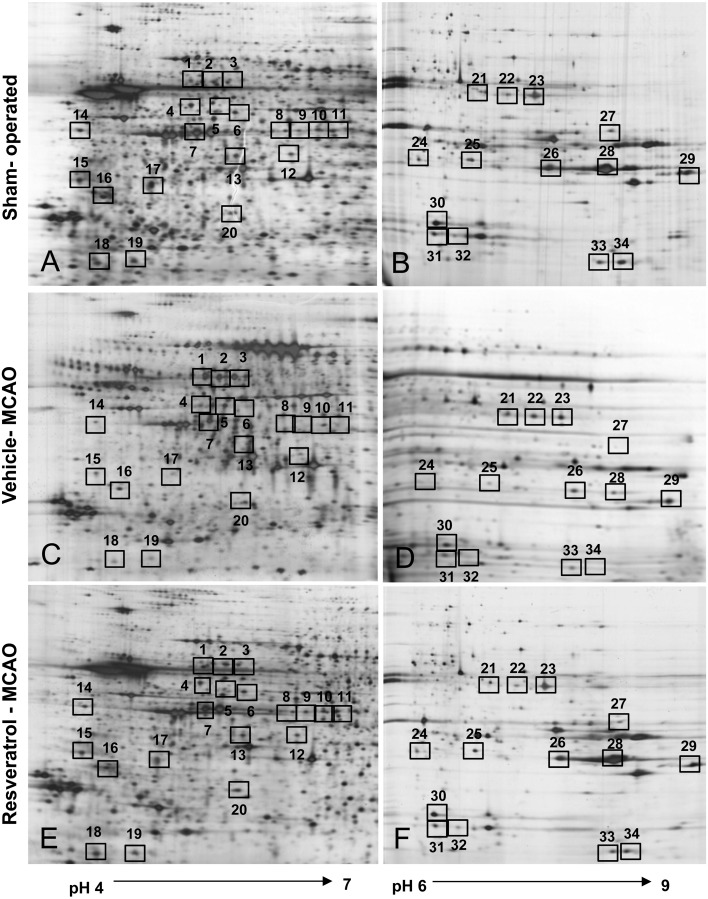
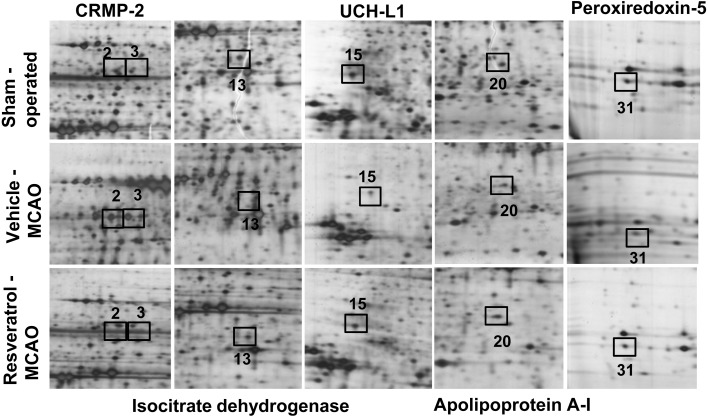
Two-dimensional electrophoresis (2-DE) maps were generated for cerebral cortex protein spots from sham-operated, vehicle-treated and resveratrol-treated animals (pH range 4–7 and pH 6–9). Approximately 980 protein spots per image were observed. We detected thirty-four protein spots with more than a 2.5-fold change in intensity. Among these protein spots, twenty-nine proteins were clearly identified by MALDI-TOF analysis with a protein sequence coverage ranging from 21–67% (Fig. 1, Table 2). However, five proteins were not identified by MALDI-TOF analysis and named as unknown proteins. Among these identified proteins, we focused on peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I, ubiquitin carboxy terminal hydrolase L1 (UCH-L1) and collapsing response mediator protein 2 (CRMP-2) (Fig. 2).
Fig. 1.
Two-dimensional SDS-PAGE analysis of proteins in the cerebral cortex from sham-operated (A and B), vehicle-treated (C and D) and resveratrol-treated (E and F) animals after middle cerebral artery occlusion (MCAO). Isoelectric focusing was performed at pH 4–7 and pH 6–9 using IPG strips, followed by second-dimensional separation on 7.5–17.5% gradient SDS gels stained with silver. Squares indicate the protein spots that were differentially expressed between vehicle- and resveratrol-treated groups.
Table 2. List of identified proteins that were significantly differentially expressed in vehicle- and resveratrol-treated animals.
| Spot No. |
Protein name | Accession No. |
Mw (kDa) |
pI | Mass matched |
Sequence coverage (%) |
Ratio Veh / Sham |
Ratio Res/ Sham |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 kDa heat shock protein | P63039 | 60.91 | 5.91 | 11/133 | 32% | 3.01 ± 0.13* | 1.15 ± 0.12* |
| 2 | Collapsing response mediator protein 2 | P47942 | 83.85 | 6.64 | 7/109 | 29% | 3.03 ± 0.14* | 1.10 ± 0.13* |
| 3 | Collapsing response mediator protein 2 | P47942 | 62.27 | 6.0 | 24/103 | 52% | 2.78 ± 0.12* | 1.09 ± 0.12* |
| 4 | Rab, GTPase-GDP dissociation stimulation stimulator 1 | P52306 | 66.40 | 5.2 | 20/156 | 53% | 0.32 ± 0.04* | 1.02 ± 0.07* |
| 5 | Unknown | 2.93 ± 0.12* | 1.08 ± 0.02* | |||||
| 6 | Unknown | 0.33 ± 0.03* | 0.97 ± 0.02* | |||||
| 7 | Eukaryotic initiation factor 4A-II | Q5RKI1 | 46.73 | 5.33 | 16/82 | 40% | 0.36 ± 0.04* | 0.89 ± 0.05* |
| 8 | Succinyl-CoA ligase subunit beta | Q9Z2I9 | 50.27 | 7.75 | 11/74 | 24% | 0.34 ± 0.03* | 1.01 ± 0.03* |
| 9 | Adenosine kinase | Q64640 | 40.10 | 5.72 | 12/95 | 41% | 0.33 ± 0.02* | 1.02 ± 0.02* |
| 10 | MAP kinase kinase 1 | Q01986 | 43.43 | 6.18 | 12/72 | 30% | 0.35 ± 0.03* | 0.98 ± 0.03* |
| 11 | Adenosylhomocysteinase | P10760 | 47.50 | 6.07 | 15/132 | 33% | 0.31 ± 0.03* | 1.01 ± 0.04* |
| 12 | Protein phosphatase 2A, subunit B | P58389 | 36.59 | 5.88 | 9/56 | 29% | 0.32 ± 0.02* | 0.89 ± 0.02* |
| 13 | Isocitrate dehydrogenase[NAD+] subunit alpha | Q99NA5 | 39.58 | 6.47 | 8/93 | 31% | 0.31 ± 0.04* | 0.87 ± 0.02* |
| 14 | γ-enolase | P07323 | 47.14 | 5.00 | 14/70 | 35% | 0.27 ± 0.03* | 0.91 ± 0.02* |
| 15 | Ubiquitin carboxy-terminal hydrolase L1 | Q7TQI3 | 31.25 | 4.85 | 11/66 | 50% | 0.30 ± 0.03* | 1.02 ± 0.02* |
| 16 | Thioredoxcin | Q920J4 | 32.23 | 4.84 | 8/87 | 42% | 0.22 ± 0.03* | 0.89 ± 0.04* |
| 17 | Mu-crystallin | Q9QYU4 | 33.53 | 5.34 | 9/86 | 24% | 0.15 ± 0.02* | 0.94 ± 0.02* |
| 18 | Hippocalcin | P62749 | 22.32 | 5.3 | 10/102 | 52% | 0.25 ± 0.03* | 0.96 ± 0.02* |
| 19 | Proteasome subunit alpha type3 | P18422 | 28.40 | 5.3 | 7/112 | 27% | 0.20 ± 0.03* | 0.97 ± 0.02* |
| 20 | Apolipoprotein A-I | P044639 | 30.04 | 5.52 | 16/116 | 50% | 0.34 ± 0.02* | 1.05 ± 0.03* |
| 21 | Pyruvate kinase isoenzyme M1/M2 | P11980 | 57.82 | 6.63 | 19/80 | 42% | 2.51 ± 0.03* | 1.08 ± 0.03* |
| 22 | Pyruvate kinase isoenzyme M1/M2 | P11980 | 57.82 | 6.63 | 19/80 | 42% | 2.73 ± 0.03* | 1.13 ± 0.04* |
| 23 | Unknown | 0.27 ± 0.03* | 1.05 ± 0.03* | |||||
| 24 | Mitogen-activated protein (MAP) kinase 1 | P63086 | 41.27 | 6.5 | 10/96 | 36% | 0.29 ± 0.03* | 0.97 ± 0.02* |
| 25 | Alcohol dehydrogenase (NADP+) | P51635 | 36.50 | 6.8 | 9/104 | 28% | 0.33 ± 0.03* | 1.02 ± 0.03* |
| 26 | Unknown | 0.32 ± 0.03* | 0.99 ± 0.02* | |||||
| 27 | Obg-like ATPase 1 | A0JPJ7 | 44.50 | 7.62 | 6/68 | 21% | 0.33 ± 0.02* | 1.02 ± 0.03* |
| 28 | Malate dehydrogenase | P04636 | 35.68 | 8.9 | 23/80 | 67% | 0.17 ± 0.04* | 1.07 ± 0.02* |
| 29 | Malate dehydrogenase | P04636 | 35.68 | 8.9 | 23/80 | 67% | 0.32 ± 0.04* | 0.99 ± 0.03* |
| 30 | Nucleoside diphosphate kinase B | P19804 | 17.28 | 6.9 | 8/86 | 49% | 0.33 ± 0.02* | 0.95 ± 0.03* |
| 31 | Peroxiredoxin-5 | Q9R063 | 22.17 | 8.9 | 9/114 | 46% | 0.24 ± 0.03* | 0.97 ± 0.02* |
| 32 | CB1 cannabinoid receptor-interacting protein 1 | Q5M7A7 | 18.64 | 7.7 | 5/120 | 31% | 0.31 ± 0.03* | 0.95 ± 0.03* |
| 33 | Adenylate kinase isoenzyme1 | P39069 | 21.58 | 7.7 | 12/95 | 34% | 0.34 ± 0.03* | 0.87 ± 0.02* |
| 34 | Unknown | 0.15 ± 0.04* | 0.79 ± 0.03* |
Protein names and accession numbers are listed according to the SWISS-PROT database. Mw, molecular weight; pI, isoelectric point. Ratio is described as spots intensity of MCAO-operated to spots intensity of sham-operated. Data (n=4) are presented as means ± SEM. *P<0.05 (vs. sham).
Fig. 2.
Image of protein spots identified by MALDI-TOF. Magnified pictures of peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I, ubiquitin carboxy terminal hydrolase L1 (UCH-L1) and collapsing response mediator protein 2 (CRMP-2) protein spots in the cerebral cortex from sham-operated, vehicle-treated and resveratrol-treated animals after middle cerebral artery occlusion (MCAO). Squares indicate the specific spots.
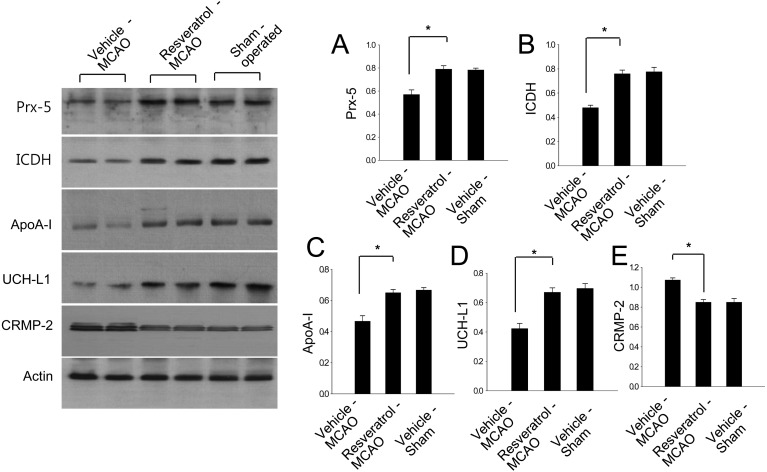
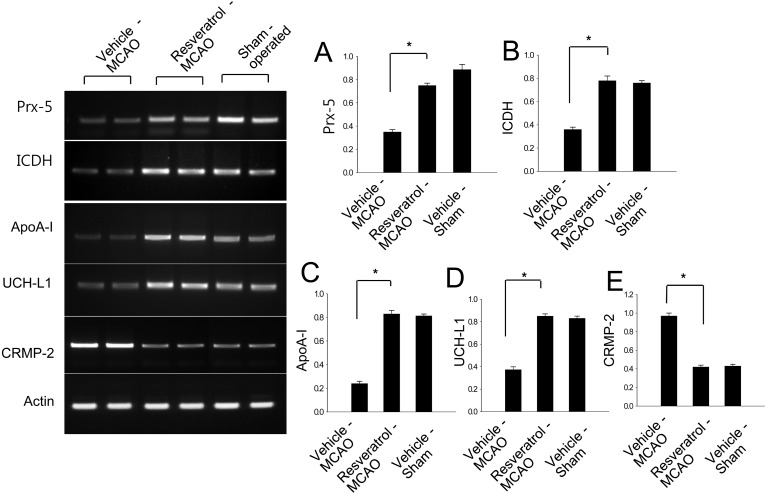
Western blot analysis showed that peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I and UCH-L1 levels decreased in vehicle-treated animals, whereas resveratrol treatment prevented the injury-induced decrease in expression of these proteins. Protein expression levels are represented as the ratio of intensity of the protein to that of actin. Peroxiredoxin-5 levels were lower in vehicle-treated animals than resveratrol- and sham-operated animals, and these proteins were expressed at similar levels in the latter two groups (Fig. 3). Peroxiredoxin-5 levels were 0.57 ± 0.04 and 0.79 ± 0.03 in vehicle- and resveratrol-treated animals. Isocitrate dehydrogenase [NAD+] levels were 0.42 ± 0.03 and 0.67 ± 0.03 in vehicle- and resveratrol-treated animals. Apolipoprotein A-I levels were 0.47 ± 0.03 and 0.65 ± 0.02 in vehicle- and resveratrol-treated animals, respectively. UCH-L1 levels were 0.48 ± 0.02 and 0.76 ± 0.03 in vehicle- and resveratrol-treated animals, respectively (Fig. 3). In contrast, CRMP-2 expression increased in vehicle-treated animals relative to resveratrol-treated animals and sham-operated animals. CRMP-2 levels were 1.07 ± 0.02 and 0.85 ± 0.03 in vehicle- and resveratrol-treated animals, respectively (Fig. 3). Moreover, RT-PCR analysis clearly demonstrated that peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I and UCH-L1 levels decreased in vehicle-treated animals, whereas resveratrol treatment attenuated the injury-induced decreases in these mRNA levels. However, CRMP-2 mRNA increased in vehicle-treated animals, whereas resveratrol treatment attenuated this increase (Fig. 4). The intensity of RT-PCR product was normalized to that of actin product. Peroxiredoxin-5 levels were 0.36 ± 0.02 and 0.73 ± 0.02 in vehicle- and resveratrol-treated animals. Isocitrate dehydrogenase [NAD+] levels were 0.35 ± 0.02 and 0.78 ± 0.03 in vehicle- and resveratrol-treated animals. Apolipoprotein A-I levels were 0.24 ± 0.02 and 0.82 ± 0.02 in vehicle- and resveratrol-treated animals, respectively. UCH-L1 levels were 0.38 ± 0.02 and 0.83 ± 0.03 in vehicle- and resveratrol-treated animals, respectively (Fig. 3). CRMP-2 levels were 0.94 ± 0.02 and 0.41 ± 0.02 in vehicle- and resveratrol-treated animals, respectively (Fig. 3).
Fig. 3.
Western blot analysis of peroxiredoxin-5 (Prx-5), isocitrate dehydrogenase [NAD+] (ICDH), apolipoprotein A-I (ApoA-I), ubiquitin carboxy terminal hydrolase L1 (UCH-L1) and collapsing response mediator protein 2 (CRMP-2) in the cerebral cortex from sham-operated, vehicle-treated and resveratrol-treated animals after middle cerebral artery occlusion (MCAO). Each lane represents an individual experimental animal. Densitometric analysis is represented as a ratio, proteins intensity to actin intensity. Data (n=4) are represented as mean ± S.D. *P<0.05.
Fig. 4.
Representative photos of RT-PCR analysis of peroxiredoxin-5 (Prx-5), isocitrate dehydrogenase [NAD+] (ICDH), apolipoprotein A-I (ApoA-I), ubiquitin carboxy terminal hydrolase L1 (UCH-L1) and collapsing response mediator protein 2 (CRMP-2) in the cerebral cortex from sham-operated, vehicle-treated and resveratrol-treated animals after middle cerebral artery occlusion (MCAO). Each lane represents an individual experimental animal. The band intensity of RT-PCR product was normalized to that of actin product. Data (n=4) are represented as mean ± S.D. *P<0.05.
DISCUSSION
We previously reported that ferulic acid exerts a neuroprotective effect against MCAO injury by modulating several proteins [32]. Such as resveratrol, ferulic acid is a phenolic compound that extracted from plant. Ferulic acid and resveratrol have similar functions, including anti-inflammatory and anti-oxidative effects. Resveratrol exerts a neuroprotective effect against experimental stroke [29]. In the present study, we identified differentially expressed proteins following resveratrol treatment in focal cerebral ischemia. Among these identified proteins, we discussed on proteins that associated with oxidative stress and energy metabolism.
Peroxiredoxins are thioredoxin peroxidases that participate the elimination of hydrogen peroxide and neutralize other reactive oxygen species (ROS). Moreover, peroxiredoxins peroxidases are anti-oxidant proteins that preserve the integrity of the cell against oxidative stress [5]. There are six peroxiredoxin isoforms. Among these isoforms, peroxiredoxin-5 has a broader activity against both ROS and reactive nitrogen species compared with other isoforms of peroxiredoxins [34]. Ischemic stroke leads to the generation of free radicals, and this excessive production of free radicals disrupts the hemostatic environment and triggers neuronal cells death [7]. Peroxiredoxin-5 plays a protective role against oxidative stress by reducing apoptosis [34]. Peroxiredoxin-5 also protects neuronal cells against excitotoxic brain lesions [26]. We found that peroxiredoxin-5 expression decreases in ischemic brain injury, whereas resveratrol attenuates the injury-induced decline in this protein. Resveratrol is an antioxidant agent that exerts a neuroprotective effect against oxidative stress [15]. Our results demonstrate that resveratrol minimizes the decrease of peroxiredoxin-5 in focal cerebral ischemia.
NAD (+)-dependent isocitrate dehydrogenase is located in the mitochondria and is associated with energy metabolism and mitochondrial function [2]. NAD (+)-dependent isocitrate dehydrogenase plays a pivotal role in the production of NADH from NAD+ in the Krebs cycle. NADH regulates various biological functions, including energy metabolism and anti-oxidation activity [13]. However, oxidative stress causes a decrease in the concentration of NAD+ and cessation of energy metabolism [11]. Resveratrol protects neuronal cells by modulating mitochondrial dysfunction in cerebral ischemia [33]. Resveratrol protects the brain by up-regulating mitochondrial superoxide dismutase, which reduces oxidative stress and decreases cell death [28]. Moreover, resveratrol increases the activities of adenylate kinase and isocitrate dehydrogenase in heart ischemia [20]. We demonstrated that ischemic brain injury results in a decrease in the expression of isocitrate dehydrogenase [NAD+] using a proteomics approach. RT-PCR and Western blot analyses clearly confirmed that resveratrol prevents the injury-induced decrease in isocitrate dehydrogenase [NAD+] expression caused by MCAO. Isocitrate dehydrogenase is involved in energy metabolism and cell survival. These results suggest that maintenance of isocitrate dehydrogenase levels by resveratrol not only has a positive effect on energy metabolism, but is also neuroprotective effect.
Apolipoprotein A-I is an essential constituent of high density lipoprotein and is a key player in the transport and delivery of lipids [9]. Apolipoprotein A-I is involved in cholesterol metabolism. It has anti-oxidant and anti-inflammatory properties and modifies various immune functions [18]. Overexpression of apolipoprotein A-I attenuates neuroinflammation and preserves cognitive function [18]. Moreover, apolipoprotein A-I expression is markedly decreased in neurodegenerative diseases [16]. Our proteomics approach revealed that apolipoprotein A-I levels decreases in response to focal cerebral ischemia. These results are consistent with the decreases in apolipoprotein A-I levels in neurodegenerative diseases. Resveratrol treatment attenuates the MCAO-induced decrease in apolipoprotein A-I expression. A previous study demonstrated that resveratrol treatment increases expression of apolipoprotein A-I and inhibits the lipid peroxidation [3]. Together, these findings suggest that maintenance of apolipoprotein A-I expression by resveratrol contributes to attenuation of cell damage in cerebral ischemia.
Ubiquitin carboxy terminal hydrolase L1 (UCH-L1) is a neuron specific de-ubiquitinating enzyme, because it is exclusive expressed in brain tissue [24]. UCH-L1 ubiquitinates damaged proteins, such as oxidized proteins and aggregated proteins, for degradation by the proteasome system [1]. Thus, UCH-L1 plays an essential role in the maintenance of cell homeostasis. UCH-L1 prevents neuronal cell death from oxidative stress, and down-regulation of UCH-L1 leads to neuronal degeneration [1, 24]. Moreover, it has been reported that down-regulation of UCH-L1 is associated with Parkinson’s disease and Alzheimer’s disease [8]. Our proteomics data revealed that UCH-L1 levels decreased in response to cerebral ischemia, whereas resveratrol treatment attenuated this decrease in expression. We confirmed these results using RT-PCR and Western blot analyses. A decrease in UCH-L1 expression would lead to an imbalance between ubiquitination and de-ubiquitination and accumulation of aggregated and compromised protein, thereby inducing cell death [31]. Our results demonstrate that resveratrol prevents the down-regulation of UCH-L1 in focal cerebral ischemia. We can make an inference from these results that regulation of UCH-L1 by resveratrol maintains the cell homeostasis and preserves neuronal cells against MCAO.
Collapsing response mediator protein 2 (CRMP-2) regulates various neuronal activities including neuronal maturation, regeneration and axonal growth [6, 27]. CRMP-2 is involved in pathological disorders of neurons and is increased by ischemic brain injury [6]. Our results showed the rise of CRMP-2 expression in MCAO injury. This is consistent with previous studies that CRMP-2 is up-regulated after focal cerebral ischemia [6]. The rise of CRMP-2 indicates neuronal dysfunction [6]. Resveratrol treatment prevents the ischemic brain injury-induced elevation of CRMP-2. These results suggest that the regulation of CRMP-2 expression by resveratrol contributes to the neuroprotective effect of resveratrol. In conclusion, we provide evidence that resveratrol modulates the expression of various specific proteins in focal cerebral ischemia. Specifically, resveratrol attenuates the injury-induced decrease in expression of peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I and UCH-L1. However, resveratrol prevents an increase in expression of CRMP-2 in response to MCAO-induced injury. Peroxiredoxin-5 is an anti-oxidant protein. Isocitrate dehydrogenase [NAD+], apolipoprotein A-I and ubiquitin carboxy terminal hydrolase L1 are known to have neuroprotective effects. CRMP-2 is involved in protein oxidation and neuronal dysfunction. The results of this study show that resveratrol prevents the injury-induced decrease of peroxiredoxin-5, isocitrate dehydrogenase [NAD+], apolipoprotein A-I and ubiquitin carboxy terminal hydrolase L1 levels and these proteins are involved in oxidative stress and energy metabolism. In conclusion, these results provide evidence that resveratrol regulates expression of various proteins that associated with oxidative stress and energy metabolism in focal cerebral ischemia.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2007300).
REFERENCES
- 1.Alves-Rodrigues A., Gregori L., Figueiredo-Pereira M. E.1998. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 21: 516–520. doi: 10.1016/S0166-2236(98)01276-4 [DOI] [PubMed] [Google Scholar]
- 2.Barnes L. D., Kuehn G. D., Atkinson D. E.1971. Yeast diphosphopyridine nucleotide specific isocitrate dehydrogenase. Purification and some properties. Biochemistry 10: 3939–3944. doi: 10.1021/bi00797a022 [DOI] [PubMed] [Google Scholar]
- 3.Berrougui H., Grenier G., Loued S., Drouin G., Khalil A.2009. A new insight into resveratrol as an atheroprotective compound: inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis 207: 420–427. doi: 10.1016/j.atherosclerosis.2009.05.017 [DOI] [PubMed] [Google Scholar]
- 4.Burns J., Yokota T., Ashihara H., Lean M. E., Crozier A.2002. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 50: 3337–3340. doi: 10.1021/jf0112973 [DOI] [PubMed] [Google Scholar]
- 5.Cimini A., Gentile R., Angelucci F., Benedetti E., Pitari G., Giordano A., Ippoliti R.2013. Neuroprotective effects of PrxI over-expression in an in vitro human Alzheimer’s disease model. J. Cell. Biochem. 114: 708–715. doi: 10.1002/jcb.24412 [DOI] [PubMed] [Google Scholar]
- 6.Chen A., Liao W. P., Lu Q., Wong W. S., Wong P. T.2007. Upregulation of dihydropyrimidinase-related protein 2, spectrin alpha II chain, heat shock cognate protein 70 pseudogene 1 and tropomodulin 2 after focal cerebral ischemia in rats—a proteomics approach. Neurochem. Int. 50: 1078–1086. doi: 10.1016/j.neuint.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Chen X. M., Chen H. S., Xu M. J., Shen J. G.2013. Targeting reactive nitrogen species: a promising therapeutic strategy for cerebral ischemia-reperfusion injury. Acta Pharmacol. Sin. 34: 67–77. doi: 10.1038/aps.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J., Levey A. I., Weintraub S. T., Rees H. D., Gearing M., Chin L. S., Li L.2004. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J. Biol. Chem. 279: 13256–13264. doi: 10.1074/jbc.M314124200 [DOI] [PubMed] [Google Scholar]
- 9.de Vries H. E., Breedveld B., Kuiper J., de Boer A. G., Van Berkel T. J., Breimer D. D.1995. High-density lipoprotein and cerebral endothelial cells in vitro: interactions and transport. Biochem. Pharmacol. 50: 271–273. doi: 10.1016/0006-2952(95)00127-L [DOI] [PubMed] [Google Scholar]
- 10.Dong W., Li N., Gao D., Zhen H., Zhang X., Li F.2008. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J. Vasc. Surg. 48: 709–714. doi: 10.1016/j.jvs.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 11.Du L., Zhang X., Han Y. Y., Burke N. A., Kochanek P. M., Watkins S. C., Graham S. H., Carcillo J. A., Szabó C., Clark R. S.2003. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J. Biol. Chem. 278: 18426–18433. doi: 10.1074/jbc.M301295200 [DOI] [PubMed] [Google Scholar]
- 12.Gim S. A., Koh P. O.2014. Ferulic acid prevents the injury-induced decrease of γ-enolase expression in brain tissue and HT22 cells. Lab. Anim. Res. 30: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartong D. T., Dange M., McGee T. L., Berson E. L., Dryja T. P., Colman R. F.2008. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 40: 1230–1234. doi: 10.1038/ng.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin F., Wu Q., Lu Y. F., Gong Q. H., Shi J. S.2008. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 600: 78–82. doi: 10.1016/j.ejphar.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 15.Kairisalo M., Bonomo A., Hyrskyluoto A., Mudò G., Belluardo N., Korhonen L., Lindholm D.2011. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci. Lett. 488: 263–266. doi: 10.1016/j.neulet.2010.11.042 [DOI] [PubMed] [Google Scholar]
- 16.Keeney J. T., Swomley A. M., Förster S., Harris J. L., Sultana R., Butterfield D. A.2013. Apolipoprotein A-I: insights from redox proteomics for its role in neurodegeneration. Proteomics Clin. Appl. 7: 109–122. doi: 10.1002/prca.201200087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh P. O.2010. Proteomic analysis of focal cerebral ischemic injury in male rats. J. Vet. Med. Sci. 72: 181–185. doi: 10.1292/jvms.09-0364 [DOI] [PubMed] [Google Scholar]
- 18.Lewis T. L., Cao D., Lu H., Mans R. A., Su Y. R., Jungbauer L., Linton M. F., Fazio S., LaDu M. J., Li L.2010. Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J. Biol. Chem. 285: 36958–36968. doi: 10.1074/jbc.M110.127829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z., Pang L., Fang F., Zhang G., Zhang J., Xie M., Wang L.2012. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 1450: 116–124. doi: 10.1016/j.brainres.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 20.Lin J. F., Wu S., Huang S. S., Lu B. Y., Lin S. M., Tsai S. K.2011. Resveratrol protects left ventricle by increasing adenylate kinase and isocitrate dehydrogenase activities in rats with myocardial infarction. Chin. J. Physiol. 54: 406–412. [DOI] [PubMed] [Google Scholar]
- 21.Longa E. Z., Weinstein P. R., Carlson S., Cummins R.1989. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20: 84–91. doi: 10.1161/01.STR.20.1.84 [DOI] [PubMed] [Google Scholar]
- 22.Marambaud P., Zhao H., Davies P.2005. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 280: 37377–37382. doi: 10.1074/jbc.M508246200 [DOI] [PubMed] [Google Scholar]
- 23.O’Farrell P. H.1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250: 4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 24.Papa L., Akinyi L., Liu M. C., Pineda J. A., Tepas J. J., 3rd, Oli M. W., Zheng W., Robinson G., Robicsek S. A., Gabrielli A., Heaton S. C., Hannay H. J., Demery J. A., Brophy G. M., Layon J., Robertson C. S., Hayes R. L., Wang K. K.2010. Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38: 138–144. doi: 10.1097/CCM.0b013e3181b788ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paschen W.1996. Disturbances of calcium homeostasis within the endoplasmic reticulum may contribute to the development of ischemic-cell damage. Med. Hypotheses 47: 283–288. doi: 10.1016/S0306-9877(96)90068-7 [DOI] [PubMed] [Google Scholar]
- 26.Plaisant F., Clippe A., Vander Stricht D., Knoops B., Gressens P.2003. Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic. Biol. Med. 34: 862–872. doi: 10.1016/S0891-5849(02)01440-5 [DOI] [PubMed] [Google Scholar]
- 27.Quinn C. C., Gray G. E., Hockfield S.1999. A family of proteins implicated in axon guidance and outgrowth. J. Neurobiol. 41: 158–164. doi: [DOI] [PubMed] [Google Scholar]
- 28.Robb E. L., Winkelmolen L., Visanji N., Brotchie J., Stuart J. A.2008. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem. Biophys. Res. Commun. 372: 254–259. doi: 10.1016/j.bbrc.2008.05.028 [DOI] [PubMed] [Google Scholar]
- 29.Sakata Y., Zhuang H., Kwansa H., Koehler R. C., Doré S.2010. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp. Neurol. 224: 325–329. doi: 10.1016/j.expneurol.2010.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanderson T. H., Reynolds C. A., Kumar R., Przyklenk K., Hüttemann M.2013. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 47: 9–23. doi: 10.1007/s12035-012-8344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun J., Ying M., Li H., Shang X., He Y., Chen K., Cheng H., Zhou R.2008. Role of UCH-L1/ubiquitin in acute testicular ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 366: 539–544. doi: 10.1016/j.bbrc.2007.11.176 [DOI] [PubMed] [Google Scholar]
- 32.Sung J. H., Cho E. H., Cho J. H., Won C. K., Kim M. O., Koh P. O.2012. Identification of proteins regulated by ferulic acid in a middle cerebral artery occlusion animal model-a proteomics approach. J. Vet. Med. Sci. 74: 1401–1407. doi: 10.1292/jvms.12-0063 [DOI] [PubMed] [Google Scholar]
- 33.Yousuf S., Atif F., Ahmad M., Hoda N., Ishrat T., Khan B., Islam F.2009. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 1250: 242–253. doi: 10.1016/j.brainres.2008.10.068 [DOI] [PubMed] [Google Scholar]
- 34.Yuan J., Murrell G. A., Trickett A., Landtmeters M., Knoops B., Wang M. X.2004. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim. Biophys. Acta 1693: 37–45. doi: 10.1016/j.bbamcr.2004.04.006 [DOI] [PubMed] [Google Scholar]