ABSTRACT
In this study, we examined the antimicrobial susceptibility of the enterococci isolated from dogs and cats in Japan during 2011–2012. Fecal samples were collected from 84 dogs and 16 cats that underwent antibiotic treatment. Enterococci were detected in 70 of 84 dogs (83.3%) and 7 of 16 cats (43.8%). The most prevalent Enterococcus species was Enterococcus faecalis (64.9%); Enterococccus faecium and Enterococcus durans were also isolated from 14 of 77 (18.2%) and 5 of 77 (6.5%) of these animals, respectively. The most active resistance was observed for erythromycin (44.2%) and oxytetracycline (44.2%), and there was considerable resistance to lincomycin (41.6%), gentamicin (31.2%) and kanamycin (31.2%). Compared with the results of a similar study conducted in 2006 and 2007, enterococci susceptibility to enrofloxacin and ampicillin had significantly increased. Enterococcus gallinarum harboring vanC1 and Enterococcus casseliflavus harboring vanC2/3 were isolated from 4 of 77 enterococcal isolates. However, no enterococcal isolates were resistant to vancomycin. Multidrug resistance was found for as few as two and as many as nine antimicrobials regardless of the class. These results demonstrate that dogs and cats treated with antibiotics are commonly colonized with antimicrobial-resistant enterococci.
Keywords: antimicrobial susceptibility, companion animal, enterococci, multidrug resistance
Enterococci are a part of the normal microbial flora in the gastrointestinal tracts of humans, animals and birds. The major enterococcal species include Enterococcus faecalis, Enterococcus faecium and Enterococcus durans[1, 2, 15]. Enterococci do not cause illness in healthy humans or animals. However, they have recently been recognized as opportunistic nosocomial pathogens that cause infections of the urinary tract and central nervous system and lead to endocarditis and bacteremia [4, 10]. In addition, enterococci can rapidly acquire antimicrobial resistance through mutations or acquisition of plasmids and transposons that contain foreign genetic material, including vancomycin-resistance genes [7, 11]. Outbreaks of vancomycin-resistant enterococci (VRE) in humans may be associated with VRE in livestock, although the transmission mechanisms remain unclear.
Numerous reports have described the transmission of pathogenic and/or antimicrobial-resistant bacteria from companion animals to their owners [3, 18]. Therefore, monitoring drug-resistant and pathogenic bacteria in companion animals has become important for public health and veterinary medicine. However, few studies have reported the presence of enterococci in companion animals in Japan or elsewhere [13, 14, 16, 21].
We previously conducted an epidemiological survey of enterococci in companion dogs and cats in Japan [16]. In particular, several enterococci species that were resistant to ampicillin (ABPC) and enrofloxacin (ERFX) were detected in a group under antibiotic selective pressure, while VRE harboring vanA or vanB were not detected. Subsequently, we surveyed the antimicrobial susceptibility of enterococci in antibiotic-treated dogs and cats. The objective of this study was to examine the antimicrobial susceptibility of enterococci isolated from dogs and cats during 2011–2012.
Fecal swabs were collected from 84 dogs and 16 cats (>2 years old) that had been treated with antibiotics (penicillins, 13/100; cephalosporins, 53/100; aminoglycosides, 4/100; quinolones, 31/100; and macrolides, 2/100) at the Nippon Veterinary and Life Science University Animal Medical Center from 2011 to 2012. All the samples were plated on Enterococcosel agar (Becton, Dickinson and Co., Tokyo, Japan) and were incubated aerobically at 37°C for 48 hr. For each plate, 1 colony with the morphological characteristics of enterococci (i.e., dark brown halo) was initially tested by Gram staining, growth in 6.5% NaCl broth and bile esculin hydrolysis. The identities of these enterococci were then confirmed using API STREP 20 (bioMerieux Japan Ltd., Tokyo, Japan) and polymerase chain reaction (PCR) [16].
Minimum inhibitory concentrations (MICs) were determined using a microdilution test according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement. Villanova, PA: CLSI; 2005 Publication No. M100-S15). The following 10 antibiotics were tested: ABPC, dihydrostreptomycin (DSM), gentamicin (GM), kanamycin (KM), erythromycin (EM), lincomycin (LCM), oxytetracycline (OTC), chloramphenicol (CP), ERFX and vancomycin (VCM). Two-fold dilutions of each antibiotic were prepared to obtain final concentrations from 512 mg/l to 0.125 mg/l. MIC breakpoints were set according to CLSI guidelines. Isolates were considered resistant when MICs for ABPC, DSM, GM, KM, EM, LCM, OTC, CP, ERFX and VCM were greater than or equal to 16, 128, 32, 128, 8, 128, 16, 32, 4 and 32 mg/l, respectively. E. faecalis ATCC 29212 was used as a control microorganism for each set of tests.
Presence of the resistance genes vanA, vanB, vanC1 and vanC2/C3 was examined for all enterococcal strains by PCR, as described by Clark et al. [6], Dutka-Malen et al. [9] and Satake et al. [19], with previously described modifications [16]. PCR products were analyzed by agarose gel electrophoresis. E. faecium ATCC 51559 (vanA- harboring reference strain), E. faecalis ATCC 51299 (vanB), Enterococcus gallinarum ATCC 49573 (vanC1) and Enterococcus casseliflavus ATCC 25788 (vanC2/3) were used as VRE controls.
Enterococci were isolated from 70 of 84 dogs (83.3%) and 7 of 16 cats (43.8%) (Table 1). The distribution of species among the 77 enterococcal isolates was as follows: E. faecalis (50/77; 64.9%), E. faecium (14/77; 18.2%) and E. durans (5/77; 6.5%). The remaining two strains were not identified with the API STREP 20 system. In most previous studies on enterococci from dogs and cats, E. faecalis and E. faecium were the predominant species [19, 20].
Table 1. Enterococcus species isolated from dogs and cats.
| Bacterial species | Dogs | Cats | Totals | Type of van gene |
|---|---|---|---|---|
| Enterococcus faecalis | 46 | 4 | 50 (64.9%) | |
| E. faecium | 11 | 3 | 14 (18.2%) | |
| E. gallinarum | 1 | 0 | 1 (1.3%) | VanC1 |
| E. casseliflavus | 3 | 0 | 3 (3.9%) | VanC2/3 |
| E. avium | 2 | 0 | 2 (2.6%) | |
| E. durans | 5 | 0 | 5 (6.5%) | |
| Others | 2 | 0 | 2 (2.6%) | |
| Totals | 70 | 7 | 77 | |
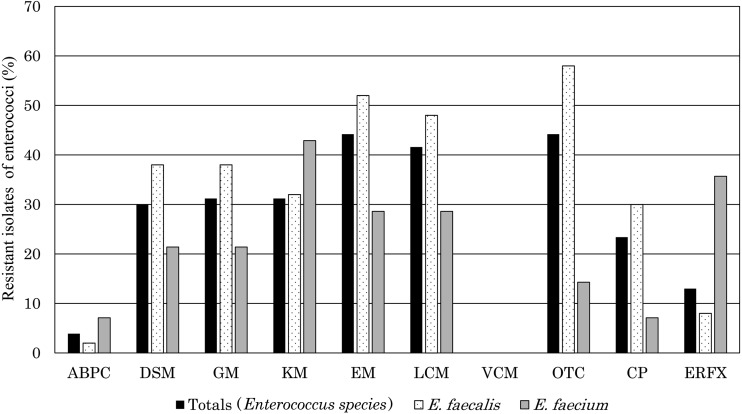
Antibiotic susceptibility results are summarized in Fig. 1. Resistance to EM and OTC was detected in 44.2% of the 77 enterococci isolates. Resistance to GM and KM was detected in>30% of isolates. In contrast, resistance to ABPC and ERFX was detected in 3.9% and 13.0% of all isolates, respectively. E. faecium isolates exhibited higher resistance to KM and ERFX than E. faecalis. Compared with a previous survey [16], resistance to DSM, GM, EM and CP was not different in this study; however, significant changes were observed in resistance to ABPC and ERFX. Although it was uncertain why the susceptibility to ABPC and ERFX was restored, it was possible that increased use of recently marketed third-generation cephalosporins for small animals accounted for the reduced occurrence of resistance to quinolones and penicillins.
Fig 1.
Antibiotic resistant isolates of enterococci isolated from dogs and cats.
Delgado et al. reported on the antimicrobial susceptibility patterns among veterinary clinical enterococci isolated from dogs and cats [8]. In their results, susceptibility was high to ABPC (95.5%), and resistance was high to TC (67.3%) and EM (50.9%). Cinquepalmi et al. reported that enterococci isolated from dog feces were resistant to TC (65.7%), EM (60.3%), ABPC (47.9%) and levofloxacin (23.3%) [5]. Thus, our results were in agreement with their results that many of the enterococci isolated from companion animals were resistant to TC and EM. We found no resistance to VCM, which was consistent with a number of studies on enterococci from dogs and cats [8, 17, 20].
The resistance patterns of 77 enterococcal isolates are shown in Table 2. Eighteen (23.4%) isolates were susceptible to all antibiotics tested. A total of 15 strains (19.5%) exhibited resistance to a single antimicrobial agent, and multidrug resistance (MDR; resistance to ≥2 antimicrobial agents) was detected in 44 of 77 isolates (57.1%). In particular, MDR of isolates from dogs (39 of 70; 50.6%) was remarkable. Poeta et al. reported similar results that susceptible strains to all tested antibiotics were isolated from 31.0% of dogs and cats in Portugal [19]. However, they also reported that the proportion of enterococci isolated from poultry that is susceptible to antimicrobial agents is extremely low (0.7%). Compared with the susceptibility of enterococci from poultry, it appears that the enterococci from dogs and cats are more susceptible to antibiotics.
Table 2. Antibiotic-resistant patterns of enterococci from dogs and cats.
| Number of antibiotics to which resistance was shown |
Antibiotic resistance pattern | Number of strains |
Species | Number of animals | |
|---|---|---|---|---|---|
| Dogs | Cats | ||||
| 9 | ABPC, DSM, GM, KM, EM, LCM, OTC, CP, ERFX | 1 | E. gallinarum | 1 | |
| 8 | ABPC, DSM, GM, KM, EM, LCM, CP, ERFX | 1 | E. faecium | 1 | |
| 7 | DSM, GM, KM, EM, LCM, OTC, CP | 2 | E. faecalis | 2 | |
| GM, KM, EM, LCM, OTC, CP, ERFX | 1 | E. faecalis | 1 | ||
| 6 | DSM, GM, KM, EM, LCM, OTC | 3 | E. faecalis | 3 | |
| DSM, KM, EM, LCM, OTC, CP | 1 | E. faecalis | 1 | ||
| DSM, KM, EM, LCM, OTC, ERFX | 2 | E. faecalis | 1 | ||
| E. faecium | 1 | ||||
| GM, KM, EM, LCM, OTC, CP | 1 | E. faecalis | 1 | ||
| 5 | DSM, EM, LCM, OTC, CP | 2 | E. faecalis | 2 | |
| GM, KM, EM, LCM, CP | 1 | E. durans | 1 | ||
| GM, KM, EM, LCM, OTC | 1 | E. faecalis | 1 | ||
| GM, EM, LCM, OTC, CP | 1 | E. faecalis | 1 | ||
| 4 | ABPC, EM, LCM, OTC | 1 | E. faecalis | 1 | |
| DSM, EM, LCM, CP | 2 | E. faecalis | 2 | ||
| DSM, EM, OTC, CP | 1 | E. faecalis | 1 | ||
| GM, EM, LCM, OTC | 1 | E. faecalis | 1 | ||
| EM, LCM, OTC, CP | 1 | E. faecalis | 1 | ||
| 3 | DSM, GM, LCM | 1 | E. faecalis | 1 | |
| DSM, GM, OTC | 2 | E. faecalis | 2 | ||
| DSM, GM, ERFX | 1 | E. faecalis | 1 | ||
| DSM, KM, OTC | 1 | E. faecalis | 1 | ||
| DSM, EM, CP | 1 | E. faecalis | 1 | ||
| DSM, EM, LCM | 1 | E. faecium | 1 | ||
| DSM, OTC, CP | 1 | E. faecalis | 1 | ||
| GM, KM, OTC | 1 | E. faecalis | 1 | ||
| KM, EM, LCM | 2 | E. faecalis | 2 | ||
| KM, OTC, ERFX | 1 | E. faecium | 1 | ||
| EM, LCM, OTC | 3 | E. faecalis | 1 | 1 | |
| E.casseliflavus | 1 | ||||
| EM, LCM, CP | 1 | E. faecalis | 1 | ||
| 2 | GM, KM | 1 | E. faecalis | 1 | |
| KM, ERFX | 1 | E. faecium | 1 | ||
| EM, LCM | 2 | E. faecium | 1 | ||
| E. avium | 1 | ||||
| EM, OTC | 1 | E. faecalis | 1 | ||
| 1 | GM | 5 | E. faecalis | 3 | |
| E. faecium | 2 | ||||
| KM | 3 | E. faecalis | 1 | ||
| E. faecium | 2 | ||||
| OTC | 5 | E. faecalis | 4 | ||
| E. durans | 1 | ||||
| ERFX | 2 | E. faecalis | 1 | ||
| E. faecium | 1 | ||||
| 0 | Susceptive | 18 | E. faecalis | 6 | 1 |
| E. faecium | 2 | 1 | |||
| E.casseliflavus | 2 | ||||
| E. durans | 3 | ||||
| E. avium | 1 | ||||
| Others | 2 | ||||
| Totals | 77 | 70 | 7 | ||
One strain of E. gallinarum harboring vanC1 and 3 strains of E. casseliflavus harboring vanC2/3 were found among our isolates (Table 1). In our previous study, enterococci that harbored vanC1 and VanC2/3 genes were isolated from only 1 of 29 (3.4%) antibiotic-treated dogs and cats [16]. These results demonstrated that VRE were rarely colonized in dogs and cats.
In conclusion, comparing the results of the present study with those of a previous study revealed a difference in the frequency of antimicrobial resistance among enterococci isolated from antibiotic-treated companion animals. This might reflect a change in the use of antimicrobials for companion animals [12]. Surveillance studies should be continued to detect any changes in antimicrobial resistance of the normal flora of the intestinal tracts of companion animals. Our follow-up studies will address the presence of antimicrobial resistance genes harbored by resistant isolates.
REFERENCES
- 1.Akhter S., Asna Z. H., Rahman M. M.2011. Prevalence and antimicrobial susceptibility of enterococcus species isolated from clinical specimens. Mymensingh Med. J. 20: 694–699. [PubMed] [Google Scholar]
- 2.Anbumani N., Menon T., Kalyani J., Mallika M.2005. Isolation, distribution and prevalence of various species of enterococci isolated from clinical specimens in a tertiary care hospital. Indian J. Pathol. Microbiol. 48: 534–537. [PubMed] [Google Scholar]
- 3.Buma R., Maeda T., Kamei M., Kourai H.2006. Pathogenic bacteria carried by companion animals and their susceptibility to antibacterial agents. Biocontrol Sci. 11: 1–9. doi: 10.4265/bio.11.1 [DOI] [PubMed] [Google Scholar]
- 4.Byappanahalli M. N., Nevers M. B., Korajkic A., Staley Z. R., Harwood V. J.2012. Enterococci in the environment. Microbiol. Mol. Biol. Rev. 76: 685–706. doi: 10.1128/MMBR.00023-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinquepalmi V., Monno R., Fumarola L., Ventrella G., Calia C., Greco M. F., de Vito D., Soleo L.2013. Environmental contamination by dog’s faeces: A public health problem? Int. J. Environ. Res. Public Health 10: 72–84. doi: 10.3390/ijerph10010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark N. C., Cooksey R. C., Hill B. C., Swenson J. M., Tenover F. C.1993. Characterization of glycopeptide-resistant enterococci from U. S. hospitals. Antimicrob. Agents Chemother. 37: 2311–2317. doi: 10.1128/AAC.37.11.2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Costa P. M., Loureiro L., Matos A. J.2013. Transfer of multidrug-resistant bacteria between intermingled ecological niches: the interface between humans, animals and the environment. Int. J. Environ. Res. Public Health 10: 278–294. doi: 10.3390/ijerph10010278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado M., Neto I., Correia J. H., Pomba C.2007. Antimicrobial resistance and evaluation of susceptibility testing among pathogenic enterococci isolated from dogs and cats. Int. J. Antimicrob. Agents 30: 98–100. doi: 10.1016/j.ijantimicag.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Dutka-Malen S., Evers S., Courvalin P.1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol.33: 24–27. Erratum 33: 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher K., Phillips C.2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology (Reading, Engl.) 155: 1749–1757. doi: 10.1099/mic.0.026385-0 [DOI] [PubMed] [Google Scholar]
- 11.Gilmore M. S., Lebreton F., van Schaik W.2013. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr. Opin. Microbiol. 16: 10–16. doi: 10.1016/j.mib.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagman R., Greko C.2005. Antimicrobial resistance in Escherichia coli isolated from bitches with pyometra and from urine samples from other dogs. Vet. Rec. 157: 193–196. [DOI] [PubMed] [Google Scholar]
- 13.Harada T., Tsuji N., Otsuki K., Murase T.2005. Detection of the esp gene in high-level gentamicin resistant Enterococcus faecalis strains from pet animals in Japan. Vet. Microbiol. 106: 139–143. doi: 10.1016/j.vetmic.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Herrero I. A., Fernandez-Garayzabal J. F., Moreno M. A., Domínguez L.2004. Dog should be included in surveillance programs for vancomycin-resistant enterococci. J. Clin. Microbiol. 42: 1384–1385. doi: 10.1128/JCM.42.3.1384-1385.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh S. R.2000. Antimicrobial susceptibility and species identification for clinical isolates of enterococci. J. Microbiol. Immunol. Infect. 33: 253–257. [PubMed] [Google Scholar]
- 16.Kataoka Y., Ito C., Kawashima A., Ishii M., Yamashiro S., Harada K., Ochi H., Sawada T.2013. Identification and antimicrobial susceptibility of enterococci isolated from dogs and cats subjected to differing antibiotic pressures. J. Vet. Med. Sci. 75: 749–753. doi: 10.1292/jvms.12-0243 [DOI] [PubMed] [Google Scholar]
- 17.Leener E. D., Decostere A., De Graef E. M., Moyaert H., Haesebrouck F.2005. Presence and mechanism of antimicrobial resistance among enterococci from cats and dogs. Microb. Drug Resist. 11: 395–403. doi: 10.1089/mdr.2005.11.395 [DOI] [PubMed] [Google Scholar]
- 18.Lloyd D. H.2007. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 45: 148–152. doi: 10.1086/519254 [DOI] [PubMed] [Google Scholar]
- 19.Poeta P., Costa D., Rodrigues J., Torres C.2006. Antimicrobial resistance and the mechanisms implicated in faecal enterococci from healthy humans, poultry and pets in Portugal. Int. J. Antimicrob. Agents 27: 131–137. doi: 10.1016/j.ijantimicag.2005.09.018 [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues J., Poeta P., Martins A., Costa D.2002. The importance of pets as reservoirs of resistant Enterococcus strains, with special reference to vancomycin. J. Vet. Med. B Infect. Dis. Vet. Public Health 49: 278–280. doi: 10.1046/j.1439-0450.2002.00561.x [DOI] [PubMed] [Google Scholar]
- 21.Satake S., Clark N., Rimland D., Nolte F. S., Tenover F. C.1997. Detection of vancomycin-resistant enterococci in fecal samples by PCR. J. Clin. Microbiol. 35: 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]