Abstract
Behavioral habituation during repeated exposure to aversive stimuli is an adaptive process. However, the way in which changes in self-reported emotional experience are related to the neural mechanisms supporting habituation remains unclear. We probed these mechanisms by repeatedly presenting negative images to healthy adult participants and recording behavioral and neural responses using functional magnetic resonance imaging. We were particularly interested in investigating patterns of activity in insula, given its significant role in affective integration, and in amygdala, given its association with appraisal of aversive stimuli and its frequent coactivation with insula. We found significant habituation behaviorally along with decreases in amygdala, occipital cortex and ventral prefrontal cortex (PFC) activity with repeated presentation, whereas bilateral posterior insula, dorsolateral PFC and precuneus showed increased activation. Posterior insula activation during image presentation was correlated with greater negative affect ratings for novel presentations of negative images. Further, repeated negative image presentation was associated with increased functional connectivity between left posterior insula and amygdala, and increasing insula–amygdala functional connectivity was correlated with increasing behavioral habituation. These results suggest that habituation is subserved in part by insula–amygdala connectivity and involves a change in the activity of bottom-up affective networks.
Keywords: habituation, emotion, functional connectivity, insula, amygdala
INTRODUCTION
When confronted with the same emotional stimulus repeatedly, it is natural to become inured to a degree. This natural process underlies many desensitization-based psychotherapies (Foa and Kozak, 1986). Habituation is a long-recognized behavioral and physiological consequence of repeated stimulus presentation across many species (Thompson and Spencer, 1966), governed at the cellular level by plasticity in synaptic transmission (Castellucci et al., 1970; Kupfermann et al., 1970; Pinsker et al., 1970; Castellucci and Kandel, 1974). Although the fact that habituation occurs is not surprising, examining the neural mechanisms that subserve this phenomenon for emotional stimuli in the human brain and their relationship to self-reported emotional experience may assist in understanding the biological basis of exaggerated or attenuated habituation responses in various forms of psychopathology.
We focused the current investigation on the connectivity of brain areas that have been closely linked to affective appraisal, principally including the insula and amygdala. Given the important role of the insula in affective, cognitive and visceral integration (Augustine, 1996; Flynn et al., 1999; Craig, 2003, 2009; Wager and Barrett, 2004; Singer et al., 2009) and emotional appraisal (Phan et al., 2002; Kober et al., 2008), and particularly given its role in negative emotional appraisal (Phillips et al., 1997; Damasio et al., 2000; Wicker et al., 2003; Ochsner and Gross, 2008; Denny et al., 2009), the insula represented an important region of interest. Further, given the central role played by the insula in affective integration as noted earlier, the insula represented an a priori seed region candidate for analyses of functional connectivity with other brain regions during habituation.
Prior anatomical (Mesulam and Mufson, 1982a,b) and resting state functional connectivity (Deen et al., 2011) work has provided evidence for functional subdivisions within insula. Posterior insula has been particularly associated with primary visceral sensation (Ploghaus et al., 1999; Wager and Barrett, 2004; Deen et al., 2011) and heteromodal integration across all five senses (Flynn et al., 1999), whereas there is evidence for a dorsal–ventral distinction in anterior insula, with dorsal anterior insula being more associated with cognitive control and ventral anterior insula being more associated with affective experience (Wager and Barrett, 2004; Deen et al., 2011). Prior work examining the neural correlates of habituation to emotional stimuli in insula has provided mixed results, however, with evidence for both decreasing activation (Feinstein et al., 2002; Ishai et al., 2004) and increasing activation (Feinstein et al., 2002) upon repeated presentation of emotional stimuli.
Amygdala activity, by contrast has consistently been shown to decrease upon repeated presentation of negative stimuli (Breiter et al., 1996; Fischer et al., 2000, 2003; Wright et al., 2001; Phan et al., 2003; Ishai et al., 2004; Britton et al., 2008), which may reflect the diminishment of an initial orienting response to salient affective stimuli (Holland and Gallagher, 1999; Fischer et al., 2003; Britton et al., 2008). Further, and importantly, amygdala has been shown to both frequently co-activate with insula (Stein et al., 2007; Kober et al., 2008; Etkin and Wager, 2010) as well as share significant structural connectivity with insula (Mufson et al., 1981; Mesulam and Mufson, 1982b; Flynn et al., 1999). Thus, the amygdala represented an additional key region of interest in this work.
Along with insula and amygdala, other brain regions have been broadly linked to habituation, including hippocampus and parahippocampal gyrus. Like amygdala, hippocampus and parahippocampal gyrus play a critical role in memory encoding and stimulus recognition (Phelps, 2004), and hippocampal activity has been shown to decrease upon repeated stimulus presentation (Fischer et al., 2000, 2003; Feinstein et al., 2002; Phan et al., 2003).
The functional interrelationship between insula, amygdala and other brain regions underlying habituation to negative images remains unclear, however, as does the way these relationships are related to habituation behavior (i.e. self-reports of affective appraisal). In order to probe these two questions as a means to understand the neural mechanisms governing habituation, we employed an event-related functional magnetic resonance imaging (fMRI) task paradigm involving repeated presentations of negative and neutral images. Although functional connectivity patterns have not been established for habituation, prior structural equation modeling work has supported a significant positive coupling between insula and amygdala activity when appraising negative stimuli a single time (Stein et al., 2007). Thus, we made this same prediction for increasing connectivity between insula and amygdala upon repeated presentation of negative stimuli, and we further predicted that such connectivity increases would be positively correlated with greater behavioral habituation.
METHODS
Participants
Participants were 29 healthy volunteers who were free from any DSM-IV Axis I or Axis II disorders (American Psychiatric Association, 1994), as assessed by the Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-IP) and the Structured Interview for Personality Disorders (SID-P). Participants with significant medical illness, contraindications to fMRI and pregnant women were excluded. Participants provided informed consent to participate in this study as approved by the Institutional Review Boards of the Mount Sinai School of Medicine and the James J Peters Veterans Affairs Medical Center. Of these, two participants were excluded for excessive head motion, one participant was excluded due to significant artifacts and poor fMRI signal quality, and one participant was excluded due to making no behavioral response for over one-third of the trials. Therefore, 25 healthy adult participants were included in the present analyses (mean age = 27.96 years, s.d. = 6.86 years, 13 female).
Materials
A total of 48 negative and 48 neutral images were used. These consisted of 22 negative [mean valence = 2.35 (1 = most negative to 9 = most positive), mean arousal = 5.80 (1 = least arousing to 9 = most arousing)] and 48 neutral (mean valence = 5.14, mean arousal = 3.81) images from the International Affective Picture System (IAPS) (Lang et al., 1993) and 26 negative images [mean valence = −1.47 (–3 = most negative to 3 = most positive)] from the Empathy Picture System (EPS) (Geday et al., 2001). The mean valence and arousal ratings were derived from the canonical data provided with each image set. Image themes were comparable across IAPS and EPS image sets (violence, death, anger, sadness, disgust).
Task design
Participants completed a habituation task in an fMRI scanner consisting of up to two presentations of a given negative or neutral image. Therefore, four conditions were present corresponding to the 2 × 2 design of valence (negative and neutral) and novelty (novel and repeat): NovelNeg, RepeatNeg, NovelNeut and RepeatNeut. Participants were told that they should view each image and respond naturally, and that some images might be repeated. Neutral images were presented as buffer stimuli so that participants would not become inured to negative image presentations overall; no specific hypotheses were made regarding habituation to neutral stimuli, which are not the focus of the present analyses.
Images were presented for 4 s each. After each image, participants were asked to rate their affective response to that image on a scale from 1 to 5 (with 1 being most negative and 5 being most positive) during a 3 s rating period. Following the rating period, each trial concluded with a 3 s inter-trial interval in which a blank screen was presented. The interval between the initial presentation of an image and its repetition was 5.3 min, and the image-repeat pairs were distributed uniformly throughout the 27 min of the task to control for potential confounds of practice, fatigue or scanner drift. This trial design is illustrated in Figure 1. Two-thirds of images were presented twice. Trials were presented in five pseudorandomly ordered blocks of 32 trials each, yielding 160 total trials (48 NovelNeg, 32 RepeatNeg, 48 NovelNeut, 32 RepeatNeut). Images were counterbalanced across participants for whether the image was repeated or not.
Fig. 1.
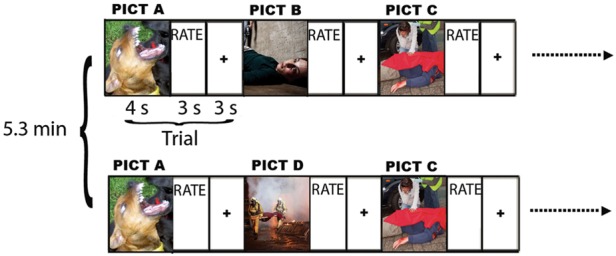
Trial design for habituation paradigm. Each image is presented for 4 s and is followed by a 3 s rating interval when the participant rates the image on a 1 (most negative) to 5 (most positive) scale. This is followed by a 3 s interstimulus interval. Images are shown in five consecutive blocks of 16 negative and 16 neutral images. Two-thirds of the images are shown a second time. The repeat images are always shown 5.3 min after the first showing of that image, and images and repeats are distributed uniformly throughout the 27 min of the task. Novel and repeat images are counterbalanced among participants. Reprinted with permission from The American Journal of Psychiatry, (Copyright © 2013). American Psychiatric Association.
Data acquisition and analysis
Behavior
Behavioral data were acquired using a 5-button hand pad during fMRI scanning and recorded using E-Prime software (Psychology Software Tools, Inc.). These data were analyzed using linear mixed models incorporating fixed effects estimates for valence (negative and neutral), novelty (novel and repeat) and their interaction, and a random effect consisting of an intercept for each participant.
Functional MRI
Whole-brain fMRI data were acquired on a 3.0 T Siemens Allegra scanner (Siemens Medical Solutions USA) with a gradient-echo echo-planar imaging sequence using the following protocol: 42 axial slices, 2.5 mm thickness, skip = 0.825 mm, repetition time = 3 s, TE = 27 ms, flip angle = 84°, field of view = 210 mm, matrix = 64 × 64. Slices were acquired in an interleaved ascending order. Data were acquired in one functional run of 594 volumes. A high-resolution T2-weighted anatomical scan was also acquired on an axial plane with a turbo spin-echo pulse sequence.
Preprocessing was carried out using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) using standard parameters: slice-timing correction, realignment and coregistration between each participant’s functional and anatomical data, normalization to a standard template [Montreal Neurological Institute (MNI)] using 3 mm isotropic voxels, and spatial smoothing with a Gaussian kernel (full-width at half maximum = 7 mm).
General linear modeling (GLM) for each participant was carried out using Neuroelf software (neuroelf.net) by convolving task event vectors (defined below) with the canonical hemodynamic response function. Five vectors were specified in the GLM: four vectors corresponding to the 4 s stimulus periods of each trial for each condition (NovelNeg, RepeatNeg, NovelNeut, RepeatNeut); and a vector modeling the 3 s rating period, which was undifferentiated by condition. Further, parametric weights corresponding to each participant’s trial-by-trial affect reports were specified for each stimulus period. Participants’ six motion parameters were also included in the GLM. Data were high-pass filtered (cut-off = 130 s), and participant timecourses underwent percent signal change transformation.
Contrast images for all participants were entered into random-effects between-subjects analyses using Neuroelf software. The primary contrast of interest was (RepeatNeg − NovelNeg). Whole-brain familywise error (FWE) multiple comparison correction thresholds were determined using Alphasim (Ward, 2000). Anatomical labels were determined using an International Consortium for Brain Mapping (ICBM) to Talairach coordinate conversion (icbm2tal.m) and the Talairach atlas (Talairach and Tournoux, 1988). Reported coordinates are in MNI space.
Insula seed region determination via parametric analysis
As noted above, given the extensive role of the insula in affective and visceral integration, we were particularly interested to interrogate insula as a seed region in subsequent functional connectivity analyses. In order to define insula seed region voxels in an unbiased, theoretically relevant manner, we performed a parametric analysis examining the covariance between within-subject fMRI timecourses and trial-by-trial affective response to novel presentations of negative images (i.e. NovelNeg) with a focus on whether such an effect would be observed in insula, as predicted. This seed region definition strategy was independent of subsequent functional connectivity to other brain regions.
Functional connectivity analyses
Functional connectivity (psychophysiological interaction, PPI) (Friston et al., 1997) analyses were then performed using the insula seed region determined via parametric analysis (and described in the results). A new GLM was computed incorporating regressors for the coupling of activity between the seed region and other brain areas, as well as a PPI term representing the interaction of the coupling of the seed region and other brain regions modulated by the psychological context of interest, in this case the change between NovelNeg and RepeatNeg conditions (i.e. RepeatNeg minus NovelNeg). Participants’ six motion parameters were also included in the GLM. Data were high-pass filtered as detailed earlier, and participant timecourses underwent percent signal change transformation. Following GLM estimation, random effects analyses were performed as above, with contrasts in this case representing areas showing a significant PPI effect. Amygdala results were masked using a Brodmann atlas-based anatomical boundary, and FWE extent thresholds were small volume-corrected using this bilateral anatomical amygdala mask. Results were then statistically thresholded as described earlier.
RESULTS
Behavior
Self-reported negative affect results are shown in Figure 2. A significant main effect of valence (neutral > negative) was present, F(1,72) = 1304.09, P < 0.01. However, there was no main effect of novelty (F < 1), nor was there a significant interaction between valence and novelty (F = 1.33, n.s.). A main effect of novelty (i.e. habituation) across image valences may have been obscured, however, by the fact that neutral images, viewed in the context of negative images, were scored more positively on first viewing, but then more neutrally on second viewing (i.e. relatively less positive appraisals on repeat viewing), whereas repeated presentation of negative images resulted in more positive appraisals. Given our explicit a priori hypothesis regarding habituation for negative images in particular, we conducted planned comparisons of potential habituation effects within each image valence using paired t-tests. There was a significant habituation effect among negative images (with RepeatNeg images rated less negatively than NovelNeg images), t(24) = 2.71, P < 0.02, two-tailed. In addition, neutral images, which were interspersed with negative images, were rated positively when first viewed and more neutrally upon repeat viewing, t(24) = −2.39, P < 0.03, two-tailed.
Fig. 2.
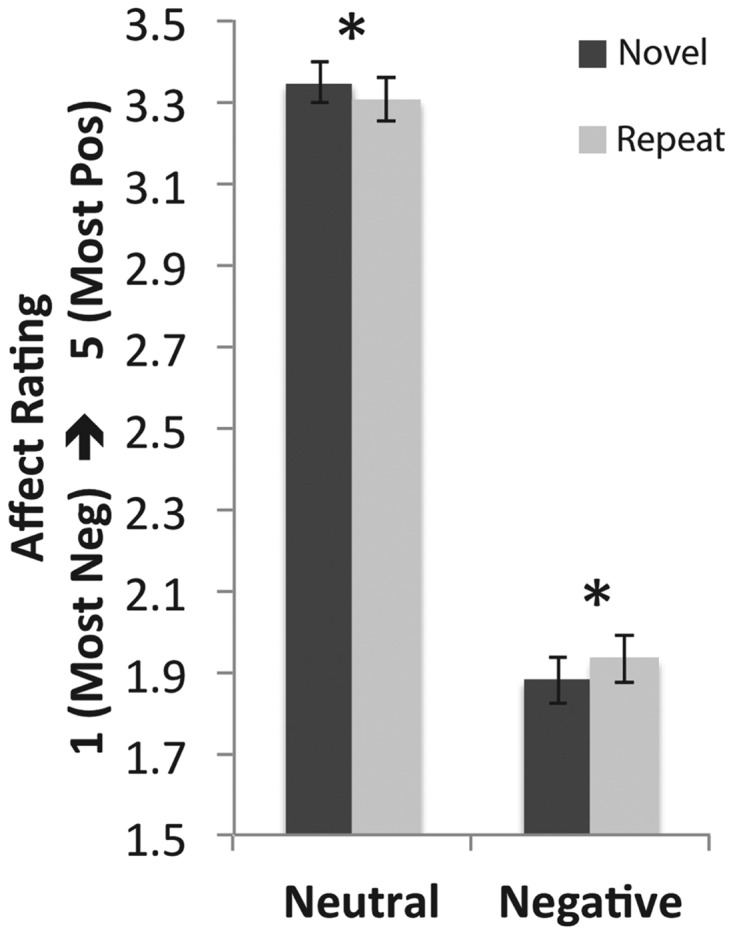
Behavioral results. Significant habituation of negative affect for negative images (P < 0.02, two-tailed) and reduction in positive affect ratings toward more neutral for neutral images (P < 0.03, two-tailed) for second image presentation relative to the first were observed. *P < 0.05 (two-tailed).
Insula seed region determination via parametric analysis
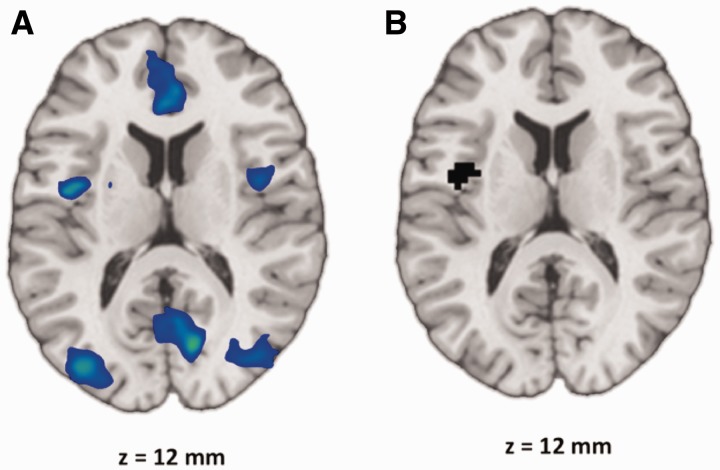
The insula was selected to provide a seed region in view of its central role in affective integration. We functionally defined the insula seed region on the basis of a parametric relationship between subjective affective self-reports and neural activation during novel presentations of negative images. Bilateral mid- and posterior insula activity showed a negative parametric relationship with trial-by-trial affective response, such that increasing insula activation was associated with increasingly negative affect reports for novel presentations of negative images (i.e. NovelNeg) (Figure 3A). Regarding seed determination, no explicit hypothesis was made with respect to insula laterality. Right posterior insula was interrogated as a seed region (using the region identified in the parametric analysis; peak at [42, 3, 0], 150 voxels, thresholded at P < 0.01, uncorrected, FWE-corrected, P < 0.05) and produced results that were consistent, albeit weaker than those reported here using the left posterior insula seed (see Supplementary Results and Supplementary Figure S4). Thus, the left posterior insula result identified in this parametric analysis became the seed region for subsequent functional connectivity analyses (local maximum of region shown in Figure 3A; peak at [−39, −3, 12], 62 voxels, thresholded at P < 0.01, uncorrected, FWE-corrected, P < 0.05). These left posterior insula seed region voxels are shown in Figure 3B.
Fig. 3.
(A) Parametric map for NovelNeg showing that increasing bilateral insula activation is associated with increasingly negative trial-by-trial affect reports. Thresholded at P < 0.01, uncorrected, extent threshold = 1539 mm3, FWE-corrected, P < 0.05. (B) Left posterior and mid-insula seed region voxels at z = 12 mm.
Whole-brain response to repeated viewing of negative pictures and correlations with behavioral habituation
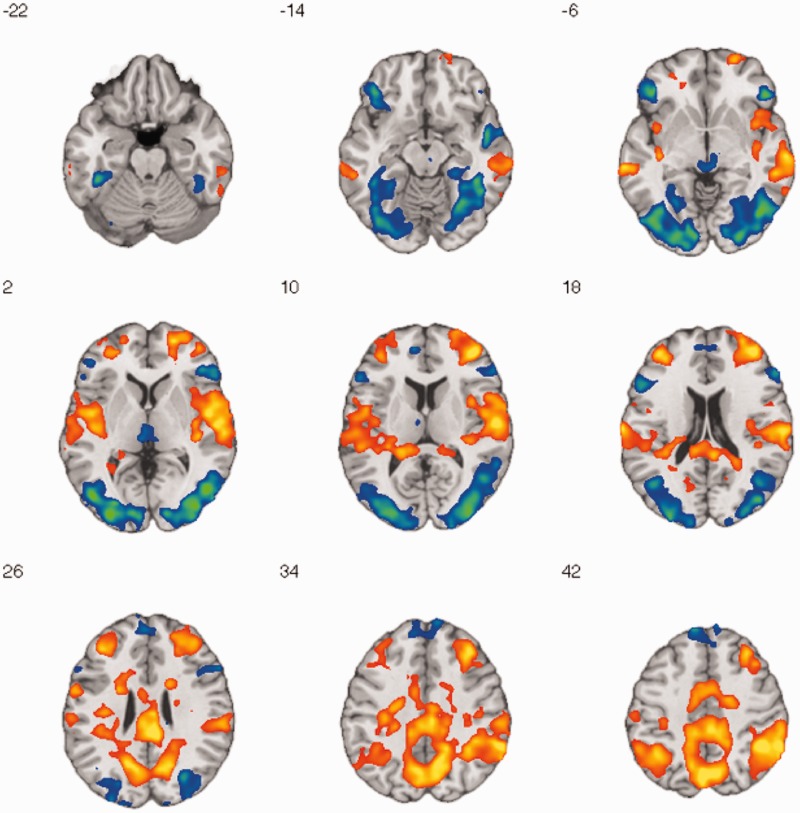
Before addressing our primary question regarding the interrelationship of insula and amygdala activity during habituation, we first conducted whole-brain analyses examining effects of repeated picture viewing and interactions with image valence. Whole-brain results for the novelty × valence interaction contrast [(RepeatNeg − NovelNeg) − (RepeatNeut − NovelNeut)] are shown in the Supplementary Results (Supplementary Figure S3). Regarding our primary interest concerning responses to negative images in particular, Figure 4 shows regions of decreased (NovelNeg > RepeatNeg) and increased (RepeatNeg > NovelNeg) activity across the brain with repeated viewing of negative images. Cool colors represent regions showing decreased activation and warm colors represent regions showing increased activation. These results are also shown in Table 1. Significant decreased activity was observed in occipital regions including the fusiform gyrus, amygdala, hippocampus, thalamus, and ventrolateral and dorsomedial PFC. Increased activity was observed in bilateral mid- and posterior insula, dorsolateral PFC, and a broad region of posterior cingulate cortex and precuneus.
Fig. 4.
Brain regions showing decreased and increased activity with repeated negative image presentation. Axial montage shows z = −22 to 42 mm, thresholded at P < 0.01, uncorrected, extent threshold = 1539 mm3, FWE-corrected, P < 0.05. Cool colors represent decreased activity (NovelNeg > RepeatNeg), whereas warm colors represent increased activity (RepeatNeg > NovelNeg). Coordinate labels are in MNI space.
Table 1.
Brain regions showing decreased (NovelNeg > RepeatNeg; top) and increased (RepeatNeg > NovelNeg; bottom) activity with repeated negative image presentation
| Region | x | y | z | k | tmax | |
|---|---|---|---|---|---|---|
| Decreased activity (NovelNeg > RepeatNeg) | ||||||
| Middle occipital gyrus (BA 37) | RH | 48 | −69 | 0 | 1615 | 7.729 |
| Middle occipital gyrus (BA 19) | LH | −45 | −75 | 0 | 1689 | 7.200 |
| Inferior frontal gyrus (BA 45) | RH | 48 | 33 | −3 | 216 | 6.137 |
| Inferior frontal gyrus (BA 47) | LH | −45 | 33 | −6 | 252 | 5.936 |
| Superior temporal gyrus (BA 22) | RH | 51 | −3 | −15 | 63 | 5.283 |
| Superior frontal gyrus (BA 8) | LH | −9 | 51 | 48 | 306 | 5.213 |
| Thalamus (Pulvinar) | LH | −3 | −30 | −3 | 113 | 4.480 |
| Amygdalaa | RH | 24 | −6 | −15 | 16 | 4.563 |
| Increased activity (RepeatNeg > NovelNeg) | ||||||
| Inferior frontal gyrus (BA 46) | RH | 42 | 48 | 12 | 862 | 9.051 |
| Inferior parietal lobule (BA 40) | RH | 45 | −45 | 42 | 8171 | 8.934 |
| Middle frontal gyrus (BA 46) | LH | −36 | 45 | 15 | 347 | 6.706 |
Thresholded at P < 0.01, uncorrected, extent threshold = 1539 mm3, FWE-corrected, P < 0.05. tmax refers to maximum t-scores for each cluster. Coordinates are in MNI space and refer to the peak activation. aFWE small volume-corrected, P < 0.05, two-tailed.
We further explored the direct relationship between increases and decreases in neural activity upon repeated presentation (i.e. neural habituation) and behavioral habituation via a whole-brain search for regions where RepeatNeg − NovelNeg beta values were correlated with RepeatNeg − NovelNeg affect reports. No correlation was observed in amygdala, and the only FWE-corrected result was found in precentral gyrus (peak at [45, −12, 51], 129 voxels, thresholded at P < 0.01, uncorrected, FWE-corrected, P < 0.05), with greater neural habituation being correlated with greater behavioral habituation. However, bilateral posterior insula did also show this effect (greater decreases in activity—i.e. neural habituation—being correlated with greater behavioral habituation) at a relaxed threshold (P < 0.05, extent = 675 mm3; Supplementary Figure S1).
Changes in functional connectivity between insula, amygdala and other brain regions when viewing repeat vs novel negative pictures
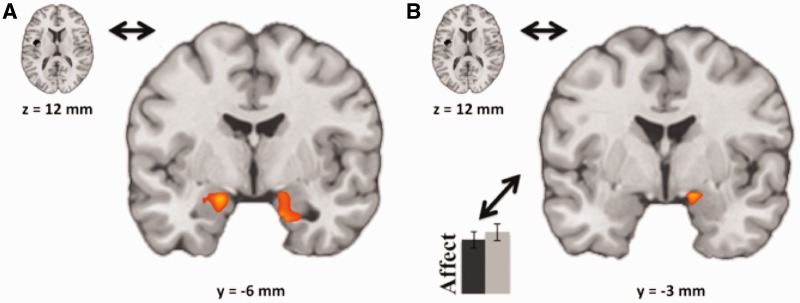
With respect to our primary question regarding the functional interrelationship between insula and amygdala during habituation, Figure 5 shows a significant and robust PPI effect representing functional connectivity between the left posterior insula seed region and bilateral amygdala (Figure 5A; left amygdala peak at [−18, −6, −18], 31 voxels; and right amygdala peak at [30, 0, −24], 42 voxels; each thresholded at P < 0.01, uncorrected, FWE small volume-corrected, P < 0.05, two-tailed). This result indicates that activity in the left posterior insula and the bilateral amygdala becomes increasingly coupled for RepeatNeg trials relative to NovelNeg trials.
Fig. 5.
(A) PPI results showing increased connectivity (warm colors) between the left posterior insula seed region and bilateral amygdala when seeing RepeatNeg relative to NovelNeg images. Thresholded at P < 0.01, uncorrected, extent threshold = 189 mm3, FWE small volume-corrected, P < 0.05, two-tailed. (B) Correlation of PPI results with behavioral habituation (affect) in right amygdala, showing that increasing connectivity between the left posterior insula seed region and amygdala when viewing RepeatNeg relative to NovelNeg images is correlated with increasing behavioral habituation. Thresholded at P < 0.05, uncorrected, extent threshold = 432 mm3, FWE small volume-corrected, P < 0.05, two-tailed.
Further, in order to better understand the relationship between overall decreased amygdala activity, overall increased insula activity and increased insula–amygdala connectivity during repeated viewing of negative pictures, we correlated RepeatNeg − NovelNeg beta values in the left posterior insula seed region (Figure 3B) with the insula–amygdala functional connectivity effects shown in Figure 5A. Although amygdala activity decreased and insula activity increased overall [including in the left posterior seed region in particular, t(24) = 3.17, P < 0.01, two-tailed; see Figure 4], and although insula–amygdala functional connectivity increased overall (see Figure 5A), a trend was observed for greater increases in insula–amygdala functional connectivity (using the independently defined 42 voxel right amygdala result in the insula–amygdala PPI analysis shown in Figure 5A and Table 2) being correlated with greater decreases in activity of the insula seed region overall (r = −0.30, P < 0.08, one-tailed). Thus, while on average amygdala activity decreased, posterior insula activity increased, and functional connectivity between the two increased with repeated viewing, a trend was observed with posterior insula decreasing activity in participants who showed the greatest increase in insula–amygdala connectivity.
Table 2.
PPI analysis results: changes in functional connectivity between left posterior insula and other brain regions when viewing repeat vs novel negative pictures
| Region | x | y | z | k | tmax | |
|---|---|---|---|---|---|---|
| Superior frontal gyrus (BA 10) | RH | 9 | 69 | 18 | 59 | 6.720 |
| Superior temporal gyrus (BA 22) | LH | −48 | −12 | −15 | 224 | 5.157 |
| Parahippocampal gyrus | RH | 27 | −18 | −21 | 241 | 4.720 |
| Precuneus (BA 7) | RH | 24 | −54 | 60 | 74 | 4.719 |
| Precentral gyrus (BA 6) | RH | 54 | 3 | 33 | 115 | 4.654 |
| Amygdalaa | RH | 30 | 0 | −24 | 42 | 4.189 |
| Amygdalaa | LH | −18 | −6 | −18 | 31 | 5.101 |
Thresholded at P < 0.01, uncorrected, extent threshold = 1539 mm3, FWE-corrected, P < 0.05. tmax refers to maximum t-scores for each cluster. Positive max values reflect increased connectivity between seed region (left posterior insula) and given brain region for second (RepeatNeg) relative to first viewing (NovelNeg). Coordinates are in MNI space and refer to the peak activation. aFWE small volume-corrected, P < 0.05, two-tailed.
With respect to the whole-brain examination of functional connectivity to the left posterior insula seed, notably little functional connectivity change between NovelNeg and RepeatNeg trials was observed in PFC areas previously associated with top-down cognitive control and explicit emotion regulation, including ventrolateral and dorsolateral PFC (Ochsner and Gross, 2008; Denny et al., 2009; Supplementary Figure S2 and Table 2).
Relationship between functional connectivity effects and behavioral habituation
Crucially, our second question was addressed by our final analysis that sought to contextualize the above functional connectivity effects regarding their relationship with self-reported behavior during habituation. Figure 5B then shows that, within right amygdala, increasing connectivity between left posterior insula and right amygdala is correlated with increasingly large amounts of average behavioral habituation (peak at [15, −3, −15], 20 voxels, thresholded at P < 0.05, uncorrected, FWE small volume-corrected, P < 0.05, two-tailed). No other brain regions showed FWE-corrected effects.
DISCUSSION
We investigated the neural mechanisms underlying habituation by examining the relationships between habituation-related brain activity and affective experience. In doing so, we examined patterns of functional connectivity among key appraisal-related brain regions including the posterior insula and amygdala. With respect to our two primary questions of interest, we have shown that functional connectivity between insula and amygdala increases between novel and repeated presentations of a negative image, and this increased connectivity is associated with increasing levels of behavioral habituation. These results elucidate the shared roles of insula and amygdala in integration of affective information.
Consistent with prior work, we found that behavioral habituation occurs with repeated viewing of negative images and, correspondingly, there is evidence of decreased activity (i.e. RepeatNeg < NovelNeg) in key subcortical loci involved in memory encoding and attentional orienting toward negative stimuli such as the amygdala (Breiter et al., 1996; Fischer et al., 2000, 2003; Wright et al., 2001; Phan et al., 2003; Ishai et al., 2004; Britton et al., 2008) and hippocampus (Fischer et al., 2000, 2003; Feinstein et al., 2002; Phan et al., 2003). Further, consistent with Wright et al. (2001), we observed habituation in right, but not left, amygdala and parahippocampal gyrus.
In addition, consistent with Feinstein et al. (2002), we observed increased rather than decreased activity (i.e. RepeatNeg > NovelNeg) in left posterior insula for repeated negative image presentation, although this effect was also observed bilaterally and extended toward mid-insula as well and was shown to be specific to negative rather than neutral images in the present results. This stands in contrast to more anterior regions of the insula that have been shown to habituate to negative stimuli (Ishai et al., 2004). Given that posterior insula has often been associated with negative emotion reactivity (Ochsner and Gross, 2008; Denny et al., 2009, and in the present results) and visceral affective sensation and integration (Augustine, 1996; Flynn et al., 1999; Wager and Barrett, 2004), we were motivated to examine the relationship between individual differences in posterior insula increases and decreases in activity and the insula–amygdala functional connectivity results described here. We found a trend such that greater increases in insula–amygdala functional connectivity were associated with greater decreases in activity of posterior insula. We also observed increases in activity in a broad region of posterior cingulate and precuneus, which may reflect episodic memory retrieval for previously seen negative stimuli (Wagner et al., 2005; Cavanna and Trimble, 2006).
The current results reflecting the neural correlates of habituation and sensitization were further clarified through examination of their relationship to task-related negative affect. Previously, greater decreases in amygdala signal when viewing fearful faces over time have been correlated with reduced trait anxiety in both adolescents and adults (Hare et al., 2008). Although we did not measure trait anxiety, we were able to test correlations between changes in neural activity and changes in subjective experience of negative affect (i.e. behavioral habituation). Although we did not observe a direct correlation between changes in amygdala activity and behavioral habituation, we did observe effects in bilateral posterior insula and insula–amygdala functional connectivity that contribute to understanding the neural mechanisms underlying the affect experienced during initial and repeated viewing of negative stimuli.
Given the insula’s extensive role in affective and visceral integration, we focused on the activity of insula as a functional connectivity seed region. Based on parametric analyses between insula activity during novel negative image viewing and trial-by-trial variation in task-related negative affect showing that posterior insula activity was correlated with increasingly negative affect, left posterior insula was chosen as the seed region for subsequent functional connectivity analyses. Further, greater insula–amygdala functional connectivity was associated with both greater decreases in insula activity and greater behavioral habituation. This result contextualizes the overall increase in insula activity with repeated presentation discussed above and suggests that posterior and mid-insula may be particularly important nodes of on-line affective salience discrimination and integration of sensory information (Ploghaus et al., 1999; Wager and Barrett, 2004; Menon and Uddin, 2010; Deen et al., 2011).
Further, the increased insula–amygdala coupling for repeated presentation means that, as posterior insula is more (or less) active upon the second presentation of a negative image, on average amygdala is likewise more (or less) active. This is consistent with data showing that amygdala and insula frequently coactivate (Kober et al., 2008; Etkin and Wager, 2010), which may owe to the fact that insula and amygdala are highly interconnected anatomically (Mufson et al., 1981; Mesulam and Mufson, 1982b) and functionally (Stein et al., 2007).
The correlation between these insula–amygdala connectivity results and changes in self-reported negative affect ratings made during the task further clarifies the neural mechanisms underlying habituation. The finding that increased coupling between insula and amygdala is associated with increasingly large behavioral habituation of negative affect yields insight into adaptive connectivity patterns when appraising emotional stimuli. Although such patterns have not previously been reported for habituation, some prior work in explicit emotion regulation (via cognitive reappraisal) has shown that increasing connectivity between amygdala and orbitofrontal cortex and amygdala and dorsomedial PFC when reappraising relative to responding naturally is associated with decreasing reports of negative affect (Banks et al., 2007), whereas other reappraisal work has shown that greater inverse coupling of amygdala to orbitofrontal and dorsomedial and dorsolateral PFC is associated with better psychophysiological indicators of regulatory success (Lee et al., 2012). Neither of these reports indicated effects of insula–amygdala coupling. It is likely that the habituation effects reported here do owe in part to some form of emotion regulatory process, more likely to be implicit than explicit as participants were not instructed to regulate emotion during the task. The coupling of insula and amygdala is consistent with their shared role in affective appraisal, particularly for negative stimuli (Etkin and Wager, 2010). Indeed, prior work using path analysis has supported a functional interconnection between insula and amygdala, with positive coupling in activity between the two, when perceiving negative stimuli (Stein et al., 2007).
Overall, the present pattern of results are consistent with the idea that habituation involves changes in bottom-up affective appraisal rather than top-down, higher-level cognitive representations. This distinction refers to whether the mechanisms driving particular appraisal processes are rooted in quick, stimulus-driven perceptual processes (bottom-up) or rather in deliberative, cognitive processes that may draw upon semantic or episodic knowledge (top-down) (Ochsner and Gross, 2007; Ochsner et al., 2009; McRae et al., 2012). Although we did not observe increases in PFC activity from the first to second presentation of negative stimuli being associated with behavioral habituation that we would expect in top-down control, we did observe significant coupling of activation in key appraisal-related regions including insula and amygdala. These results, taken together with a key finding from prior work (Ochsner et al., 2009) that manipulated top-down vs bottom-up appraisal frames and found that behavioral self-reports of emotion correlated most strongly with amygdala activity when using the bottom-up frame and with PFC activity when using the top-down frame, provide support for a bottom-up appraisal mechanism for the behavioral habituation we observed in this study.
In summary, the present results suggest that posterior insula activity may be an informative correlate of both amygdala activity and affective response when repeatedly experiencing negative stimuli. A limitation of this study is that habituation was examined to a single repetition of the stimulus. Multiple repetitions might yield stronger behavioral effects as well as different time series effects in neural activity. Future investigations may uncover whether habituation to multiple repetitions involves a greater overall habituation response as well as a graded modulation of some of the effects reported here. Further, in this study, no explicit instructions to change or regulate responses upon repeated image presentations were given. In that case, habituation responses would need to be separated from responses attributable to better implementation of the regulation strategy. Future work may investigate whether such a paradigm yields evidence of top-down control networks driving behavioral habituation to repeated images or continued support for a bottom-up framework. In addition, future work may examine these effects in clinical populations that exhibit behavioral sensitization or attenuated habituation to negative stimuli.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (R01 MH077813 to Dr Koenigsberg); by the National Center for Research Resources, National Institutes of Health for the Mount Sinai General Clinical Research Center (5M01 RR00071) and by the James J Peters Veterans Affairs Medical Center.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. American Psychiatric Publishing: Washington, DC; 1994. [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews. 1996;22(3):229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Britton JC, Shin LM, Barrett LF, Rauch SL, Wright CI. Amygdala and fusiform gyrus temporal dynamics: responses to negative facial expressions. BMC Neuroscience. 2008;9:44. [Google Scholar]
- Castellucci V, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proceedings of the National Academy of Sciences of the United States of America. 1974;71(12):5004–8. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, Kandel ER. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167(3926):1745–8. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews. Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3(10):1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21(7):1498–506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Silvers JA, Ochsner KN. How we heal what we don’t want to feel: the functional neural architecture of emotion regulation. In: Kring AM, Sloan DM, editors. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. New York: Guilford Press; 2009. pp. 59–87. [Google Scholar]
- Etkin A, Wager TD. Brain systems underlying anxiety disorders: a view from neuroimaging. In: Simpson HB, Schneier F, Neria Y, Lewis-Fernandez R, editors. Anxiety Disorders: Theory, Research and Clinical Perspectives. Cambridge, UK: Cambridge University Press; 2010. pp. 192–203. [Google Scholar]
- Feinstein JS, Goldin PR, Stein MB, Brown GG, Paulus MP. Habituation of attentional networks during emotion processing. Neuroreport. 2002;13(10):1255–8. doi: 10.1097/00001756-200207190-00007. [DOI] [PubMed] [Google Scholar]
- Fischer H, Furmark T, Wik G, Fredrikson M. Brain representation of habituation to repeated complex visual stimulation studied with PET. Neuroreport. 2000;11(1):123–6. doi: 10.1097/00001756-200001170-00024. [DOI] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Research Bulletin. 2003;59(5):387–92. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Flynn FG, Benson DF, Ardila A. Anatomy of the insula functional and clinical correlates. Aphasiology. 1999;13(1):55–78. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychologicl Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Geday J, Ehlers L, Boldsen AS, Gjedde A. The inferior temporal and orbitofrontal cortex in analysing emotional pictures. Neuroimage. 2001;13(6):S406. [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences. 1999;3(2):65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(26):9827–32. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I, Castellucci V, Pinsker H, Kandel E. Neuronal correlates of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167(3926):1743–5. doi: 10.1126/science.167.3926.1743. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62(3):1575–81. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: implications for emotion regulation. Social Cognitive and Affective Neuroscience. 2012;7(3):253–62. doi: 10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. The Journal of Comparative Neurology. 1982a;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of Comparative Neurology. 1982b;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6(7):1231–48. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17(2):153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychological Science. 2009;20(11):1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28(7):1344–50. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167(3926):1740–2. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–81. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. Neuroimage. 2007;36(3):736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System—An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Wager TD, Barrett LF. From affect to control: functional specialization of the insula in motivation and regulation. PsycExtra. 2004 http://www.apa.org/pubs/databases/psycextra. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Science. 2005;9(9):445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf (Retrieved 3 July 2011) [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.