Abstract
Depending on threat proximity, different defensive behaviours are mediated by a descending neural network involving forebrain (distal threat) vs midbrain areas (proximal threat). Compared to healthy subjects, it can be assumed that phobics are characterized by shortened defensive distances on a behavioural and neural level. This study aimed at characterizing defensive reactivity in two subtypes of specific phobia [snake (SP) and dental phobics (DP)]. Using functional magnetic resonance imaging (fMRI), n = 39 subjects (13 healthy controls, HC; 13 SP; 13 DP) underwent an event-related fMRI task employing an anticipation (5–10 s) and immediate perception phase (phobic pictures and matched neutral stimuli; 1250 ms) to modulate defensive distance. Although no differential brain activity in any comparisons was observed in DP, areas associated with defensive behaviours (e.g. amygdala, hippocampus, midbrain) were activated in SP. Decreasing defensive distance in SP was characterized by a shift to midbrain activity. Present findings substantiate differences between phobia types in their physiological and neural organization that can be expanded to early stages of defensive behaviours. Findings may contribute to a better understanding of the dynamic organization of defensive reactivity in different types of phobic fear.
Keywords: specific phobia, defensive behaviour, fMRI, amygdala, midbrain
INTRODUCTION
Evidence suggests a cross-species conservation of basic defensive behaviours that are organized depending upon the proximity of threat (Fanselow, 1994; Blanchard et al., 2001). Post-encounter defence (being signalled by conditioned or contextual cues of a potential predator) results in enhanced vigilance and freezing; circa-strike defence under threat imminence (predator attack) is characterized by escape or defensive fighting. The neural organization of defensive behaviours has been suggested to follow a functional gradient from higher-order cortical and forebrain structures in response to distal threat (post-encounter) vs midbrain structures mediating proximal (circa-strike) threat (Fanselow, 1994; McNaughton and Corr, 2004; Mobbs et al., 2007, 2009).
Psychologically, defensive distance represents a cognitive construct linked to the intensity of perceived threat (McNaughton and Corr, 2004). In other words, defensive distance and associated types of defensive behaviours depend upon the subjective evaluation of threat intensity. Defensive behaviours like exaggerated anticipatory anxiety and phobic avoidance in the presence of the feared stimulus are hallmark features of specific phobia (American Psychiatric Association, 2000). Specific phobia can be regarded as a paradigmatic condition to study pathologically altered defensive reactivity in humans. It may be assumed that identical defensive mechanisms act in healthy subjects and in phobics, but at different defensive distances. Shortened defensive distances in phobics could be indicated by lowered thresholds for post-encounter or circa-strike defensive behaviours, such as hypervigilance, anticipatory anxiety, avoidance or flight behaviour. In line with this, first evidence for enhanced right amygdala reactivity during subconscious processing of phobic stimuli indicates increased vigilance towards potential threat in phobic subjects (Lipka et al., 2011). Although neural substrates of defensive behaviour during exposure (e.g. proximal threat) to phobic stimuli have been repeatedly investigated (see Etkin and Wager, 2007; Shin and Liberzon, 2010; Linares et al., 2012 for reviews), less evidence is available on anticipatory anxiety (Straube et al., 2007) as an indicator of a post-encounter defence mode. Moreover, the vast majority of studies has been conducted on a particular animal subtype of specific phobia, namely spider phobia. Despite sharing core clinical features, the animal and BII subtype differ in psychophysiological response characteristics (Hamm et al., 1997; Globisch et al., 1999). On a neural level, they are also characterized by distinct functional activation patterns (Hermann et al., 2007; Caseras et al., 2010a; Lueken et al., 2011b). Recent studies do however show that these differences may be explained by the mode of stimulation. Immediate stimulus processing using short presentation times in animal and BII phobics has been associated with similar neural response patterns for both groups (albeit more strongly pronounced in animal phobics), but critical differences emerged during sustained processing using longer presentation times (Caseras et al., 2010a, b). In line with this, a recent study by Schienle et al. (2013) reported enhanced brain activation in the prefrontal and orbitofrontal cortex (PFC, OFC), the anterior cingulate cortex (ACC), insula and basal ganglia during immediate stimulus perception in a large sample of dental phobics (DP; as a subgroup of the BII type; Lebeau et al., 2010). These findings underline that temporal characteristics of defensive behaviours could constitute a critical factor when comparing neural substrates of phobia subtypes.
We aimed to investigate neural correlates of defensive reactivity in two types of specific phobia. Assuming that phobic subjects exhibit shorter defensive distances, we hypothesized that phobics compared to controls will show enhanced activation in brain structures associated with a post-encounter defence mode (amygdala, hippocampus, insula, thalamus, ACC and PFC/OFC) during the anticipation of phobic stimuli. Based upon recent findings from Caseras et al. (2010b) and Schienle et al. (2013) on increased neural activation during immediate processing of phobic stimuli in BII and DP, enhanced defensive reactivity was expected in both phobic groups compared to controls, but with stronger activation in snake phobics (SP) when compared to DP. Based upon research on the neural organization of defensive behaviours we assumed that midbrain activity would be particularly present during the circa-strike defence mode (perception), but not during post-encounter (anticipation of phobic stimuli). Anxiety reports and skin conductance as subjective and autonomic markers of defensive behaviour were expected to positively correlate with the magnitude of neural activation in key regions of the defensive network (e.g. ACC, amygdala, midbrain).
EXPERIMENTAL PROCEDURES
Subjects
Extending previous work (Lueken et al., 2011b), an independent student sample was recruited for this study via an online-screening. Clinically relevant snake phobia was indicated by a cut-off ≥20 points on the Snake Questionnaire (SNAQ; Hamm, 2006); DP were selected scoring two standard deviations above the mean of the Dental Fear Survey (DFS; Tönnies et al., 2002), resulting in a cut-off ≥75 points, thus being marginally below the reported cut-off ≥76 points indicating severe dental phobia (Tönnies et al., 2002). Healthy controls (HC) scored in the lower quartiles of both questionnaires. Exclusion criteria encompassed current use of psychotropic medication, any lifetime neurological disease, functional magnetic resonance imaging (fMRI)-related exclusion criteria, left-handedness (self-report) and 12 month prevalence of comorbid psychiatric disorders (HC: any psychiatric disorder; phobic groups: bipolar disorder, obsessive–compulsive disorder, psychotic disorder, posttraumatic stress disorder, substance disorder, and comorbid snake and dental phobia as indicated by the above-mentioned cut-offs). Psychiatric exclusion criteria were assessed by a standardized clinical interview according to DSM-IV-TR criteria (Composite International Diagnostic Interview; Wittchen and Pfister, 1997) and where validated by clinical experts. Phobia groups did not differ in the number of subjects with comorbid disorders [six per group χ2(1) = 0.000, P = 1.000] or comorbid anxiety disorders [SP: n = 5, DP: n = 6, χ2(1) = 0.158, P = 0.698]. The final sample consisted of n = 39 subjects (13 subjects per group). Subjects received 25€ or course credit. The study protocol was approved by the ethics committee of the Technische Universität Dresden.
Procedure
The fMRI task was based upon a previously validated video set (Lueken et al., 2011a, b). Individually tailored control conditions for each anxiety condition that were matched in terms of information complexity, movements, timing and background textures provided an optimal baseline to control for non-anxiety-specific processes. For example, in the anxiety condition, a dentist puts on a latex glove in order to prepare for the treatment, whereas in the neutral condition a different person puts on a white wool glove (see Supplemental Figure S1 for an illustration). The videos showed typical dentist actions that exhibit anxiety-provoking stimulus characteristics (Oosterink et al., 2008; see Lueken et al., 2011a for a full description of the video contents). A similar approach was chosen for the snake stimuli. We used publicly available video material to assemble snake-anxiety videos of living snakes. The respective neutral conditions were custom-fit videotaped and matched for environmental textures (e.g. stones, trees or leaves), movements and timing of cuts within the sequences. For this study, pictures (two per video, selected from matched scenes of the phobic and respective neutral material) were extracted. During the perception phase (PP), stimuli were presented for 1250 ms in randomized order. Preceding the PP, an anticipation phase (AP) was implemented, announcing the type of stimulus with a letter centred on the middle of the screen [‘Z’ for dental stimulus (German: Zahnarzt), ‘S’ for snake stimulus and ‘N’ for neutral stimulus; always-true relation]. A varying AP interval between 5 and 10 s was used to avoid prediction of PP onset, followed by a jittered inter-stimulus interval (5.8, 9.3 or 11.9 s). The design consisted of eight conditions in randomized order for each subject [AP: 20 snake neutral (AP-SN), 20 snake anxiety (AP-SA), 20 dental neutral (AP-DN) and 20 dental anxiety (AP-DA) stimuli; PP: 20 snake neutral (PP-SN), 20 snake anxiety (PP-SA), 20 dental neutral (PP-DN) and 20 dental anxiety (PP-DA) stimuli]. Subjects were instructed to attentively view the stimuli and to press a button as fast as possible upon PP onset with their right index finger. Reaction time (RT) data were extracted as a measure of selective attention and hypervigilance towards the phobic vs neutral stimulus. Those shorter than 100 ms (premature responding) or after stimulus offset were scored as missing. One subject (HC) exhibiting 24% missings was excluded from RT analyses, whereas all other subjects had below 10% missings. The paradigm was programmed using Presentation 12.0 (Neurobehavioral Systems, Albany, CA, USA) and presented on video goggles (VisuaStim Digital, Northridge, CA, USA). After completion, subjects rated each picture on a nine-point Likert scale regarding valence (‘The picture was: negative/neutral/positive’), arousal (‘The picture made me nervous: not at all/very’), anxiety (‘The picture made me anxious: not at all/very’), disgust (‘The picture was disgusting: not at all/very’) and pain (‘The picture made me feel/remember pain: not at all/very’). Subjective ratings from n = 4 subjects (DP: n = 1, HC: n = 3) were lost due to technical failure.
Skin conductance (SC) was recorded during scanning using Ag/AgCl electrodes (MES Medizintechnik, Munich, Germany) that were attached to the second phalanx of the index and middle fingers of the non-dominant hand, using isotonic electrode paste as contact medium (Synapse, Kustomer Kinetics, Arcadia, CA, USA) and Brain Vision hard- and software for data acquisition (Brain Vision ExG Amplifier and Brain Vision Recorder; Brain Products, Munich, Germany). Data were recorded with an initial sampling rate of 1000 Hz (downsampled to 10 Hz), applying a low cut-off filter of 10 s and a high cut-off filter of 250 Hz. A Matlab based application (Ledalab Version 3.3.4; Benedek and Kaernbach, 2010) was employed to run a discrete decomposition analysis from which through-to-peak values were used to calculate the sum amplitude of the first interval response within a time window of 1–5 s after stimulus onset (AMP.SCR; response criterion 0.02 μS). SC data were range-corrected according to Lykken (1972) and did not differ significantly from the normal distribution as indicated by Kolmogorov–Smirnov tests.
fMRI data acquisition and analysis
MRI data were collected on a 3 Tesla Trio-Tim MRI whole-body scanner (Siemens, Erlangen, Germany). A T2* weighted gradient echo planar imaging sequence covering the whole brain was used to acquire functional images [560 volumes, repetition time (TR) 2500 ms, echo time (TE) 25 ms, field of view (FOV) 192 × 192 mm, matrix 64 × 64] in tilted angle (AC − PC + 20°) to reduce susceptibility artefacts in inferior brain areas (Deichmann et al., 2003), with each volume including 44 axial slices (interleaved acquisition, no gap, slice thickness 3 mm, in-plane resolution 3 × 3 mm). The first four volumes were excluded due to T1 equilibration effects. A magnetization-prepared rapid gradient echo imaging sequence was used to acquire a structural reference image (176 sagittal slices, slice thickness = 1 mm, TE = 2.26 ms, TR = 1900 ms, flip angle = 9°, FOV = 256 × 256 mm, matrix = 256 × 256). A 12 channel head coil and headphones were used. fMRI data were analyzed using SPM5 (Wellcome Trust Centre for Neuroimaging, UCL, London, UK). Following slice timing correction, realignment and unwarping of the images was applied to correct for movement artefacts. Structural and functional images were coregistered in order to use tissue segmentation maps of the structural image for normalization to the MNI reference brain (Montreal Neurological Institute, Quebec, Canada). Functional data were spatially smoothed with a Gaussian kernel of 8 mm full-width half-maximum.
Applying the general linear model, first level modelling included the eight regressors of interest and the six movement parameters from the rigid body transformation. A high-pass filter (128 s cut-off period) was applied to remove low frequency fluctuations in the BOLD signal. Contrasts against tailored baseline stimuli (SA > SN; DA > DN) were computed and introduced into a flexible factorial second-level random effects analysis including a subjects factor and the interaction between group (HC, SP, DP) and stimulus material (AP-snake, AP-dental, PP-snake, PP-dental). Contrasts of interest were: AP: SP (SA > SN) > HC (SA > SN), AP: SP (SA > SN) > DP (SA > SN), AP: DP (DA > DN) > HC (DA > DN), AP: DP (DA > DN) > SP (DA > DN), and PP: SP (SA > SN) > HC (SA > SN), PP: SP (SA > SN) > DP (SA > SN), PP: DP (DA > DN) > HC (DA > DN), PP: DP (DA > DN) > SP (DA > DN). Differences in functional activation patterns between both phobia groups were directly compared when confronted with their respective stimulus material [AP: SP (SA > SN) > DP (DA > DN), AP: DP (DA > DN) > SP (SA > SN), and PP: SP (SA > SN) > DP (DA > DN), PP: DP (DA > DN) > SP (SA > SN)]. Due to a priori hypotheses based upon previous findings on fear network components in specific phobia, a region of interest (ROI) approach was employed (exploratory whole brain results are reported in Supplementary Table S2). ROIs encompassed the bilateral ACC, amygdala, insula, thalamus, hippocampus, orbitofrontal gyrus, inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus and midbrain as defined by the WFU Pickatlas (Maldjian et al., 2003) using the Talairach daemon (midbrain) and anatomic automatic labelling (aal; Tzourio-Mazoyer et al., 2002) masks. Anatomical regions were assigned to peak voxel coordinates using the aal toolbox. Threshold significance was P < 0.05 corrected for family-wise errors (FWE) with a minimum cluster size of 15 contiguous voxels (5 voxels for the exploratory whole brain analysis). Estimated beta values were extracted from peak voxels for box plots and correlational analyses.
Analysis of demographic and behavioural data
Demographic and clinical data were compared using chi-square tests and one-factorial analyses of variance (ANOVAs). Difference scores (anxiety minus neutral) were used for all behavioural data. Reactivity in RT and subjective rating data was investigated using two-factorial ANOVAs, with the between-subjects factor group (HC, SP, DP) and stimulus material (snake, dental); a three-factorial ANOVA including an additional within-subjects factor phase (AP, PP) was calculated for AMP.SCR. Pairwise comparisons were used as post hoc tests. Markers of phobic fear (SNAQ) and autonomic arousal (AMP.SCR) were correlated (Pearson’s r; Bonferroni corrected) with the magnitude of neural activation in the ACC, amygdala and midbrain representing different nodes within the descending network mediating defensive reactivity. SPSS 20 was used for all analyses, with the level of significance being set at P < 0.05.
Results
Sample characteristics and behavioural data
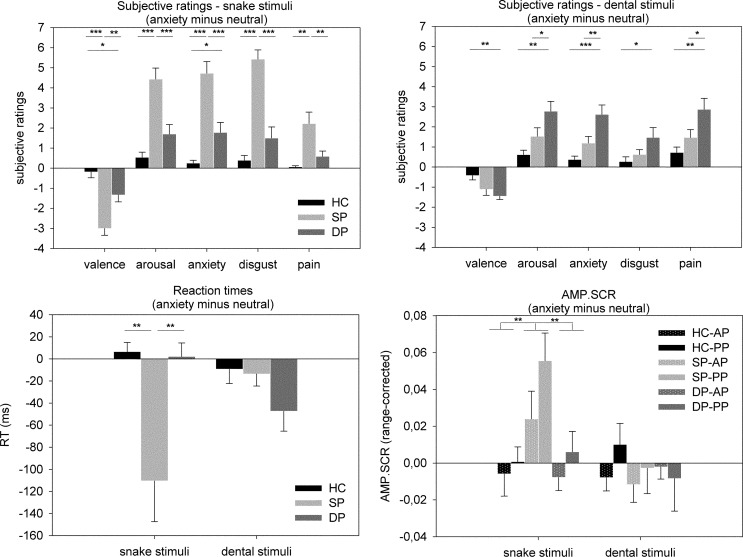
Sample characteristics are given in Table 1. Phobic groups were more reactive towards their phobic stimulus material than HC [interaction effect group*stimulus material: valence: F(2, 32) = 17.735, P < 0.001; arousal: F(2, 32) = 14.895, P < 0.001; anxiety: F(2, 32) = 20.941, P < 0.001; disgust: F(2, 32) = 28.753, P < 0.001; pain: F(2, 32) = 8.433, P = 0.001]. Although SP scored higher in all snake stimuli dimensions than DP, DP differed from SP in the arousal, anxiety and pain dimension for dental stimuli (Figure 1; see Supplementary Table S1 for complete baseline and anxiety scores). Significant main effects of group [stronger reactions of phobia groups towards both stimulus classes than HC; valence: F(2, 32) = 12.047, P < 0.001; arousal: F(2, 32) = 11.826, P < 0.001; anxiety: F(2, 32) = 15.584, P < 0.001; disgust: F(2, 32) = 18.413, P < 0.001; pain: F(2, 32) = 5.893, P = 0.007] and stimulus material [higher ratings for snake than dental stimuli in the valence, anxiety and disgust dimension, and for dental stimuli in the pain dimension; valence: F(1, 32) = 9.203, P = 0.005; arousal: F(1, 32) = 3.263, P = 0.080; anxiety: F(1, 32) = 7.745, P = 0.009; disgust: F(1, 32) = 29.520, P < 0.001; pain: F(1, 32) = 5.481, P = 0.026] were observed.
Table 1.
Sample characteristics. Means (s.d.) except where noted
| HC (n = 13) | SP (n = 13) | DP (n = 13) | chi2/F (df) | P | |
|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||
| Female sex (n, %) | 10 (76.92) | 11 (84.62) | 10 (76.92) | 0.315 (2) | 0.854 |
| Unmarried (n, %) | 12 (92.31) | 13 (100.00) | 12 (92.31) | 1.054 (2) | 0.590 |
| Non-smoker (n, %) | 13 (100.00) | 13 (100.00) | 11 (84.62) | 4.216 (2) | 0.121 |
| Age (years) | 21.46 (1.85) | 21.85 (1.95) | 23.00 (3.37) | 1.346 (38) | 0.273 |
| Clinical characteristics | |||||
| DFSa | 25.62 (3.71) | 41.85 (12.73) | 80.31 (4.55) | 156.493 (38) | <0.001 |
| SNAQb | 3.92 (2.81) | 23.38 (1.39) | 6.92 (6.25) | 87.246 (38) | <0.001 |
| ASI | 13.54 (5.24) | 20.62 (9.71) | 18.85 (8.62) | 2.699 (38) | 0.081 |
DP, dental phobia group; SP, snake phobia group; ASI, Anxiety Sensitivity Index. aDental phobics > snake phobics > controls: P < 0.001. bSnake phobics > dental phobics, controls: P < 0.001. Please note that questionnaire data relate to the date of screening that was used for study inclusion.
Fig. 1.
Behavioural data. Depicted are difference scores (anxiety minus neutral). Upper half: subjective ratings for snake (left) and dental (right) stimuli. Lower half: reaction times (RT; left) and skin conductance amplitudes (AMP.SCR; right). SP, snake phobia group; DP, dental phobia group; HC: healthy controls; AP: anticipation phase; PP: perception phase. *P < 0.05; **P < 0.01; ***P < 0.001.
SP exhibited reduced RT in response to the snake, but not dental stimuli [main effect group: F(2, 35) = 4.356, P = 0.020; interaction effect group*stimulus material: F(2, 35) = 9.391, P = 0.001; Figure 1]. In DP, RT only yielded a non-significant trend (DP < HC; P = 0.071). The main effect of stimulus material remained insignificant [F(1, 35) = 0.510, P = 0.480].
SP yielded higher AMP.SCR towards snake, but not dental stimuli during anticipation and perception [interaction effect group*stimulus material: F(2, 36) = 4.786, P = 0.014; Figure 1]. The main effect of stimulus [snake > dental, F(1, 36) = 4.901, P = 0.033] was driven by this interaction. No further interaction effects yielded significance [main effect group: F(1, 36) = 2.596, P = 0.091; main effect phase: F(1, 36) = 3.791, P = 0.059; group*phase: F(2, 36) = 0.582, P = 0.564; stimulus material*phase: F(1, 36) = 0.774, P = 0.385; group*stimulus material*phase: F(2, 36) = 0.841, P = 0.440].
fMRI results
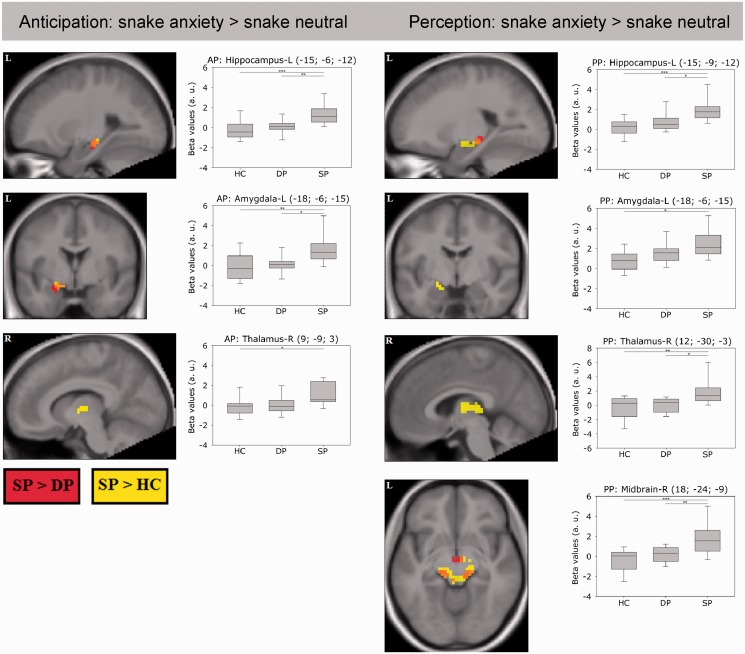
ROI analyses are given in Table 2. Results were not indicative of differential activation in DP compared to HC during AP or PP [AP: DP (DA > DN) > HC (DA > DN); PP: DP (DA > DN) > HC (DA > DN)] or to the phobic control group [AP: DP (DA > DN) > SP (DA > DN); PP: DP (DA > DN) > SP (DA > DN)]. In contrast, SP activated a medial-temporal network encompassing the bilateral amygdala, hippocampus and right thalamus during AP when compared to HC. When compared to their phobic control group (DP) differences during the anticipation of snake phobic stimulus material were less pronounced and encompassed the left amygdala and hippocampus only. Direct comparisons of the respective phobic stimulus material [AP: SP (SA > SN) > DP (SA > SN)] again yielded enhanced brain activation during anticipation in SP encompassing the left amygdala, bilateral hippocampus and thalamus. During PP, SP subjects compared to HC exhibited enhanced activation along the entire defensive network including the bilateral ACC, the right insula, the bilateral amygdala, hippocampus, thalamus and the right midbrain. SP compared to DP showed increased activation in the bilateral ACC, left hippocampus and bilateral midbrain. Direct comparisons of the respective phobic stimulus material indicated enhanced activation in SP involving the bilateral inferior frontal gyrus, the left OFC, bilateral insula, amygdala, hippocampus and midbrain during perception (Figure 2). Exploratory whole brain analyses are given in Supplementary Table S2.
Table 2.
ROI analysis on brain activation for group differences, separated for AP and PP
| Group/phase | Region | Side | Voxels | t | P corr. | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Phase: Anticipation | ||||||||
| Stimulus: snake (SA > SN) | ||||||||
| SP > HC | Amygdala | L | 22 | 4.42 | <0.001 | −18 | −6 | −15 |
| Amygdala | R | 15 | 4.07 | 0.001 | 30 | 0 | −27 | |
| Hippocampus | L | 20 | 4.93 | <0.001 | −15 | −6 | −12 | |
| Hippocampus | L | 21 | 4.87 | <0.001 | −21 | −24 | −12 | |
| Hippocampus | R | 15 | 4.08 | 0.004 | 30 | −9 | −24 | |
| Thalamus | R | 16 | 3.53 | 0.023 | 9 | −9 | 3 | |
| SP > DP | Amygdala | L | 25 | 3.99 | 0.001 | −15 | −6 | −18 |
| Hippocampus | L | 17 | 3.95 | 0.007 | −21 | −24 | −12 | |
| Stimulus: dental (DA > DN) | ||||||||
| DP > HC | No differential activation | |||||||
| DP > SP | No differential activation | |||||||
| Stimulus: snake (SA > SN) vs dental (DA > DN) | ||||||||
| SP > DP | Amygdala | L | 32 | 3.93 | 0.002 | −18 | 0 | −18 |
| Hippocampus | L | 16 | 4.45 | 0.001 | −27 | −33 | −3 | |
| Hippocampus | R | 15 | 4.02 | 0.005 | 18 | −30 | −3 | |
| Thalamus | L | 36 | 4.02 | 0.006 | −9 | −15 | 18 | |
| Thalamus | L | 20 | 3.65 | 0.018 | −6 | −27 | 3 | |
| Thalamus | R | 36 | 4.42 | 0.002 | 15 | −6 | 0 | |
| Thalamus | R | 16 | 4.22 | 0.003 | 15 | −27 | −3 | |
| Thalamus | R | 22 | 4.03 | 0.005 | 9 | −24 | 12 | |
| Stimulus: dental (DA > DN) vs snake (SA > SN) | ||||||||
| DP > SP | No differential activation | |||||||
| Phase: Perception | ||||||||
| Stimulus: snake (SA > SN) | ||||||||
| SP > HC | ACC | L | 123 | 5.34 | <0.001 | 0 | 33 | 9 |
| ACC | R | 43 | 4.84 | <0.001 | 3 | 27 | 21 | |
| Insula | R | 61 | 4.50 | 0.002 | 36 | 18 | −6 | |
| Amygdala | L | 26 | 4.19 | 0.001 | −18 | −6 | −15 | |
| Amygdala | R | 20 | 4.66 | <0.001 | 21 | −6 | −12 | |
| Hippocampus | L | 42 | 5.27 | <0.001 | −15 | −9 | −12 | |
| Hippocampus | R | 40 | 5.14 | <0.001 | 21 | −9 | −12 | |
| Thalamus | L | 33 | 4.34 | 0.002 | −3 | −9 | 6 | |
| Thalamus | L | 17 | 3.84 | 0.010 | −21 | −18 | 9 | |
| Thalamus | R | 81 | 4.87 | <0.001 | 12 | −30 | −3 | |
| Midbrain | R | 163 | 5.41 | <0.001 | 18 | −24 | −9 | |
| SP > DP | ACC | L | 78 | 5.12 | <0.001 | 0 | 24 | 18 |
| ACC | R | 29 | 5.22 | <0.001 | 3 | 24 | 15 | |
| Hippocampus | L | 18 | 4.49 | 0.001 | −18 | −27 | −9 | |
| Midbrain | R | 59 | 5.00 | <0.001 | 0 | −33 | −3 | |
| Midbrain | L | 49 | 4.52 | 0.002 | −15 | −24 | −12 | |
| Stimulus: dental (DA > DN) | ||||||||
| DP > HC | No differential activation | |||||||
| DP > SP | No differential activation | |||||||
| Stimulus: snake (SA > SN) vs dental (DA > DN) | ||||||||
| SP > DP | Inferior frontal gyrus | L | 18 | 4.65 | 0.003 | −27 | 15 | −21 |
| Inferior frontal gyrus | R | 34 | 5.49 | <0.001 | 39 | 9 | 30 | |
| Inferior frontal gyrus | R | 30 | 4.71 | 0.003 | 48 | 27 | 0 | |
| Inferior frontal gyrus | R | 17 | 4.62 | 0.004 | 48 | 27 | 15 | |
| OFC | L | 21 | 4.65 | 0.003 | −27 | 15 | −21 | |
| Insula | L | 40 | 5.49 | <0.001 | −27 | 15 | −18 | |
| Insula | R | 15 | 4.12 | 0.007 | 45 | 24 | −3 | |
| Amygdala | L | 42 | 5.37 | <0.001 | −18 | −6 | −15 | |
| Amygdala | R | 36 | 5.62 | <0.001 | 21 | −6 | −12 | |
| Hippocampus | L | 19 | 5.72 | <0.001 | −15 | −6 | −12 | |
| Hippocampus | L | 26 | 4.50 | 0.001 | −18 | −27 | −9 | |
| Hippocampus | R | 26 | 5.83 | <0.001 | 18 | −6 | −12 | |
| Midbrain | L | 41 | 4.91 | 0.001 | −12 | −9 | −12 | |
| Midbrain | R | 20 | 4.28 | 0.005 | 21 | −24 | −6 | |
| Stimulus: dental (DA > DN) vs snake (SA > SN) | ||||||||
| DP > SP | No differential activation | |||||||
DP, dental phobia group; SP, snake phobia group; SN, snake neutral stimuli; SA, snake anxiety stimuli; DN, dental neutral stimuli; DA, dental anxiety stimuli; R, right side; L, left side; Voxels, number of voxels per cluster; x, y, z: MNI coordinates of peak voxel; analysis: P < 0.05 FWE-corrected using a minimum cluster size of 15 voxels.
Fig. 2.
Functional activation patterns (ROI analysis) for the contrast snake anxiety > snake neutral comparing snake phobics (SP) with healthy controls (HC; yellow blobs) vs a phobic control group of dental phobics (DP; red blobs). Activation patterns are separately displayed for the AP (left) and PP (right; P < 0.05 FWE-corrected using a minimum cluster size of 15 voxels). R, right; L, left.
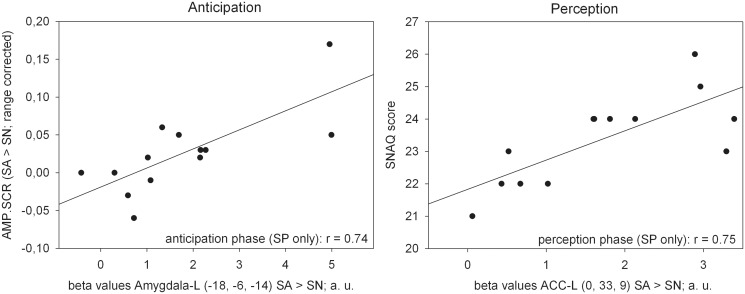
During anticipation, significant correlations were observed between the magnitude of neural activation in the left amygdala and AMP.SCR and between left ACC and phobic fear (SNAQ) during perception of phobic stimuli in the SP group (Table 3 and Figure 3).
Table 3.
Pearson’s correlations between neural (SA > SN) and behavioural data, separately displayed for AP and PP (snake phobics only; n = 13)
| AP: Brain areas (MNI coordinates) | SNAQ scores |
AP: AMP.SCR |
|||
|---|---|---|---|---|---|
| R | P corr | r | P corr | ||
| Amygdala-L | (−18, −6, −14) | 0.31 | 0.312 | 0.74 | 0.004 |
| PP: Brain areas (MNI coordinates) | SNAQ scores | PP: AMP.SCR | |||
|---|---|---|---|---|---|
| ACC-L | (0, 33, 9) | 0.75 | 0.003 | 0.21 | 0.945 |
| Amygdala-R | (21, −6, −12) | 0.54 | 0.056 | 0.65 | 0.017 |
| Midbrain-R | (18, −24, −9) | 0.41 | 0.162 | 0.06 | 0.862 |
SNAQ: Snake Questionnaire; AMP.SCR, sum amplitude of stimulus-specific skin conductance reactions; AP: anticipation phase; PP: perception phase; L, left side; R, right side. Please note that questionnaire data relate to the screening. All P’s Bonferroni-corrected (threshold for significance set at P = 0.00625); significant correlations are bolded.
Fig. 3.
Scatterplots for the magnitude of neural activation in the left amygdala and autonomic arousal (left) during anticipation, and the left anterior cingulate cortex (ACC) and subjective anxiety (right) during perception (snake phobics only; n = 13). SP, snake phobia group; SA, snake anxiety stimuli; SN, snake neutral stimuli; AMP.SCR, sum amplitude of stimulus-specific skin conductance reactions; SNAQ: Snake Questionnaire.
DISCUSSION
This study investigated the neural correlates of defensive reactivity according to different modes of threat imminence in phobia subtypes and yielded the following findings: (i) brain structures associated with the processing of fearful stimuli encompassing the amygdala and hippocampus were activated in SP during both post-encounter (anticipation) and circa-strike (exposure during perception) stages of defence; (ii) only proximal threat during circa-strike defence was associated with enhanced midbrain activity in SP; and (iii) no differential brain activity was observed during any mode of threat imminence in DP.
We applied the concept of defensive distance to specific phobia, assuming that although all humans share a common set of defensive mechanisms, phobics exhibit shorter defensive distances and thus higher threat imminence than non-phobic controls. Present findings support the notion of altered defensive reactivity, at least in animal phobics. Enhanced midbrain activity was detected during circa-strike (PP) of a phobic stimulus in SP, possibly indicating the preparation and/or execution of freezing, fight or flight reactions that are associated with the dorsal periaqueductal grey in animal studies (Brandao et al., 2008). Results support the notion that different networks mediating defensive behaviours were activated with decreasing defensive distance: although medial temporal lobe structures such as the amygdala and hippocampus subserved the detection of potential threats during anticipation and perception, midbrain activity was recruited during confrontation (circa-strike) with a feared object only. Studying the neural organization during anticipation and avoidance of artificial predators in healthy subjects, Mobbs et al. (2009) identified a functional gradient from higher forebrain areas (including the ACC and amygdala) to midbrain structures with decreasing predatory distance. Present results are in line with these findings, indicating that snake phobia may be characterized by enhanced defensive reactivity, including its neural substrates.
Hyperactivation of the amygdala and hippocampus was detected in SP during different modes of defensive reactivity. The amygdala is known to be a core region for the detection of biologically significant stimuli and downstream activation of defensive behaviours (LeDoux, 2007). Activity in this structure has been shown to be abnormally enhanced in animal phobics (Dilger et al., 2003; Schienle et al., 2005, 2007; Straube et al., 2006; Etkin and Wager, 2007; Lipka et al., 2011; Schweckendiek et al., 2011) and sensitive to treatment (Goossens et al., 2007). Furthermore, Lipka et al. (2011) reported enhanced amygdala activation in phobics vs controls not only during conscious but also unconscious processing of phobic stimuli. Anticipation of aversive events (monetary loss) has moreover been reported to activate the amygdala–hippocampal circuit (Hahn et al., 2010). Although medial temporal lobe structures are associated with fear conditioning (Sehlmeyer et al., 2009), the hippocampus is particularly involved in context conditioning (Kalisch et al., 2006). In this regard, anticipation cues could have served as conditioned stimuli announcing a particular context (e.g. anxiety or neutral stimuli of dental or snake conditions). As hippocampus function is also associated with encoding temporal and spatial information (Byrne et al., 2007), jittered APs could have resulted in increased processing of temporal and spatial features of the anticipation cue and the feared stimulus, resembling hypervigilance and environmental scanning for phobic objects. Future studies employing other cognitive or affective probes beyond symptom provocation should further elucidate the role of the hippocampus in the etiopathogenesis of specific phobia.
Although anticipation of aversive events in healthy or anxiety–prone subjects has been associated with insular activity (Simmons et al., 2006; Carlson et al., 2011), this study yielded increased insular activation during the perception, but not anticipation of phobic stimuli in SP. Findings may indicate that the insula is involved in a neurocircuit underlying anticipation in general, but may not necessarily represent pathologically enhanced anticipatory anxiety in specific phobia. However, Straube et al. (2007) reported abnormally enhanced activation in the insula, ACC, thalamus, fusiform gyrus and bed nucleus of the stria terminalis (but not amygdala) during long APs varying between 10 and 28 s. Amygdala activation exhibits a fast habituation profile, being involved in the initial processing of threat (Larson et al., 2006). The choice of an event-related design could have contributed to the detection of amygdala activity, whereas long anticipation blocks as used by Straube et al. (2007) may have increased the power to detect neural activation in other structures not exhibiting fast habituation such as the insula (Lueken et al., 2011b).
Regarding the comparison of phobia types, behavioural data suggested significant differences in stimulus processing between SP and DP. Although subjective ratings indicated successful symptom provocation in both phobic groups, a dissociation of subjective and autonomic arousal was observed in DP, confirming previous reports on missing sympathetically mediated defensive reactions in the BII subtype (Friedman et al., 1993; Hamm et al., 1997). Also, only SP showed faster stimulus-specific RT, indicating hypervigilance and an attentional bias towards threat (Mogg and Bradley, 1998). Neuroanatomically, enhanced attentional resources are likely to be recruited via noradrenergic modulation of attentional networks initialized by downstream amygdala activity (LeDoux, 2007). The presence of sympathetic arousal and amygdala activity in SP but not DP may have contributed to faster RT in the former, but not the latter group.
Based upon recent findings from Caseras et al. (2010b) and Schienle et al. (2013) focusing on immediate stimulus processing, we hypothesized that both SP and DP would show enhanced neural activation during anticipation and immediate processing of a feared stimulus.
Present findings do however indicate rejection of this hypothesis for DP, suggesting critical differences between phobia types also during anticipation and immediate stimulus processing. Using slightly longer presentation times of 4 s (Caseras et al., 2010b) and 3 s (Schienle et al., 2013) (compared to 1250 ms used here), significant activation in brain structures associated with phobic stimulus processing was detected in BII phobics and DP in these studies. However, Caseras et al. (2010b) also reported faster immediate BOLD responses in animal phobics. Lack of activation during immediate processing in DP may be attributable to the shorter presentation times used in the current design, maximizing differences between BOLD response dynamics in SP vs DP. Unlike previous reports (Lueken et al., 2011b), no enhanced OFC activation could be observed in DP. Activation of the lateral OFC in DP has been suggested to represent appraisal of aversive (e.g. dental) situations such that DP process phobic stimuli rather on higher-order evaluation processes than guided by autonomic arousal. Divergent findings in this study could be attributed to the task design (block design vs event-related; static vs dynamic stimuli) that may not involve OFC controlled affective evaluation processes. The relatively small sample size (compared to n = 45 DP from the Schienle et al. study) may have also resulted in insufficient power to detect smaller scale effects in DP in this study, although Schienle et al. (2013) did not detect enhanced amygdala activity in their sample either. Present findings could however indicate that differences in the neural substrates underlying SP and DP are not restricted to sustained processing phases (Lueken et al., 2011b).
Study limitations
Subjects were selected based upon clinical cut-offs; findings should be applied to treatment-seeking samples. Results are further limited to the subgroup of DP and do not apply to BII phobics in general. Due to the small sample size, the study could have been underpowered which might have prevented the detection of smaller-scale effects particularly in the DP group. Inclusion of a RT task to assess basic attentional biases could have altered neural activation patterns when compared to passive viewing only. However, amygdala activation has been reported to be enhanced during conscious and unconscious stimulus processing in phobic subjects (Lipka et al., 2011). Full randomization of stimuli controlled for potential expectation and order effects; the resulting regressors could however been differentially affected by the high-pass filter. The study would have benefited from a multimodal assessment of defensive behaviours. Startle response is a promising measure for future studies to include, as this index is selectively inhibited under proximal, but not distal threat (Richter et al., 2012). Also, other cognitive–affective probes such as active avoidance could be included into the task design. In this regard, recent findings (Caseras et al., 2013) on the anatomical and functional overlap between symptom provocation and interoceptive processing in animal, but not BII phobia are of particular interest.
In conclusion, investigation of defensive reactivity in specific phobia substantiated neural correlates indicative of shortened defensive distance in SP, who exhibited elevated activation in key neural networks for fear processing focusing on the amygdala and hippocampus during anxious apprehension and immediate confrontation with the feared object. With decreasing defensive distance, enhanced defensive reactivity in SP was characterized by a shift to midbrain activity. Results further indicate differences between SP and DP in the physiological and neural organization extending to the anticipation and immediate processing of phobic stimuli. Findings may contribute to a better understanding of the dynamic organization of defensive behaviours in different types of phobic fear and help to characterize pathological forms of anxiety based on a mechanism-based approach.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
The following authors report no conflicts of interest concerning the content of this paper: U.L., K.H., V.S., N.I.M., and K.B.-B. H.-U.W. has been member of advisory boards of several pharmaceutical companies. He received travel reimbursements and research grant support from Essex Pharma, Sanofi, Pfizer, Organon, Servier, Novartis, Lundbeck, Glaxo Smith Kline.
Supplementary Material
REFERENCES
- American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders IV, text revision. Washington, DC: American Psychiatric Association.
- Benedek M, Kaernbach C. Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology. 2010;47:647–58. doi: 10.1111/j.1469-8986.2009.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Hynd AL, Minke KA, Minemoto T, Blanchard RJ. Human defensive behaviours to threat scenarios show parallels to fear- and anxiety-related defense patterns of non-human mammals. Neuroscience and Biobehavioral Reviews. 2001;25:761–70. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behavioural Brain Research. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychological Review. 2007;114:340–75. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Social Cognitive and Affective Neuroscience. 2011;6:74–81. doi: 10.1093/scan/nsq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Giampietro V, Lamas A, et al. The functional neuroanatomy of blood-injection-injury phobia: a comparison with spider phobics and healthy controls. Psychological Medicine. 2010a;40:125–34. doi: 10.1017/S0033291709005972. [DOI] [PubMed] [Google Scholar]
- Caseras X, Mataix-Cols D, Trasovares MV, et al. Dynamics of brain responses to phobic-related stimulation in specific phobia subtypes. European Journal of Neuroscience. 2010b;32:1414–22. doi: 10.1111/j.1460-9568.2010.07424.x. [DOI] [PubMed] [Google Scholar]
- Caseras X, Murphy K, Mataix-Cols D, et al. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Human Brain Mapping. 2013;34:1220–9. doi: 10.1002/hbm.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Dilger S, Straube T, Mentzel HJ, et al. Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neuroscience Letters. 2003;348:29–32. doi: 10.1016/s0304-3940(03)00647-5. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–38. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF, Borkovec TD, Tyrrell RA, Johnson BH, Columbo R. Autonomic characteristics of nonclinical panic and blood phobia. Biological Psychiatry. 1993;34:298–310. doi: 10.1016/0006-3223(93)90087-t. [DOI] [PubMed] [Google Scholar]
- Globisch J, Hamm AO, Esteves F, Ohman A. Fear appears fast: temporal course of startle reflex potentiation in animal fearful subjects. Psychophysiology. 1999;36:66–75. doi: 10.1017/s0048577299970634. [DOI] [PubMed] [Google Scholar]
- Goossens L, Sunaert S, Peeters R, Griez EJL, Schruers KRJ. Amygdala hyperfunction in phobic fear normalizes after exposure. Biological Psychiatry. 2007;62:1119–25. doi: 10.1016/j.biopsych.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Plichta MM, et al. Functional amygdala-hippocampus connectivity during anticipation of aversive events is associated with Gray’s trait “sensitivity to punishment”. Biological Psychiatry. 2010;68:459–64. doi: 10.1016/j.biopsych.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Hamm A. Spezifische Phobien. Göttingen: Hogrefe; 2006. [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and the startle reflex: blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Hermann A, Schafer A, Walter B, Stark R, Vaitl D, Schienle A. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biological Psychology. 2007;75:124–30. doi: 10.1016/j.biopsycho.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of Neuroscience. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–7. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Lebeau RT, Glenn D, Liao B, et al. Specific phobia: a review of DSM-IV specific phobia and preliminary recommendations for DSM-V. Depression and Anxiety. 2010;27:148–67. doi: 10.1002/da.20655. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Current Biology. 2007;17:R868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Linares IMP, Trzesniak C, Chagas MHN, Hallak JEC, Nardi AE, Crippa JAS. Neuroimaging in specific phobia disorder: a systematic review of the literature. Revista Brasileira de Psiquiatria. 2012;34:101–11. [PubMed] [Google Scholar]
- Lipka J, Miltner WHR, Straube T. Vigilance for threat interacts with amygdala responses to subliminal threat cues in specific phobia. Biological Psychiatry. 2011;70:472–8. doi: 10.1016/j.biopsych.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Lueken U, Hoyer J, Siegert J, Gloster AT, Wittchen HU. Symptom provocation in dental anxiety using cross-phobic video stimulation. European Journal of Oral Sciences. 2011a;119:61–8. doi: 10.1111/j.1600-0722.2010.00790.x. [DOI] [PubMed] [Google Scholar]
- Lueken U, Kruschwitz JD, Muehlhan M, Siegert J, Hoyer J, Wittchen HU. How specific is specific phobia? Different neural response patterns in two subtypes of specific phobia. Neuroimage. 2011b;56:363–72. doi: 10.1016/j.neuroimage.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Lykken DT. Range correction applied to heart rate and to GSR data. Psychophysiology. 1972;9:373–9. doi: 10.1111/j.1469-8986.1972.tb03222.x. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neuroscience and Biobehavavioral Reviews. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, et al. From threat to fear: the neural organization of defensive fear systems in humans. The Journal of Neuroscience. 2009;29:12236–43. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, et al. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–83. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behavioural Research Therapy. 1998;36:809–48. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Oosterink FMD, De Jongh A, Aartman IHA. What are people afraid of during dental treatment? Anxiety-provoking capacity of 67 stimuli characteristic of the dental setting. European Journal of Oral Sciences. 2008;116:44–51. doi: 10.1111/j.1600-0722.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- Richter J, Hamm AO, Pané-Farré C, et al. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: implications for the etiology of panic disorder. Biological Psychiatry. 2012;72:512–20. doi: 10.1016/j.biopsych.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:486–93. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Walter B, Stark R, Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neuroscience Letters. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Schienle A, Scharmuller W, Leutgeb V, Schafer A, Stark R. Sex differences in the functional and structural neuroanatomyof dental phobia. Brain Structure and Function. 2013;218:779–87. doi: 10.1007/s00429-012-0428-z. [DOI] [PubMed] [Google Scholar]
- Schweckendiek J, Klucken T, Merz CJ, et al. Weaving the (neuronal) web: fear learning in spider phobia. Neuroimage. 2011;54:681–8. doi: 10.1016/j.neuroimage.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. Plos One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60:402–9. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Tönnies S, Mehrstedt M, Eisentraut I. Die Dental Anxiety Scale (DAS) und das Dental Fear Survey (DFS)—Zwei Messinstrumente zur Erfassung von Zahnbehandlungsängsten. Zeitschrift fur Medizinische Psychologie. 2002;11:63–72. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Pfister H. DIA-X Interview. Frankfurt: Swets & Zeitlinger; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.