Abstract
Background
Thrombotic microangiopathy (TMA) in the kidney is a histopathologic lesion that occurs in a number of clinical settings and is often associated with poor renal prognosis. The standard test for the diagnosis of TMA is the renal biopsy; noninvasive parameters such as potential biomarkers have not been developed.
Methods
We analyzed routine parameters in a cohort of 220 patients with suspected TMA and developed a diagnostic laboratory panel by logistic regression. The levels of candidate markers were validated using an independent cohort (n = 46), a cohort of systemic lupus erythematosus (SLE) (n = 157) and an expanded cohort (n = 113), as well as 9 patients with repeat biopsies.
Results
Of the 220 patients in the derivation cohort, 51 patients with biopsy-proven TMA presented with a worse renal prognosis than those with no TMA (P = 0.002). Platelet and L-lactate dehydrogenase (LDH) levels showed an acceptable diagnostic value of TMA (AUC = 0.739 and 0.756, respectively). A panel of 4 variables - creatinine, platelets, ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats 13) activity and LDH - can effectively discriminate patients with TMA (AUC = 0.800). In the validation cohort, the platelet and LDH levels and the 4-variable panel signature robustly distinguished patients with TMA. The discrimination effects of these three markers were confirmed in patients with SLE. Moreover, LDH levels and the 4-variable panel signature also showed discrimination values in an expanded set. Among patients undergoing repeat biopsy, increased LDH levels and panel signatures were associated with TMA status when paired evaluations were performed. Importantly, only the 4-variable panel was an independent prognostic marker for renal outcome (hazard ratio = 3.549; P<0.001).
Conclusions
The noninvasive laboratory diagnostic panel is better for the early detection and prognosis of TMA compared with a single parameter, and may provide a promising biomarker for clinical application.
Introduction
Thrombotic microangiopathy (TMA) is a pathological lesion that results in thrombosis in capillaries and arterioles, due to an endothelial injury [1]–[3]. TMA lesions in the kidney usually present in two different forms with considerable overlap: (1) glomerular involvement with capillary thrombi, capillary loops with double contours and mesangiolysis with microhemorrhage, that is most frequently seen in patients with hemolytic uremic syndrome; or (2) predominant arteriolar involvement with thrombi and fibrinoid necrosis, particularly in thrombotic thrombocytopenic purpura and malignant hypertension [4], [5].
TMA lesions occur in a number of other kidney diseases as well, including IgA nephropathy, systemic lupus erythematosus (SLE), antiphospholipid antibody syndrome, systemic sclerosis, preeclampsia, infections, medications and post transplantation [2], [6], [7]. The presence of TMA in the kidney has been proven to be associated with poor renal prognosis [8], [9]. Nephrologists often face a typical situation in which patients are suspected of having TMA lesions on the basis of renal disorders. In such cases, it is difficult to decide on an early therapy before the results of a renal biopsy are obtained [10]. While the interpretation of the renal biopsy has become more standardized and the invasive procedure safer over time, bleeding and subsequent functional impairment nevertheless still occur, especially in the patients with coagulopathy [11].
Heterogeneous disorders with TMA are usually characterized by microangiopathic hemolytic anemia (MAHA) [12], thrombocytopenia and/or ischemic organ failure. It is questionable as to whether these abnormalities can predict TMA, since they are also observed in patients without TMA, and studies have been inconsistent [13], [14]. Hence, we enrolled cohorts of patients with renal damage and MAHA and/or thrombocytopenia to determine whether a single parameter or a diagnostic panel could predict the histological TMA. In addition, the association between the noninvasive prediction and a poor renal outcome was further investigated.
Methods
Ethics statement
The study was approved by the Ethics Committee of Jinling Hospital. Because this study was retrospective, the Ethics Committee agreed to waive the requirement for the informed consent, and the data were analyzed anonymously.
Patient selection and study design
We enrolled a cohort of suspected patients from the Research Institute of Nephrology, Jinling Hospital, Nanjing University School of Medicine, PR China during July 2011 to July 2012 (n = 266). The enrollment criteria were: renal damage (proteinuria, hematuria or renal dysfunction) and microangiopathic hemolytic anemia (hemoglobin level below 120 g/L for males, 110 g/L for females, at least 5 schistocytes per high power field in a peripheral blood smear, elevated LDH above 240 u/L [normal 60–240 u/L]) or thrombocytopenia (platelet counts below 100×109/L of blood at any time in the course of the disease) [15]. Patients were randomly divided into two cohorts: (1) derivation cohort (n = 220) and (2) validation cohort (n = 46).
In addition, 157 patients diagnosed with SLE were selected from the suspected patients, and 113 patients were enrolled in an expanded group for external validation. SLE was defined according to the 1997 American College of Rheumatology revised criteria for SLE [16]. The independent patients were enrolled in the expanded group according to the criteria: (1) renal damage (proteinuria, hematuria or renal dysfunction) and (2) fever (temperature of >38°C, no infection), elevated LDH (more than 240 u/L) or non-renal anemia. The information and profiles of patients during the follow-up sessions were also reviewed through October 2013.
Definitions
TMA in the kidney was defined by the histologic feature of occlusive fibrin-platelet thrombi in at least one glomerulus or one arteriole, with one or more of the following: (1) glomerular endothelial swelling and detachment, capillary wall thickening and double contour formation, mesangial lysis with microhemorrhage, and erythrocytolysis, and/or (2) obliterative arteriolopathy defined as luminal occlusion with mural myxoid or fibrinoid change, thickening of the vessel wall, with or without erythrocytolysis, luminal thrombosis and concentric spindle cell proliferation or hypercellularity [2], [5].
All patients accepted adjunctive treatments, including protecting the organ function, symptomatic and immunosuppressive treatment. Glomerularfiltration rate was estimated (eGFR) using the simplified MDRD (Modification of Diet in Renal Disease) formula. End stage renal disease (ESRD) was defined as eGFR<15 mL/min/1.73 m2 or a need for permanent dialysis therapy. During follow-up, the combined event defined as ESRD or a doubling of the serum creatinine level and death. The renal survival rate was defined as the percentage of patients who had preservation of renal function independent of ESRD and death, while the survival rate was defined as the percentage of live patients.
Laboratory features
Physical exams and routine laboratory tests were performed on the suspected patients. Serum ADAMTS13 activity was measured by the Fluorescence Resonance Energy Transfer (FRET) assay (United States Patent No.7270976) [17]. Serum anti-ADAMTS13 IgG antibodies (Sekisui Diagnostics, USA), vWF (von Willebrand factor) (Sunbiote, Shanghai, China), thrombomodulin (Diaclone Research, Besancon, France), E-selectin (R&D Systems, Minneapolis, Minnesota, USA) and soluble vascular cellular adhesion molecule-1 (sVCAM-1, VCAM) (R&D Systems, Minneapolis, Minnesota, USA) were measured using enzyme linked immunosorbent assays. The concentrations of endothelial cells in the circulation were sorted using a magnetic microbead sorting system (MACS, Miltenyi Biotec, Germany), according to the manufacturer’s instructions [18].
Statistical analysis
All data were given a numerical code and statistical analysis was performed using SPSS software, version 19.0 or RMS software, version 2.12.2. Comparisons of proportions or mean values between patients with or without TMA in the kidney were calculated by the Mann-Whitney test or chi-square (χ2) test.
Logistic regression was used to identify 6 parameters - hemoglobin, platelets, serum creatinine, LDH, ADAMTS13 activity and THBD - that discriminated between patients with and without TMA in the kidney. Regression estimated from this panel defined a diagnostic signature or individual predictors, and the greatest area under the receiver-operating-characteristic (ROC) curve as the best-fitting model was used. The area under the curve (AUC) was calculated, and sensitivity and specificity were used to evaluate the ability of these to discriminate TMA in the kidney. The best sub-set panel was then fit to 1000 additional bootstrap samples. Cross-validated measures of discrimination (i.e., the AUC), model fit (i.e., calibration-curve intercept and slope) and a locally estimated scatterplot-smoothed (loess) calibration plot were obtained [19].
Cumulative incidence of poor renal outcome was calculated using Kaplan-Meier survival probabilities (1- survival probabilities) and comparisons were made using the log-rank test. Cox regression was performed to test the association of the pathologic findings and the 4-variable panel signature with the renal outcome. A two-tailed P value less than 0.05 is considered to be statistically significant.
Results
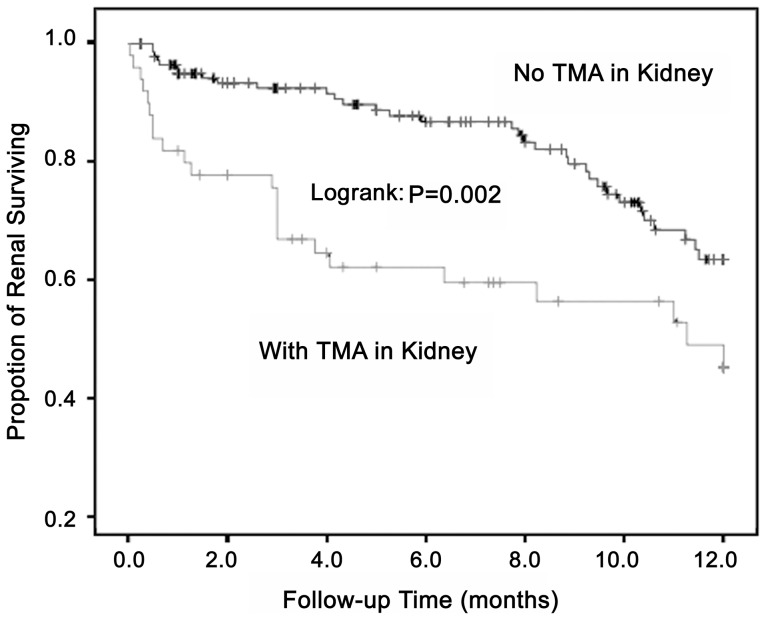
In the derivation cohort of 220 patients, 51 (23.2%) patients were histologically proven to have TMA by renal biopsy. Compared with patients without TMA, the patients with TMA presented a worse renal outcome (P = 0.002, log-rank test; Figure 1) at the 12-month follow-up, with a renal survival rate of 54.9% (P = 0.015; Table 1).
Figure 1. Comparison of renal outcome in suspected patients.
After 12 months of follow-up, the patients with TMA had a worse outcome of renal survival than those without TMA (Log rank: P = 0.002).
Table 1. Clinical laboratory data for suspected patients in derivation cohort (n = 220).
| TMA | No TMA | P Value | |
| Number of patients | 51 | 169 | |
| Age (year) | 26 (963) | 28 (1370) | 0.450 |
| Male (n, %) | 10 (19.6) | 45 (26.6) | 0.310 |
| Clinical diagnosis | |||
| HUS/TTP (n, %) | 15 (29.4) | 0 (0) | <0.001 |
| Primary glomerulonephritis & acute interstitial nephritis (n, %) | 0 (0) | 22 (13.0) | 0.003 |
| Autoimmune diseases (n, %) | 29 (56.9) | 144 (85.2) | <0.001 |
| SLE (n, %) | 27/29 (93.1) | 130/144 (90.3) | 1.000 |
| Pregnancy/postpartum (n, %) | 5 (9.8) | 1 (0.6) | 0.003 |
| Malignant hypertension (n, %) | 1 (2.0) | 0 (0) | 0.232 |
| Post-transplantation (n, %) | 1 (2.0) | 2 (1.2) | 0.549 |
| Laboratory profiles | |||
| Urine protein (g/24 h) | 1.5 (0.310.6) | 2.5 (0.29.8) | 0.073 |
| Erythrocyturia (×104/mL) | 18 (110000) | 24 (19000) | 0.364 |
| Hemoglobin (g/L) | 74.2±16.1 | 87.1±21.6 | <0.001 |
| Platelets (×109/L) | 73 (10275) | 108 (34441) | <0.001 |
| Serum creatinine (umol/L) | 347.2 (52.21697.5) | 162.7 (33.61556.0) | <0.001 |
| Globulin (g/L) | 22.0±5.7 | 23.3±6.6 | 0.289 |
| C3 (g/L) | 0.62±0.29 | 0.57±0.30 | 0.219 |
| C4 (g/L) | 0.16±0.10 | 0.14±0.10 | 0.098 |
| L-lactate dehydrogenase (u/L) | 418 (1292920) | 267 (64878) | <0.001 |
| ADAMTS13 antibody (Au/mL) | 16.6 (2.184.9) | 16.6 (1.681.3) | 0.862 |
| ADAMTS13 activity (ng/mL) | 616±331 | 772±255 | 0.001 |
| NEC (/ml) | 19.7±6.8 | 20.7±8.9 | 0.654 |
| E-selectin (ng/mL) | 65.2±37.3 | 73.1±72.8 | 0.636 |
| VCAM (ng/mL) | 2011±1070 | 2460±1340 | 0.073 |
| Thrombomodulin (ng/mL) | 6.3±4.3 | 4.4±2.8 | <0.001 |
| vWF (%) | 184±127 | 215±120 | 0.080 |
| After 12-months of follow up | |||
| Renal survival rate (%) | 54.9 (28/51) | 74.6 (126/169) | 0.015 |
| Survival rate (%) | 94.1 (48/51) | 98.2 (166/169) | 0.138 |
Values are expressed as medians (range), means ± standard deviation or percentages. P values were calculated by Mann-Whitney U test, Fisher’s exact test or chi-square test as appropriate. HUS: hemolytic uremic syndrome; TTP: thrombotic thrombocytopenic purpura; SLE: systemic lupus erythematosus; C3: Complement component 3; C4: Complement component 4; ADAMTS13: A Disintegrin and Metalloprotease with ThromboSpondin type 1 repeats 13; NEC: normal endothelial cells; VCAM: vascular cell adhesion molecule; vWF: von Willebrand factor.
Correlation between laboratory parameters and TMA lesions
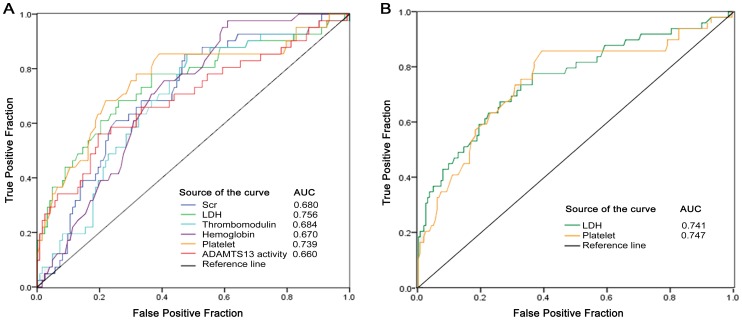
The levels of serum creatinine, LDH and thrombomodulin (THBD) were significantly higher in patients with TMA than those without TMA, while levels of hemoglobin, platelets and ADAMTS13 activity were remarkably lower (all P<0.05; Table 1). With a cutoff of 0.05 (P value, in Table 1), 6 laboratory parameters were included in the next binary logistic regression analysis. The ROC curve showed that these 6 parameters individually can distinguish patients with TMA in the derivation set (all P<0.05; Figure 2A). Only the platelet and LDH levels showed acceptable discrimination values (0.7≤AUC<0.8), while levels of hemoglobin, serum creatinine, ADAMSTS13 activity and THBD had low discrimination accuracy (0.5≤AUC<0.7). With the use of the cutoff point of 97.5×109/L, platelets had 82.4% sensitivity and 60.9% specificity, and with the cutoff point of 289 u/L, LDH had 67.3% sensitivity and 74.1% specificity (Table S1 in File S1).
Figure 2. Receiver operating characteristic curves of laboratory parameters.
(A) The fraction of true positive results (sensitivity) and the fraction of false positive results (1-specificity) for LDH, HGB, SCr, PLT, THBD and ADAMTS13 activity were developed in 220 patients (all P≤0.001), and the levels of platelet and LDH showed acceptable discrimination, with AUC 0.739 and 0.756, respectively. (B) The levels of platelet and LDH could discriminate patients with TMA from those with no TMA in the validation cohort (n = 46), with AUC 0.747 and 0.741, respectively.
In an independent validation cohort, platelet and LDH levels also could discriminate patients with TMA from those without TMA (Figure 2B; Table S2 in File S1). Thus, the levels of platelets and LDH showed acceptable predictive probabilities of patients with TMA in the kidney.
Correlation between the 4-variable panel and TMA lesions
Furthermore, multiple logistic regression analysis in the derivation set indicated that levels of serum creatinine (SCr), platelets (PLT), LDH and ADAMTS13 activity were valid predictors of renal TMA lesions (all P<0.05; Table 2), with the final panel signature:
Table 2. Results of logistic regression analysis (n = 220).
| Variable | B | S.E | Sig. | Exp (B) | 95% CI |
| Lower-Upper | |||||
| Serum creatinine | 0.140 | 0.060 | 0.020 | 1.150 | 1.022–1.295 |
| LDH | 0.004 | 0.001 | 0.002 | 1.004 | 1.001–1.006 |
| Platelet | −0.010 | 0.004 | 0.021 | 0.990 | 0.981–0.998 |
| ADAMTS 13 Activity | −0.002 | 0.001 | 0.014 | 0.998 | 0.996–1.000 |
Abbreviations: B: coefficient of regression; S.E.: Standard Error; Sig: P value; Exp (B): odds ratio; CI: confidence interval. LDH: L-lactate dehydrogenase; ADAMTS 13: a disintegrin and metalloprotease with thrombospondin type 1 repeats 13.
The units of measurement are as listed in Table 1. In the equation, −0.371 was the intercept, and −0.002, 0.140, 0.004 and −0.010 were the slopes (coefficients), respectively, for the ADAMTS13 activity, SCr (mg/dL), LDH and PLT values in the best-fitting logistic-regression model. The intercept and slopes have no intrinsic units of measurement.
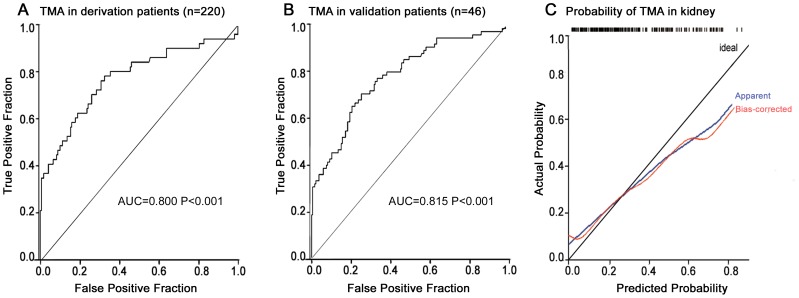
The ROC curve showed that this 4-variable model yielded an AUC of 0.800 (95% confidence interval [CI], 0.723 to 0.877; P<0.001), suggesting a good discrimination between patients with TMA in the kidney and those without TMA (Figure 3A). With the use of the cutoff point of 0.248, this diagnostic panel has 81.6% sensitivity and 66.9% specificity. The diagnostic panel for the prediction of TMA was validated in the independent cohort, with an AUC of 0.815 (P<0.001; Figure 3B). The use of the cut-off of 0.248 predicted the presence of TMA with 80.0% sensitivity and 61.5% specificity.
Figure 3. Receiver operating characteristic curves and calibration curve for a diagnostic panel.
(A) The diagnostic panel was developed in the derivation group of 220 suspected patients, with AUC 0.800, P<0.001. (B) This marker was validated in 46 independent patients, with AUC 0.815, P<0.001. (C) Bootstrap validation shows the calibration curve of the diagnostic panel. Cross-validated estimates of the AUC, calibration-curve intercept and slope were 0.777, 0.07 and 0.64, respectively. The loess-smoothed estimates of the cross-validated and unadjusted calibration curves are overlaid on a diagonal reference line representing good model calibration.
Bootstrap validation of this 4-variable panel yielded a cross-validated estimate of the AUC of 0.777, which is an estimate of the expected value of the AUC in the combined derivation and validation cohorts. The calibration-curve intercept and slope were 0.07 and 0.64, respectively. It was revealed that the predicted probabilities of a biopsy showing TMA in the kidney tended to be relatively higher than the actual probabilities (Figure 3C). The loess-smoothed estimates of the unadjusted and cross-validated calibration curves were overlaid on a diagonal reference line representing good model calibration (P = 0.489). The close correspondence of the two curves to the reference line shows good fit and reflects the above interpretation of the intercept and slope estimates of the calibration curve (Figure 3C).
Evaluation of LDH, platelet and the 4- variable panel in extra cohorts
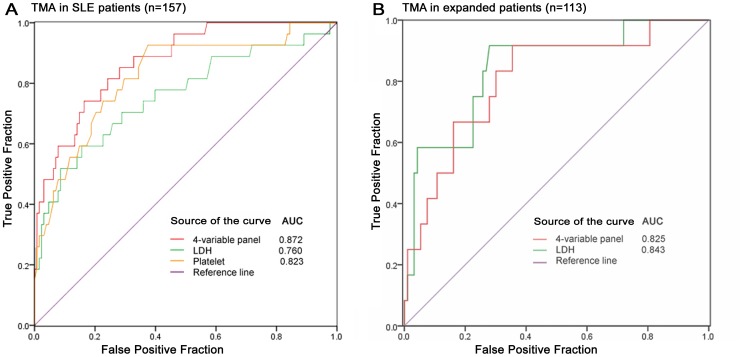
Since renal TMA lesions occur in a number of kidney diseases, we focused on a specific condition and 157 renal patients diagnosed with SLE were selected in extra validation (Table S3 in File S1). All levels of platelets, LDH and the 4-variable panel could discriminate patients with TMA in SLE. The ability of discrimination in the 4-variable panel was higher than the levels of platelets and LDH, with an AUC of 0.872 (Figure 4A). With the use of the cutoff point of 0.248, the diagnostic panel has 74.1% sensitivity and 83.6% specificity.
Figure 4. Evaluation of LDH, platelet and 4-variable panel in extra cohorts.
(A) All levels of platelet, LDH and the 4-variable panel were evaluated in 157 suspected patients diagnosed with SLE and could discriminate patients with TMA, with an AUC of 0.823, 0.76 and 0.872, respectively. (B) To further validate in another independent group of 113 patients and ROC curve analysis showed that the levels of LDH and the 4-variable panel yielded an AUC of 0.843 and 0.825, showing an good discrimination of TMA (both P<0.001).
To further enhance the application of these markers, we enrolled another independent group of 113 patients and determined the association between the levels of LDH, platelets and the 4-variable panel and TMA lesions (Table S4 in File S1). ROC curve analysis showed that the levels of LDH and the 4-variable panel yielded an AUC of 0.843 and 0.825, showing a good discrimination of TMA (both P<0.001; Figure 4B). However, platelet levels could not predict patients with TMA lesions in this cohort (P = 0.068).
Evaluation of LDH and the 4-variable panel in patients with repeat biopsy
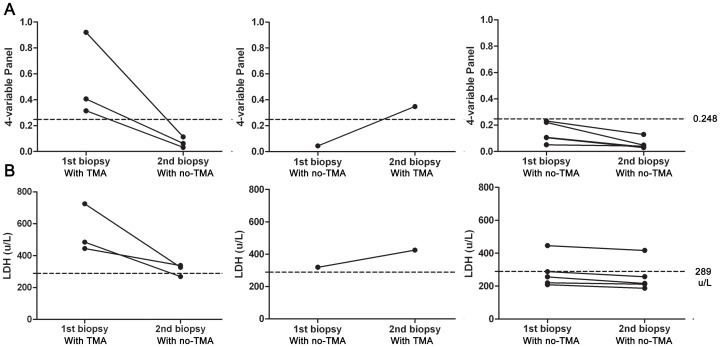
We then analyzed, in a subset of 9 patients who underwent repeat renal biopsy, levels of LDH and the 4-variable panel in paired blood samples taken at the time of the first and second biopsy (Table S5 in File S1). It is interesting to note that the 4-variable panel signature plummeted from 0.248 or higher in the same subset of patients when they presented with TMA at the first biopsy, but with no TMA at second biopsy (Figure 5A). On the contrary, for the only patient who had no TMA at first biopsy but exhibited TMA at the second biopsy, the 4-variable panel dramatically increased to greater than 0.248. For the other 5 patients, the 4-variable signature was significantly decreased after treatment (P = 0.030), although they each presented without TMA at the two biopsies and the signature was 0.248 or less.
Figure 5. Evaluation of LDH and 4-variable panel in patients with repeat biopsy.
(A)We analyzed paired first and second levels of LDH and 4-variable panel in a subset of 9 patients, who underwent repeat renal biopsy. The first figure shows 4-variable panel signature plummeted from 0.248 or greater in the same subset of patients, when three patients presented TMA at first biopsy but no TMA at second biopsy. In the second figure, only one patient had no TMA at first biopsy and TMA at second biopsy and 4-variable panel dramatically increased to 0.348. For other 5 patients, 4-variable signature was significantly decreased after treatment, although they consistently presented without TMA at two biopsies and the signature was 0.248 or less. (B) Similar pattern of LDH changes was observed in the patients with repeat biopsy, and increased LDH levels were associated with TMA status.
A similar pattern of LDH changes were observed in the patients with repeat biopsy, and increased LDH levels were associated with TMA status (Figure 5B). However, an LDH value of 289 u/L was not a good cut-off level to discriminate TMA status in this group of patients.
Association of LDH levels and the 4-variable panel with renal survival
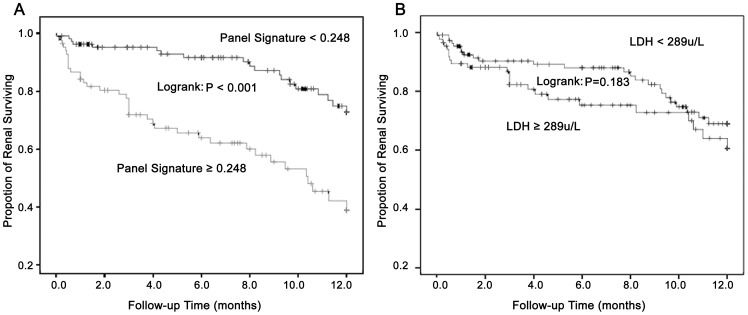
To further evaluate whether LDH levels and the 4-variable panel can serve as a predictor of renal survival in patients with suspected TMA, we performed a Kaplan-Meier survival analysis. As suspected, patients with a higher 4-variable panel signature had a statistically significant worse renal outcome (P<0.001, log-rank test; Figure 6A). However, the pattern of increased LDH concentrations was not associated with a poorer renal outcome (P = 0.183, log-rank test; Figure 6B).
Figure 6. Association of LDH levels and 4-variable panel with renal survival.
(A) Using the cutoff value of 0.248, suspected patients were divided into two groups by more or less than the value and the renal survival of two groups followed up 12 months was significantly different (P<0.001). (B) The pattern of increased LDH concentrations (more than the cutoff point of 289 u/L) associated statistically poorer renal outcome was not observed (P = 0.183).
Furthermore, the renal survival was only 53.9% in patients with a panel signature of more than 0.248, but was 79.5% in patients with a panel signature of less than 0.248. The Cox proportional hazard survival regression model revealed that TMA lesions increased the risk for a poor renal outcome, with a hazard ratio of 2.235 (95% CI 1.306 to 3.826, P = 0.003), while a panel signature 0.248 or greater had a hazard ratio of 3.549 (95% CI 2.034 to 3.549, P<0.001). These two diagnostic factors showed a similar predictive effect on renal outcome (P = 0.964).
Discussion
Histological TMA in the kidney has been frequently described in association with a large number of underlying diseases [20]–[22]. Although the causes of TMA in the kidney are unclear, it has been reported that there is a significantly worse renal outcome among patients with TMA [16], [22]. Kaplan et al. [23] reported that death rates were as high as 25% and progression to ESRD occurred in half of the patients with TMA within 10 years after diagnosis. In agreement with published studies [24], we confirmed that patients with renal biopsy-proven TMA presented with a poor outcome in the kidney and the patients with TMA had a 2.24-fold higher risk of renal failure than those without TMA.
The renal biopsy represents the gold standard in the management of patients with TMA, but a noninvasive diagnostic method for detection of TMA would be a valuable clinical application. Few studies have focused on the prediction of the histopathologic lesions, although abnormalities in the urinalysis and an increase in serum creatinine have been observed in patients with TMA lesions [25]. In addition, reports have demonstrated that laboratory variables were involved in clinical conditions associated with TMA [26]–[28], including hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Increased levels of LDH are associated with the severity of hemolysis and tissue ischemia, which might increase the risk of tissue lesions [29]. A deficiency of ADAMTS13 was related to thrombus formation and was subsequently noted in patients with thrombocytopenic purpura, who often suffered from TMA lesions in the kidney [30], [31].
This study is the first to demonstrate the potential of routine laboratory parameters to be used in the detection of TMA without renal biopsy. Six parameters - serum creatinine, platelets, hemoglobin, THBD, ADAMTS13 activity and LDH - had moderate diagnostic value for TMA. The strengths of this study are that we developed a diagnostic panel based on 4 laboratory variables (levels of serum creatinine, LDH, platelets and ADAMTS13 activity). This panel can noninvasively and accurately predict histological TMA. This is supported by the high AUC values of 0.800 derived from patients with and without TMA (sensitivity 81.6%; specificity 66.9%). Similar effects were noted when the predictive panel was validated using an independent population. The cross-validation further revealed that the 4-variable model tended to have high sensitivity, but a relatively low specificity. To the best of our knowledge, this is the first noninvasive biomarker that can detect TMA in the kidney. In addition, each individual parameter of the 5 or 6 variable model could not reach statistical significance (P<0.05, data not shown). The model of fewer variables could discriminate patients with TMA in derivation cohort, including the levels of LDH and platelets, but this effect was not proven by all different validation cohorts.
The 3 markers, LDH, platelets and the 4-variable panel, were further evaluated in a group of patients with SLE and expanded sets of patients. SLE is often reported with TMA lesions in the kidney [6], [22], and there were 157 patients diagnosed with SLE among the suspected patients investigated. Focusing on one particular disease, all 3 markers showed discrimination values for patients with TMA. Because some patients with TMA exhibit little or no signs of clinical manifestations [31]–[33], we expanded the application of these markers. LDH levels and the 4-variable panel still demonstrated good discrimination, while platelets did not. Thus, LDH levels and the 4-variable panel might have wide usage in different cohorts of patients. In addition, data from patients with a repeat biopsy confirmed the association of increased LDH levels and the 4-variable signature with TMA status.
Another important finding of our study was that the 4-variable panel also serves as a prognostic biomarker for renal patients. The increased signature of the 4-variable panel was an independent prognostic parameter. The prognostic value of the 4-variable panel was similar with the histological diagnosis of TMA. As a noninvasive biomarker, the 4-variable panel showed an advantage in clinical application. However, LDH levels did not exhibit a prognostic value for renal outcome. Therefore, the 4-variable panel might not only diagnose TMA but also help predict renal outcome with a higher accuracy than other single laboratory parameter.
Although our current assay may become a promising diagnostic tool for TMA, we acknowledge three potential limitations of this study. It is retrospective and will need to be validated with a prospective cohort of patients. Furthermore, the evaluation of prognosis was rendered difficult because of the variable nature of the treatments received. In addition, the number of patients with certain diseases was small and some validations were calculated using non-independent data, which prevented an in-depth evaluation of the usefulness of the diagnostic panel for specifically predicting TMA in the kidney.
Conclusions
In conclusion, our results provide compelling evidence for the potential use of laboratory parameters as a noninvasive diagnostic and prognostic tool for TMA in the kidney. The 4-variable panel showed an advantage for the early detection of renal TMA lesions without renal biopsy and in directly predicting renal prognosis. In order for this concept to be incorporated into routine clinical practice in the near-future, validation is needed in large-scale prospective trials.
Supporting Information
Includes table S1–S5. Table S1, Diagnosis of TMA in kidney among patients (n = 220). Table S2, Laboratory variables in the validation group of internal patients (n = 46). Table S3, Clinical laboratory data for suspected patients with SLE (n = 157). Table S4, Clinical laboratory data for patients in expanded group (n = 113). Table S5, The laboratory feature of patients with/without TMA in the kidney at first renal biopsy and repeat biopsy.
(DOCX)
Acknowledgments
We thank all the patients and their families who participated in this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported in part by National Basic Research Program of China 973 Program No.2012CB517600 (No.2012CB517606) (to Z.L.)(http://www.973.gov.cn/AreaMana.aspx), National Natural Science Foundation (NSF) Grant 81370788 (to H.C.) (http://www.nsfc.gov.cn/), The National Key Technology R&D Program (2011BAI10B04) (to Z.L.) (http://kjzc.jhgl.org/) and Six Talent Peaks Project of Jiangsu Province (2012-WSN-071) (to H.C.) (http://archives.nuist.edu.cn/rcgf/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moake JL (2002) Thrombotic microangiopathies. N Engl J Med 347: 589–600. [DOI] [PubMed] [Google Scholar]
- 2. El Karoui K, Hill GS, Karras A, Jacquot C, Moulonguet L, et al. (2012) A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol 23: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldberg RJ, Nakagawa T, Johnson RJ, Thurman JM (2010) The role of endothelial cell injury in thrombotic microangiopathy. Am J Kidney Dis 56: 1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van den Born BJ, van der Hoeven NV, Groot E, Lenting PJ, Meijers JC, et al. (2008) Association between thrombotic microangiopathy and reduced ADAMTS13 activity in malignant hypertension. Hypertension 51: 862–866. [DOI] [PubMed] [Google Scholar]
- 5.Laurence J (2000) Pathological and therapeutic distinctions in HUS/TTP. Lancet 355: 497; author reply 497–498. [DOI] [PubMed]
- 6. Hughson MD, Nadasdy T, McCarty GA, Sholer C, Min KW, et al. (1992) Renal thrombotic microangiopathy in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Kidney Dis 20: 150–158. [DOI] [PubMed] [Google Scholar]
- 7. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, et al. (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, et al. (2003) Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 290: 1360–1370. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds JC, Agodoa LY, Yuan CM, Abbott KC (2003) Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis 42: 1058–1068. [DOI] [PubMed] [Google Scholar]
- 10. Clark WF (2012) Thrombotic microangiopathy: current knowledge and outcomes with plasma exchange. Semin Dial 25: 214–219. [DOI] [PubMed] [Google Scholar]
- 11. Stiles KP, Yuan CM, Chung EM, Lyon RD, Lane JD, et al. (2000) Renal biopsy in high-risk patients with medical diseases of the kidney. Am J Kidney Dis 36: 419–433. [DOI] [PubMed] [Google Scholar]
- 12. Symmers WS (1952) Thrombotic microangiopathic haemolytic anaemia (thrombotic microangiopathy). Br Med J 2: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. George JN, Vesely SK, James JA (2007) Overlapping features of thrombotic thrombocytopenic purpura and systemic lupus erythematosus. South Med J 100: 512–514. [DOI] [PubMed] [Google Scholar]
- 14. D'Angelo WA, Fries JF, Masi AT, Shulman LE (1969) Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 46: 428–440. [DOI] [PubMed] [Google Scholar]
- 15. Cataland SR, Yang SB, Witkoff L, Kraut EH, Lin S, et al. (2009) Demographic and ADAMTS13 biomarker data as predictors of early recurrences of idiopathic thrombotic thrombocytopenic purpura. Eur J Haematol 83: 559–564. [DOI] [PubMed] [Google Scholar]
- 16. Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725. [DOI] [PubMed] [Google Scholar]
- 17. Peng J, Gong L, Si K, Bai X, Du G (2011) Fluorescence resonance energy transfer assay for high-throughput screening of ADAMTS1 inhibitors. Molecules 16: 10709–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. White LE, Cui Y, Shelak CM, Lie ML, Hassoun HT (2012) Lung endothelial cell apoptosis during ischemic acute kidney injury. Shock 38: 320–327. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC, Tu JV (2004) Bootstrap methods for developing predictive models. Am Stat 58: 131–137. [Google Scholar]
- 20. Baliga RS, Wingo CS (2003) Quinine induced HUS-TTP: an unusual presentation. Am J Med Sci 326: 378–380. [DOI] [PubMed] [Google Scholar]
- 21. Noris M, Remuzzi G (2010) Thrombotic microangiopathy after kidney transplantation. Am J Transplant 10: 1517–1523. [DOI] [PubMed] [Google Scholar]
- 22. Hu WX, Liu ZZ, Chen HP, Zhang HT, Li LS, et al. (2010) Clinical characteristics and prognosis of diffuse proliferative lupus nephritis with thrombotic microangiopathy. Lupus 19: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 23. Kaplan B, Meier-Kriesche HU (2002) Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974. [DOI] [PubMed] [Google Scholar]
- 24. Michael M, Elliott EJ, Craig JC, Ridley G, Hodson EM (2009) Interventions for hemolytic uremic syndrome and thrombotic thrombocytopenic purpura: a systematic review of randomized controlled trials. Am J Kidney Dis 53: 259–272. [DOI] [PubMed] [Google Scholar]
- 25. Droz D, Nochy D, Noel LH, Heudes D, Nabarra B, et al. (2000) Thrombotic microangiopathies: renal and extrarenal lesions. Adv Nephrol Necker Hosp 30: 235–259. [PubMed] [Google Scholar]
- 26. Vesely SK, George JN, Lammle B, Studt JD, Alberio L, et al. (2003) ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 102: 60–68. [DOI] [PubMed] [Google Scholar]
- 27. Park YA, Waldrum MR, Marques MB (2010) Platelet count and prothrombin time help distinguish thrombotic thrombocytopenic purpura-hemolytic uremic syndrome from disseminated intravascular coagulation in adults. Am J Clin Pathol 133: 460–465. [DOI] [PubMed] [Google Scholar]
- 28. Cataland SR, Yang S, Wu HM (2012) The use of ADAMTS13 activity, platelet count, and serum creatinine to differentiate acquired thrombotic thrombocytopenic purpura from other thrombotic microangiopathies. Br J Haematol 157: 501–503. [DOI] [PubMed] [Google Scholar]
- 29. Cohen JA, Brecher ME, Bandarenko N (1998) Cellular source of serum lactate dehydrogenase elevation in patients with thrombotic thrombocytopenic purpura. J Clin Apher 13: 16–19. [DOI] [PubMed] [Google Scholar]
- 30. Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, et al. (1997) Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood 89: 3097–3103. [PubMed] [Google Scholar]
- 31. Galbusera M, Noris M, Rossi C, Orisio S, Caprioli J, et al. (1999) Increased fragmentation of von Willebrand factor, due to abnormal cleavage of the subunit, parallels disease activity in recurrent hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and discloses predisposition in families. The Italian Registry of Familial and Recurrent HUS/TTP. Blood 94: 610–620. [PubMed] [Google Scholar]
- 32. George JN (2006) Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med 354: 1927–1935. [DOI] [PubMed] [Google Scholar]
- 33. Hastings MC, Wyatt RJ, Ault BH, Jones DP, Lau KK, et al. (2007) Diagnosis of de novo localized thrombotic microangiopathy by surveillance biopsy. Pediatr Nephrol 22: 742–746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes table S1–S5. Table S1, Diagnosis of TMA in kidney among patients (n = 220). Table S2, Laboratory variables in the validation group of internal patients (n = 46). Table S3, Clinical laboratory data for suspected patients with SLE (n = 157). Table S4, Clinical laboratory data for patients in expanded group (n = 113). Table S5, The laboratory feature of patients with/without TMA in the kidney at first renal biopsy and repeat biopsy.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.