Abstract
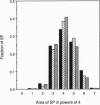
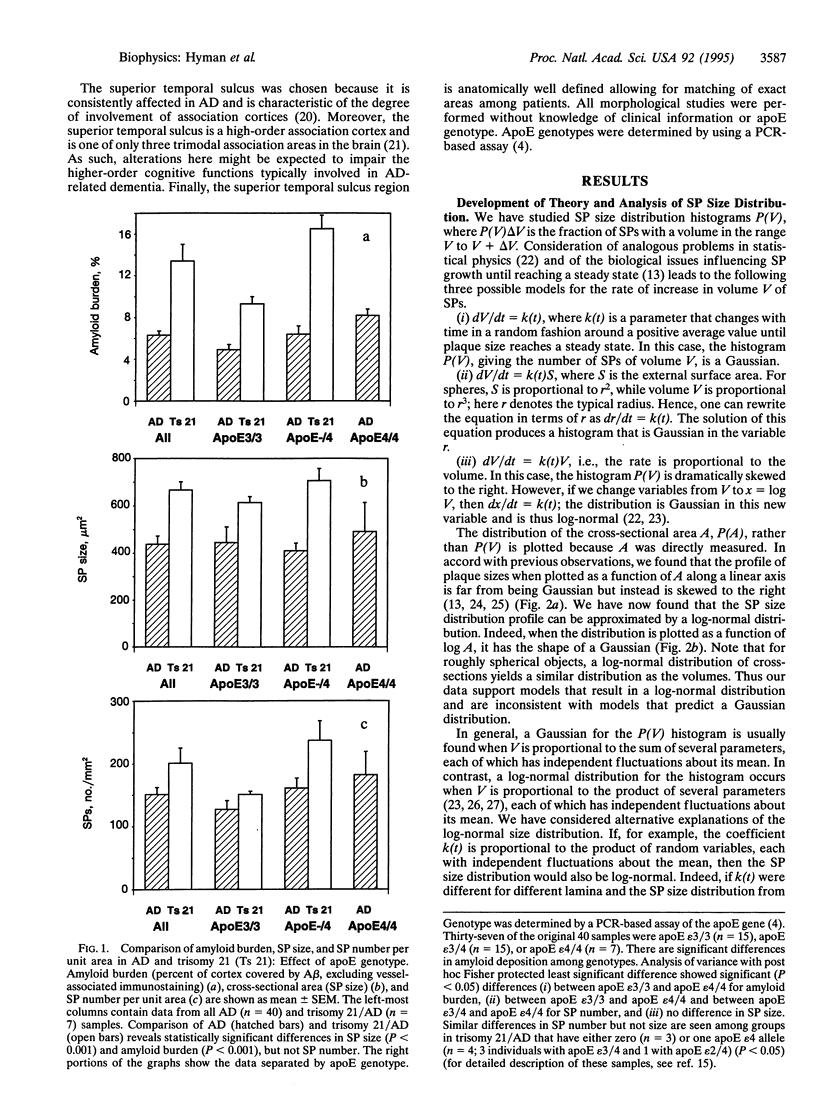
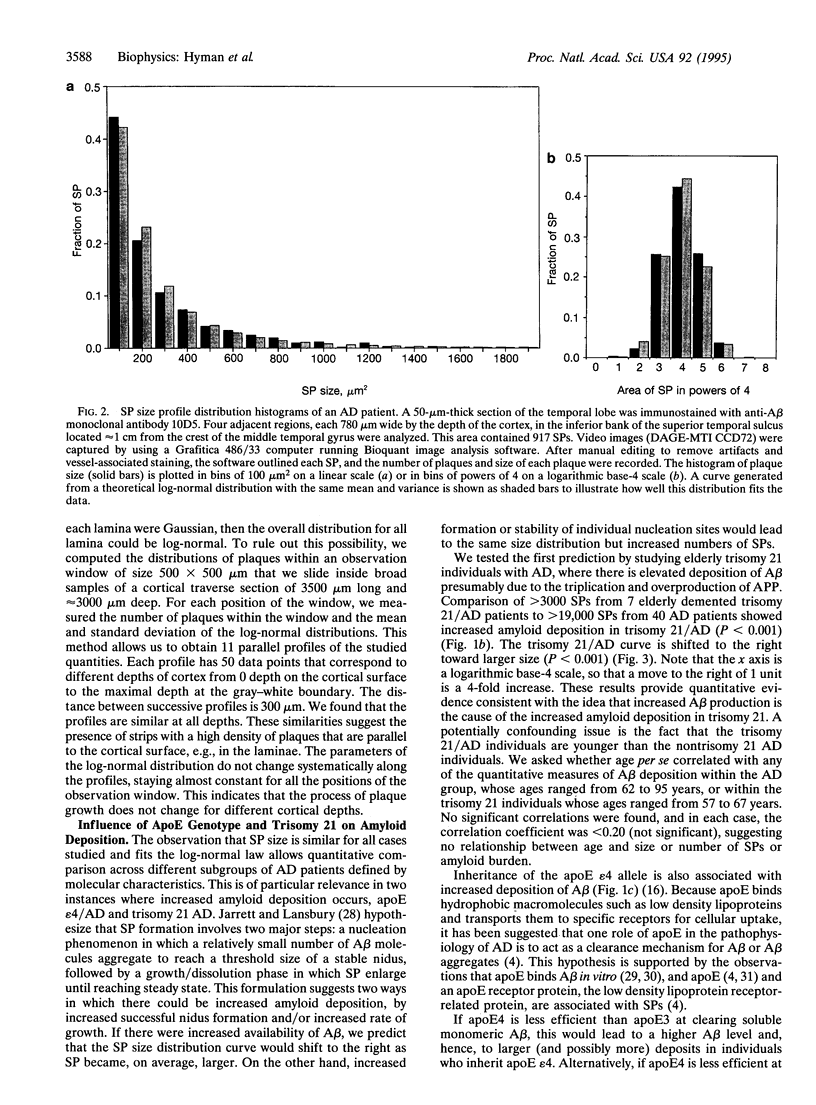
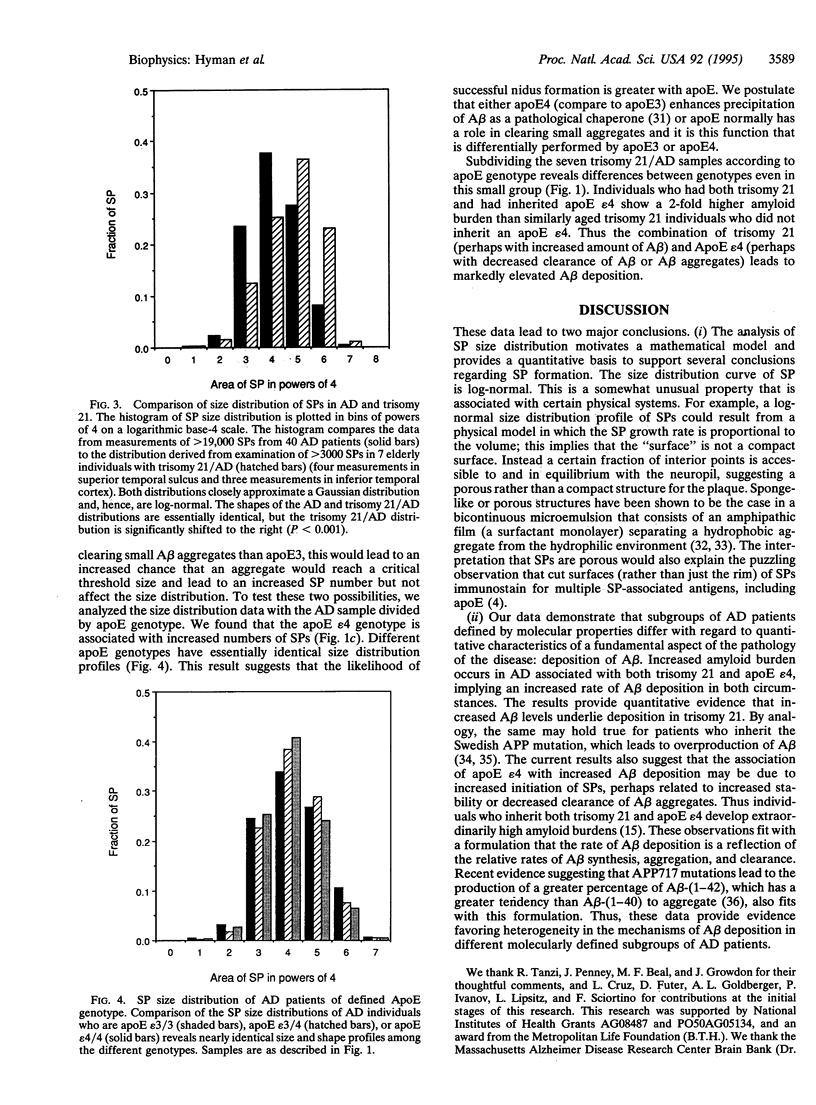
The discovery that the epsilon 4 allele of the apolipoprotein E (apoE) gene is a putative risk factor for Alzheimer disease (AD) in the general population has highlighted the role of genetic influences in this extremely common and disabling illness. It has long been recognized that another genetic abnormality, trisomy 21 (Down syndrome), is associated with early and severe development of AD neuropathological lesions. It remains a challenge, however, to understand how these facts relate to the pathological changes in the brains of AD patients. We used computerized image analysis to examine the size distribution of one of the characteristic neuropathological lesions in AD, deposits of A beta peptide in senile plaques (SPs). Surprisingly, we find that a log-normal distribution fits the SP size distribution quite well, motivating a porous model of SP morphogenesis. We then analyzed SP size distribution curves in genotypically defined subgroups of AD patients. The data demonstrate that both apoE epsilon 4/AD and trisomy 21/AD lead to increased amyloid deposition, but by apparently different mechanisms. The size distribution curve is shifted toward larger plaques in trisomy 21/AD, probably reflecting increased A beta production. In apoE epsilon 4/AD, the size distribution is unchanged but the number of SP is increased compared to apoE epsilon 3, suggesting increased probability of SP initiation. These results demonstrate that subgroups of AD patients defined on the basis of molecular characteristics have quantitatively different neuropathological phenotypes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. A., Myers D., Smith C. U. Alzheimer's disease: size class frequency distribution of senile plaques: do they indicate when a brain tissue was affected? Neurosci Lett. 1991 Jun 24;127(2):223–226. doi: 10.1016/0304-3940(91)90799-y. [DOI] [PubMed] [Google Scholar]
- Arnold S. E., Hyman B. T., Flory J., Damasio A. R., Van Hoesen G. W. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991 Jan-Feb;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Arriagada P. V., Growdon J. H., Hedley-Whyte E. T., Hyman B. T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992 Mar;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Marzloff K., Arriagada P. V. The lack of accumulation of senile plaques or amyloid burden in Alzheimer's disease suggests a dynamic balance between amyloid deposition and resolution. J Neuropathol Exp Neurol. 1993 Nov;52(6):594–600. doi: 10.1097/00005072-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Tanzi R. E. Amyloid, dementia and Alzheimer's disease. Curr Opin Neurol Neurosurg. 1992 Feb;5(1):88–93. [PubMed] [Google Scholar]
- Hyman B. T., Tanzi R. E., Marzloff K., Barbour R., Schenk D. Kunitz protease inhibitor-containing amyloid beta protein precursor immunoreactivity in Alzheimer's disease. J Neuropathol Exp Neurol. 1992 Jan;51(1):76–83. doi: 10.1097/00005072-199201000-00009. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Amyloid fibril formation requires a chemically discriminating nucleation event: studies of an amyloidogenic sequence from the bacterial protein OsmB. Biochemistry. 1992 Dec 15;31(49):12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Lansbury P. T., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993 Jun 18;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Kawai M., Cras P., Perry G. Serial reconstruction of beta-protein amyloid plaques: relationship to microvessels and size distribution. Brain Res. 1992 Oct 2;592(1-2):278–282. doi: 10.1016/0006-8993(92)91686-9. [DOI] [PubMed] [Google Scholar]
- Mann D. M. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988 May;43(2):99–136. doi: 10.1016/0047-6374(88)90041-3. [DOI] [PubMed] [Google Scholar]
- Mirra S. S., Heyman A., McKeel D., Sumi S. M., Crain B. J., Brownlee L. M., Vogel F. S., Hughes J. P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991 Apr;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montroll E. W., Shlesinger M. F. On 1/f noise and other distributions with long tails. Proc Natl Acad Sci U S A. 1982 May;79(10):3380–3383. doi: 10.1073/pnas.79.10.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordovas J. M., Litwack-Klein L., Wilson P. W., Schaefer M. M., Schaefer E. J. Apolipoprotein E isoform phenotyping methodology and population frequency with identification of apoE1 and apoE5 isoforms. J Lipid Res. 1987 Apr;28(4):371–380. [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Rebeck G. W., Reiter J. S., Strickland D. K., Hyman B. T. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993 Oct;11(4):575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Strittmatter W. J., Schmechel D., George-Hyslop P. H., Pericak-Vance M. A., Joo S. H., Rosi B. L., Gusella J. F., Crapper-MacLachlan D. R., Alberts M. J. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Orr H. T., Anderson L., Nemens E., White J. A., Bonnycastle L., Weber J. L., Alonso M. E. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992 Oct 23;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Saunders A. M., Strittmatter W. J., Crain B. J., Hulette C. M., Joo S. H., Pericak-Vance M. A., Goldgaber D., Roses A. D. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Golabek A., Matsubara E., Ghiso J., Frangione B. Apolipoprotein E: binding to soluble Alzheimer's beta-amyloid. Biochem Biophys Res Commun. 1993 Apr 30;192(2):359–365. doi: 10.1006/bbrc.1993.1423. [DOI] [PubMed] [Google Scholar]