Abstract
3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy’) releases serotonin and norepinephrine. MDMA is reported to produce empathogenic and prosocial feelings. It is unknown whether MDMA in fact alters empathic concern and prosocial behavior. We investigated the acute effects of MDMA using the Multifaceted Empathy Test (MET), dynamic Face Emotion Recognition Task (FERT) and Social Value Orientation (SVO) test. We also assessed effects of MDMA on plasma levels of hormones involved in social behavior using a placebo-controlled, double-blind, random-order, cross-over design in 32 healthy volunteers (16 women). MDMA enhanced explicit and implicit emotional empathy in the MET and increased prosocial behavior in the SVO test in men. MDMA did not alter cognitive empathy in the MET but impaired the identification of negative emotions, including fearful, angry and sad faces, in the FERT, particularly in women. MDMA increased plasma levels of cortisol and prolactin, which are markers of serotonergic and noradrenergic activity, and of oxytocin, which has been associated with prosocial behavior. In summary, MDMA sex-specifically altered the recognition of emotions, emotional empathy and prosociality. These effects likely enhance sociability when MDMA is used recreationally and may be useful when MDMA is administered in conjunction with psychotherapy in patients with social dysfunction or post-traumatic stress disorder.
Keywords: MDMA, ecstasy, empathy, emotion recognition, social cognition, social behavior
INTRODUCTION
3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and similar phenethylamines release brain serotonin [5-hydroxytryptamine (5-HT)] and norepinephrine and are classified as ‘entactogens’ or ‘empathogens’. MDMA produces subjective prosocial feelings (Dumont et al., 2009), and the enhancement of empathy and sociability is also considered a major reason for the recreational use of MDMA and its therapeutic effects in psychotherapy (Bedi et al., 2010; Hysek et al., 2012b; Mithoefer et al., 2013). However, it is unknown whether MDMA indeed increases empathic concern for others or prosocial behavior when measured objectively.
The empathy construct includes cognitive and emotional aspects (Blair, 2005; Dziobek et al., 2008). Cognitive empathy is defined as the ability to recognize emotional states in others, and emotional empathy refers to the emotional response to another person’s emotional state (Blair, 2005). Two previous studies assessed the effects of MDMA on emotion recognition which relates to the cognitive aspects of empathy using a static Face Emotion Recognition Task (FERT) (Bedi et al., 2010) and the Reading the Mind in the Eyes Test (RMET) (Bedi et al., 2010; Hysek et al., 2012b). MDMA did not improve emotion recognition overall in any of these tests (Bedi et al., 2010; Hysek et al., 2012b). However, MDMA impaired the recognition of fearful faces (Bedi et al., 2010) and mind reading of negative emotions and enhanced the identification of positive emotions in the RMET (Hysek et al., 2012b). Thus, MDMA may differentially alter emotion recognition, depending on the emotional valence of the stimuli. Whether MDMA modulates the emotional aspects of empathy such as empathic concern and whether it changes social behavior has not yet been tested.
This study investigated the effects of MDMA using the Multifaceted Empathy Test (MET) (Dziobek et al., 2008), a test specifically designed to assess different aspects of empathy. As an additional assessment of the cognitive component of empathy, and to confirm previously documented effects of MDMA on emotion recognition, we used a novel FERT, which uses more naturalistic dynamic presentations of facial affect (Domes et al., 2008). Furthermore, we assessed the effects of MDMA on prosocial behavior using the Social Value Orientation (SVO) test (Murphy et al., 2011). Because several neuropeptides and steroid hormones are involved in the regulation of social cognition and behaviors (Kosfeld et al., 2005; Thompson et al., 2006; Domes et al., 2007; Guastella et al., 2010), we determined the plasma concentrations of oxytocin (Dumont et al., 2009; Hysek et al., 2012b), C-terminal provasopressin (copeptin) (Simmler et al., 2011), cortisol, prolactin (Harris et al., 2002) and testosterone in all of the subjects before and after MDMA or placebo administration.
We hypothesized that MDMA enhances both emotional empathy and prosocial behavior consistent with the self-rated social effects of the drug. The study included equal numbers of both sexes to test the modulatory effects of the MDMA response by sex, which has been observed for oxytocin (Hurlemann et al., 2010).
MATERIALS AND METHODS
Participants
Thirty-two healthy subjects (16 men, 16 women) with a mean age of 25 ± 3 years (mean ± s.d.; range 20–31 years) were recruited from the University of Basel campus. Subjects with a personal or first-degree relative history of psychiatric disorders or chronic or acute physical illness were excluded as previously described (Hysek et al., 2012c). Additional exclusion criteria were smoking, a lifetime history of using illicit drugs more than five times, with the exception of past cannabis use, and any illicit drug use including cannabis within the last 2 months or during the study period, determined by repeated urine tests conducted during screening and before each test session using TRIAGE 8 (Biosite, San Diego, CA, USA). Nineteen subjects had used cannabis more than five times in the past. Fifteen participants reported using other illicit drugs one to four times. Most of the subjects (n = 22) were completely MDMA naïve while 10 subjects had less than five previous experiences with MDMA. The use of a within-subjects study design avoided confounding of the acute MDMA effect by drug history in this study. Female subjects were investigated during the follicular phase (Day 2–14) of their menstrual cycle to account for cyclic changes in the reactivity to amphetamines.
Experimental protocol
We used a double-blind, placebo-controlled, cross-over design where all 32 subjects were treated with both MDMA (125 mg) and placebo (64 assessments). The use of a within-subject design eliminated inter-individual differences and increased the power of the study considerably above that of a parallel design (n > 64). The washout period was at least 10 days. The study was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonization Guidelines in Good Clinical Practice and approved by the local Ethics Committee. The study was registered at ClinicalTrials.gov (NCT01386177 and NCT01465685). All the subjects provided written informed consent before participating in the study and were paid for their participation.
Study drug
MDMA (Lipomed AG, Arlesheim, Switzerland) was prepared as gelatin capsules with mannitol as filler. Identical placebo capsules contained only mannitol. MDMA was administered orally at a dose of 125 mg, corresponding to a mean dose of 1.89 ± 0.30 mg/kg body weight (mean ± s.d.).
Measures
Subjective effects
Visual analog scales (VASs) (Hysek et al., 2012b) were repeatedly used to assess subjective effects related to prosociality, including feeling ‘happy’, ‘open’ and ‘close to others’. In addition, the 60-item Adjective Mood Rating Scale (AMRS) was used to assess subjective mood effects (Janke and Debus, 1978; Hysek et al., 2011).
Multifaceted empathy test
The MET was used to assess the cognitive and emotional aspects of empathy (Dziobek et al., 2008; Hurlemann et al., 2010). The test consisted of 40 photographs that showed people in emotionally charged situations (Hurlemann et al., 2010). To assess cognitive empathy, the participants were required to infer the mental state of the subject in each scene and indicate the correct one from a list of four responses. Cognitive empathy was defined as the percentage of correct responses in the total responses. To measure emotional empathy, the subjects were asked to rate how much they were feeling for the individual in each scene (i.e. explicit emotional empathy) and how much they were aroused by each scene (i.e. implicit emotional empathy) on a 1–9 point scale. The latter rating provides an inherent assessment of emotional empathy, which is considered to reduce the likelihood of socially desirable answers (Dziobek et al., 2008). The three aspects of empathy were each tested with 20 stimuli with positive valence and 20 stimuli with negative valence, resulting in a total of 120 trials. The MET was performed 3 h after drug administration and after the initial intense subjective peak drug effects had reached a stable level.
Interpersonal reactivity
A validated German version (Paulus, 2009) of the interpersonal reactivity index (IRI) (Davis, 1983) was used once to assess trait empathy.
Social Value Orientation test
The paper-based SVO measure was used to assess social behavior (Murphy et al., 2011). In such a resource allocation task, prosociality is defined as a behavior that maximizes the sum of resources for the self and others and minimizes the difference between the two (Haruno and Frith, 2010; Murphy et al., 2011). The test consists of six primary and nine secondary SVO slider items with a resource allocation choice over a defined continuum of joint payoffs (Murphy et al., 2011). The participants were instructed to choose their allocation that defines their most preferred joint distribution between themselves and another person. Allocated funds had real value, and two randomly selected subjects received the funds they earned.
For the primary items, mean allocations for self and the other were calculated. The inverse tangent of the ratio of these two means then produced an angle that indicated the participants’ SVO index. A greater SVO angle indicates that the participant more often chose the option that maximized the allocation for the other person, consistent with prosocial or altruistic behavior. The nine secondary items were used to differentiate between two prosocial motivations, inequality aversion and joint gain maximization. The inequality-aversion index was calculated as previously described (Murphy et al., 2011). An index of 0 indicates perfect inequality aversion, and 1 indicates maximal preference for joint gain maximization. The test was administered after the MET at 4 h after drug administration.
Facial affect recognition
Facial affect recognition was tested using a dynamic FERT (Domes et al., 2008) (Supplementary data). As dependent variables, the emotional intensity at which the trial was stopped for correct answers was recorded. The emotion recognition accuracy was then assessed, defined as the percentage of correctly identified emotions (Domes et al., 2008). The FERT was performed 2 h after MDMA or placebo administration during the peak effect of MDMA.
Endocrine measures and pharmacokinetics of MDMA
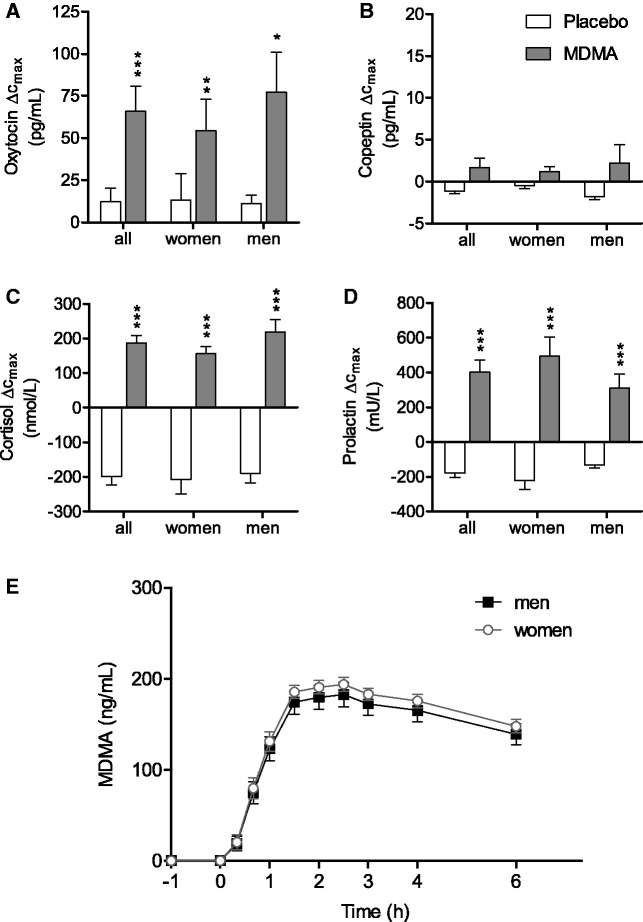
Plasma levels of oxytocin and copeptin were determined before and 1 and 2 h and levels of cortisol, prolactin and testosterone before and 2 h after drug administration using different immunoassays (Morgenthaler et al., 2006; Simmler et al., 2011; Neumann et al., 2013) and Supplementary material. The concentrations of MDMA were determined repeatedly (Figure 4E) using HPLC-tandem mass spectrometry according to (Hysek et al., 2012a,d).
Fig. 4.
(A–D) Endocrine effects of MDMA and (E) plasma concentration-time curve of MDMA. MDMA increased the plasma concentrations of (A) oxytocin, (C) cortisol and (D) prolactin but not (B) copeptin. The data are expressed as mean ± s.e.m. of differences from baseline in 32 subjects. *P < 0.05, **P < 0.01, ***P < 0.001, significant difference (change in Cmax) from placebo.
Statistical analysis
Repeated measures were expressed as peak changes from baseline (ΔEmax). Drug effects were first analyzed by an overall analysis of variance (ANOVA), with drug as a within-subject factor. The modulatory effects of sex were then analyzed by ANOVAs, with drug as within- and sex as between-subjects factors. The effects of trait empathy in the IRI on MDMA-induced changes in state empathy in the MET were analyzed using the IRI scale scores (low vs high median split) as between-subject factor. Tukey post hoc comparisons were based on significant main effects or interactions in the ANOVAs. Order effects were excluded by ANOVAs, with session order as a factor. Confounding effects of previous cannabis use on the sex–drug interaction were excluded by ANOVAs, with drug experience as additional factor. Pearson’s correlation coefficients were used to determine associations between measures.
RESULTS
Mean ± s.e.m. values and detailed statistics for all outcomes are shown in Supplementary Table S1.
Subjective effects
Significant MDMA treatment effects were found on VAS scores for ‘happy’ (F1,31 = 120.59, P < 0.001), ‘open’ (F1,31 = 80.77, P < 0.001) and ‘close to others’ (F1,31 = 67.52, P < 0.001; Figure 1). MDMA also increased AMRS scores for activity (F1,31 = 24.48, P < 0.001), inactivity (F1,31 = 7.72, P = 0.009), extroversion (F1,31 = 40.58, P < 0.001), introversion (F1,31 = 10.05, P = 0.003), well-being (F1,31 = 37.00, P < 0.001), emotional excitation (F1,31 = 28.46, P < 0.001) and dreaminess (F1,31 = 25.48, P < 0.001) but not for anxiety (Supplementary Figure S1). A significant sex × treatment interaction was found for ‘happy’ ratings (F1,31 = 10.49, P = 0.003), but the post hoc tests showed no significant differences between men and women in ‘happy’ ratings after MDMA administration. No other sex × treatment interactions were found.
Fig. 1.
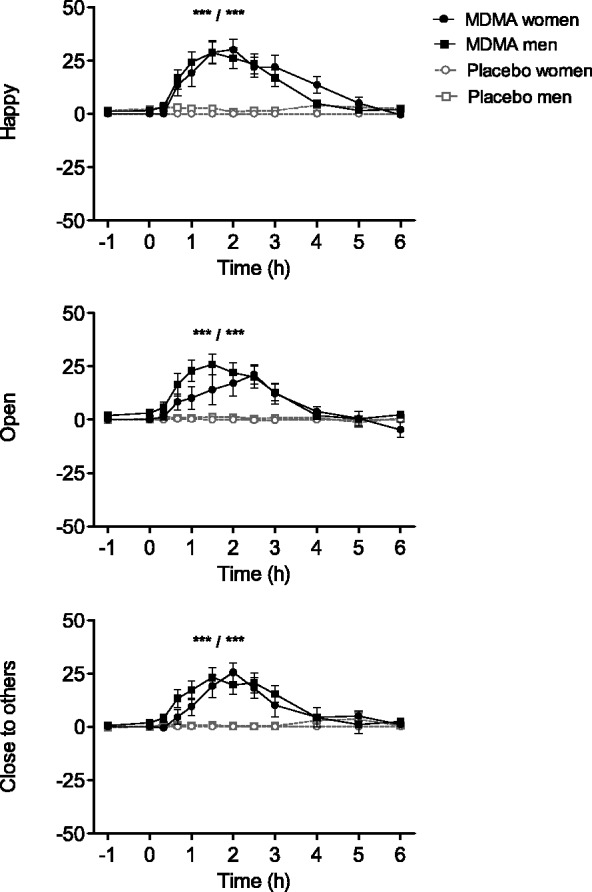
Subjective effects of MDMA measured using VASs. The data are expressed as mean ± s.e.m. score changes from predrug baseline in 32 subjects. ***P < 0.001, significant differences (Emax) from placebo in women/men.
Empathy
MDMA significantly increased explicit and implicit emotional empathy ratings for all stimuli (F1,31 = 6.05, P = 0.019 and F1,31 = 4.29, P = 0.047, respectively) (Figure 2A and B). For both explicit and implicit empathy, the MDMA-induced increase was significant for positive valence stimuli (F1,31 = 8.60, P = 0.006 and F1,31 = 5.02, P = 0.032, respectively) but not negative valence stimuli (Supplementary Figure S2A–D). MDMA influenced emotional empathy differently in male and female subjects as evidenced by a significant treatment × sex interaction for implicit emotional empathy (F1,31 = 4.68, P = 0.039) and a similar trend effect for explicit emotional empathy (F1,31 = 3.03, P = 0.092). The post hoc tests showed that MDMA increased explicit and implicit emotional empathy ratings only in men (P = 0.025 and P = 0.022, respectively) and not in women (Figure 2A and B). Men tended to score non-significantly lower on both measures of emotional empathy compared with women after placebo administration. MDMA increased empathy ratings in men to the levels of empathy in women after placebo administration. No effect of treatment was found on cognitive empathy scores (Figure 2C). Trait empathy in the IRI did not moderate the state empathy response to MDMA in the MET. As expected and validating the tasks, IRI trait empathy ratings of fantasy and empathic concern were associated with explicit empathy scores in the MET (Rp = 0.60, P < 0.05 and Rp = 0.47, P < 0.05, respectively; n = 32), and IRI trait empathy ratings of personal distress were associated with implicit emotional empathy ratings in the MET (Rp = 0.63, P < 0.01; n = 32) after placebo administration.
Fig. 2.
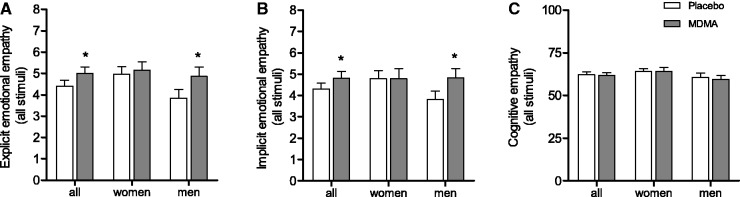
Effect of MDMA on (A) explicit and (B) implicit emotional empathy and (C) cognitive empathy in the MET. MDMA significantly increased emotional empathy in all subjects due to increases in men but not in women. The data are expressed as mean ± s.e.m. in 32 subjects. *P < 0.05, significant difference from placebo.
Social Value Orientation
MDMA increased prosociality. A significant MDMA treatment effect was found on the SVO angle (F1,31 = 4.42, P = 0.044; Figure 3A), with a significant treatment × sex interaction (F1,31 = 5.52, P = 0.026). The post hoc tests showed that MDMA significantly increased prosocial behavior in men (P = 0.008) but not women. In men, prosocial behavior increased after MDMA administration to the levels of placebo-treated women (Figure 3A). Moreover, MDMA tended to reduce the inequality-aversion index (F1,20 = 3.39, P = 0.079) in subjects with a prosocial orientation, indicating that MDMA promoted the shift from joint gain maximization to inequality aversion (Figure 3B).
Fig. 3.
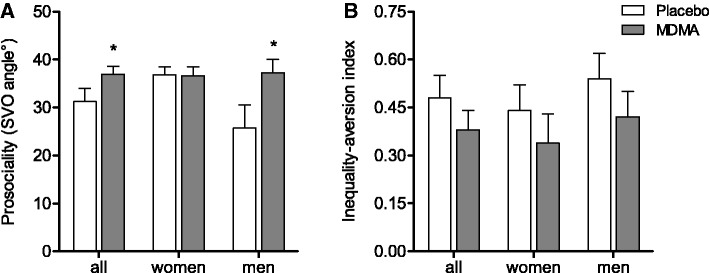
Effect of MDMA on prosociality and inequality-aversion in the SVO test. (A) MDMA had prosocial effects in men, resulting in levels of prosociality equal to those of placebo-treated women. (B) MDMA tended to reduce the inequality-aversion index (P = 0.079), consistent with an increased preference for fairness. The data are expressed as mean ± s.e.m. *P < 0.05, significant difference from placebo.
Facial emotion recognition
MDMA impaired the accuracy of emotion recognition compared with placebo (F1,31 = 28.63, P < 0.001) when all of the stimuli were analyzed together, regardless of valence (Supplementary Figure S3A). MDMA differently affected emotion recognition in male and female subjects (F1,31 = 6.04, P = 0.020). Women performed significantly worse after MDMA treatment compared with placebo treatment (P < 0.001), whereas no significant effect of MDMA was found in men (Supplementary Figure S3A). Valence-specific analyses of recognition accuracy showed that MDMA significantly impaired the correct recognition of fearful (F1,31 = 14.90, P < 0.001), angry (F1,31 = 18.60, P < 0.001), disgusted (F1,31 = 5.81, P = 0.022) and surprised (F1,31 = 9.79, P = 0.004) faces compared with placebo (Supplementary Figure S3D, E, G and H). In contrast, MDMA did not alter the correct identification of happy (F1,31 = 0.27, P = 0.607) faces compared with placebo (Supplementary Figure S3C). MDMA affected recognition accuracy for fearful and sad faces differently in male and female subjects (F1,31 = 6.61, P = 0.015 and F1,31 = 9.42, P = 0.005, respectively). Significant impairments in the recognition accuracy for fearful (P < 0.001), angry (P = 0.007) and sad (P = 0.010) faces after MDMA treatment compared with placebo were found only in women (Supplementary Figure S3E and F). Fear recognition accuracy inversely correlated with the Cmax and AUC0–6 h of MDMA in women (Rp = −0.62, P = 0.014 and Rp = −0.66, P = 0.006, respectively; n = 16).
Consistent with labeling errors for fearful faces, MDMA increased the detection threshold for fearful faces compared with placebo (F1,31 = 4.92, P < 0.032). MDMA did not alter the detection threshold for any other valence or all of the emotions together (Supplementary Figure S3B). Accuracy in the MET significantly correlated with overall emotion recognition accuracy in the FERT (Rp = 0.42, P = 0.018; n = 32) after placebo treatment, thus cross-validating both tasks. No correlations were found between the effects of MDMA on emotion recognition and the endocrine effects of MDMA.
Endocrine effects and pharmacokinetics of MDMA
MDMA significantly increased the plasma levels of oxytocin (F1,31 = 19.84, P < 0.001), cortisol (F1,31 = 98.70, P < 0.001) and prolactin (F1,31 = 127.81, P < 0.001) compared with placebo (Figure 4A, C, and D). In contrast, MDMA did not alter the plasma concentrations of copeptin (Figure 4B) or testosterone (Supplementary Table S1). No correlations were found between the neuroendocrine and empathogenic or prosocial effects of MDMA.
Both the maximum concentration (Cmax) and area under the plasma concentration-time curve (AUC0–6 h) were higher in women compared with men (F1,15 = 35.73, P < 0.001 and F1,15 = 20.77, P < 0.001, respectively) (Figure 4E). The mean Cmax values of MDMA were 209 ± 6.4 ng/ml (mean ± s.e.m.) in men and 269.9 ± 9.3 ng/ml (mean ± s.e.m.) in women. Mean AUC0–6 h values were 926.8 ± 29.3 ng/ml h (mean ± s.e.m.) in men and 1146.5 ± 37.9 ng/ml h (mean ± s.e.m.) in women. The relative doses of MDMA were 1.68 ± 0.14 mg/kg body weight (mean ± s.d.) in men and 2.09 ± 0.29 mg/kg body weight (mean ± s.d.) in women. Higher plasma exposure to MDMA was significantly associated with deficits in the recognition of fearful faces in women as described above. No other correlations were found between plasma exposure to MDMA and the pharmacodynamic effects of MDMA. The time to maximum concentration (Tmax) was reached after a mean time of 2.44 ± 0.14 h (mean ± s.e.m.) after MDMA administration. Mean Tmax values were 2.10 ± 0.20 h (mean ± s.e.m.) and 2.75 ± 0.17 h (mean ± s.e.m.) in men and women, respectively. The pharmacokinetic data were consistent with previous studies (Hysek et al., 2011; Hysek and Liechti, 2012).
DISCUSSION
The novel findings of this study are that MDMA increased emotional empathy and prosocial behavior. This effect was observed primarily in men. Consequently, male subjects showed more empathic concern and less competitive behavior and exhibited a more prosocial orientation after MDMA treatment, equal to that observed in women with placebo. In addition, MDMA tended to increase the preference for fairness, reflected by a trend reduction in inequality-aversion compared with placebo. Although MDMA is reported to be an ‘empathogen’ and has been shown to produce increased self-ratings of prosocial feelings (Dumont et al., 2009) and sociability (Bedi et al., 2009; Bedi et al., 2010), this is the first study that actually observed enhanced emotional empathy in men using an empathy test. In addition, the study also documented increased MDMA-induced prosociality in men in a behavioral task.
MDMA did not alter cognitive empathy in the MET and impaired emotion recognition of basic emotions in the FERT especially in women, consistent with impaired cognitive empathy with regard to decoding of basic emotions. Specifically, MDMA reduced the recognition of negative facial emotions, including fear, anger and disgust, consistent with the reduced recognition of fearful faces in a static FERT (Bedi et al., 2010) and the impaired mind-reading accuracy of negative emotions in the RMET (Hysek et al., 2012b). MDMA did not affect the recognition of happy faces as previously shown (Bedi et al., 2010), while improved recognition of happy expressions in the RMET was found in another study (Hysek et al., 2012b). MDMA reduced affect recognition accuracy particularly in women. The largest MDMA-induced deficit in women was found in fear recognition and involved both accuracy and intensity detection thresholds. A functional imaging study showed that MDMA enhanced the response to happy faces in the ventral striatum (Bedi et al., 2009), a structure activated by expected rewards (Knutson and Cooper, 2005), and attenuated the response to angry faces in the amygdala, which is a core region for fear processing (Zald, 2003). Because women generally exhibit greater left amygdala activation to negative emotional stimuli than men (Stevens and Hamann, 2012), MDMA may alter emotional processing in a valence- and sex-specific manner by modulating the brain circuits involved in the processing of reward and anxiety.
The findings from the MET and FERT indicate that MDMA overall enhances the emotional but not cognitive components of empathy. More specifically, MDMA appears to reduce the recognition of negative but not positive emotions in others across different tests and studies. Altogether, these effects of MDMA likely result in a shift in the processing of social–emotional information toward enhanced perception and possibly responses to positive emotional stimuli. Both the positive and prosocial mood effects and valence-specific social cognitive effects of MDMA likely enhance sociability when MDMA is used as a club drug. The effects of MDMA on social cognition may also facilitate the processing of emotionally distressing material when MDMA is used in combination with psychotherapy for patients with social dysfunction and social threat such as post-traumatic stress disorder and social anxiety disorder (Mithoefer et al., 2013).
Sex differences in various effects of MDMA have previously been described. MDMA produced more intense acute subjective effects (Liechti et al., 2001) and greater negative long-term effects (Reneman et al., 2001; Ogeil et al., 2013) in women compared with men. Women also more frequently developed hyponatremia in association with ecstasy use compared with men (Rosenson et al., 2007; van Dijken et al., 2013). These findings indicate that women may be generally more susceptible to the effects MDMA compared with men. Consistently, we observed MDMA-induced deficits in the recognition of sad faces only in women but not in men and significantly greater deficits in the recognition of fearful faces in women compared with men. A reduced ability to detect and process negative emotional information is likely therapeutically relevant when MDMA is used in the treatment of post-traumatic stress disorder. It will be of interest to see whether there are sex differences in the treatment response to MDMA in clinical studies, which have so far included 85% women (Mithoefer et al., 2010; Mithoefer et al., 2013; Oehen et al., 2013).
In this study, we documented increased levels of oxytocin, cortisol and prolactin along with alterations in emotional cognition. However, we found no correlations between MDMA-induced endocrine and emotional changes. The lack of associations does not exclude a role for oxytocin in the empathogenic and prosocial effects of MDMA as discussed below. There are several possible reasons for the lack of significant correlations. First, circulating levels of neurohormones may not reflect their brain levels (Neumann, 2007). Second, blood drawings to determine the endocrine markers had to be done before or after the computer tasks for practical reasons. Third, the use of only one relatively high dose of MDMA likely resulted in maximal threshold effects precluding the detection of correlations between the endocrine biomarkers and emotional measures across subjects. We have previously documented an identical lack of correlations between the subjective and autonomic effects of MDMA across a large number of subjects once peak drug effects are reached while there are strong associations over time within subjects (Hysek and Liechti, 2012).
Which neurotransmitters or hormones may contribute to the effects of MDMA on social cognition? The primary mechanism of action of MDMA is to release serotonin and norepinephrine in the brain, and both neurotransmitters have been shown to mediate most of the acute psychotropic effects of MDMA (Hysek et al., 2011, 2012d). Serotonin and norepinephrine release also likely mediates the effects of MDMA on emotional processing. In fact, we previously demonstrated that the inhibition of MDMA-induced serotonin and norepinephrine release with duloxetine not only prevented the subjective effects of MDMA (Hysek et al., 2012d) but also tended to reduce the effects of MDMA on emotion identification in the RMET (Hysek et al., 2012b). Similar to MDMA, the 5-HT1A/2A receptor agonist psilocybin impaired the recognition of negative facial expressions in healthy subjects, and this effect was prevented by a 5-HT2A antagonist (Kometer et al., 2012). In addition, serotonin transporter inhibitors, such as citalopram, also alter emotional processing, depending on emotional valence (Anderson et al., 2011), and generally increase the recognition of positive facial emotions (Harmer et al., 2003a) and diminish the perception of negative emotions (Pringle et al., 2013), including fear (Harmer et al., 2004) and sadness (Hinkelmann et al., 2010). Furthermore, polymorphisms in the serotonin transporter gene were also associated with altered emotion recognition, particularly of fearful faces (Hinkelmann et al., 2010). Serotonin is also a supposed regulator of social behavior. Enhancing serotonin via transporter inhibition increases aspects of prosocial behavior (Knutson et al., 1998; Crockett et al., 2010), whereas tryptophan depletion decreases cooperative behavior (Wood et al., 2006). Finally, the norepinephrine transporter inhibitor reboxetine increased the recognition of happy faces and impaired the recognition of fearful faces (Harmer et al., 2003b, 2004), similar to MDMA. Altogether, the effects of the serotonin and norepinephrine releaser MDMA on emotional processing and social behavior are consistent with the effects reported for other pharmacological manipulations of these neurotransmitters. However, the extent to which downstream stimulating effects on social neuropeptides and hormones are involved is unclear. Oxytocin is a key candidate for the mediation of the empathic and prosocial effects of MDMA (Thompson et al., 2007; Hysek et al., 2012b). MDMA activates oxytocin neurons, increases plasma oxytocin levels through 5-HT1A receptors and increases social interaction in rats (Thompson et al., 2007). Blocking oxytocin receptors in the brain reduced the prosocial effects of MDMA in rats (Thompson et al., 2007). In this study, MDMA increased the plasma levels of oxytocin in parallel with its empathogenic and prosocial effects. Increases in plasma oxytocin have previously been shown after MDMA administration (Dumont et al., 2009; Hysek et al., 2012b). The empathogenic effects of MDMA in the MET are also strikingly similar to those of oxytocin in the same test (Hurlemann et al., 2010). Analogous to MDMA, oxytocin enhanced emotional but not cognitive empathy in the MET in men (Hurlemann et al., 2010). In the RMET, oxytocin improved emotion recognition in healthy subjects (Domes et al., 2007) and patients with autism (Guastella et al., 2010). MDMA similarly improved emotion recognition in the RMET, although only for positive stimuli (Hysek et al., 2012b). A comparable selective increase in the sensitivity in detecting positive vs negative facial expressions was also reported for oxytocin (Marsh et al., 2010). Similar to the effects of MDMA in the FERT in this study, oxytocin slowed reaction times for identifying fearful faces (Di Simplicio et al., 2009). Both MDMA and oxytocin reduced the response of the amygdala to negative emotional stimuli (Kirsch et al., 2005; Bedi et al., 2009). Comparable to the prosocial effects of MDMA, oxytocin has been shown to increase generosity (Zak et al., 2007) and trust (Kosfeld et al., 2005). The MDMA-induced release of oxytocin and overall very similar emotional-cognitive effects of oxytocin and MDMA might implicate oxytocin as a crucial mediator of the effects of MDMA on empathy and social behavior. Directly testing the role of oxytocin in the effects of MDMA in humans will be difficult because clinically used oxytocin receptor antagonists or PET ligands do not cross well the blood–brain barrier (Smith et al., 2012). MDMA response modulation by genetic polymorphisms in the oxytocin receptor gene (e.g. rs53576 and rs1042778) (Kumsta and Heinrichs, 2013) could be tested. In addition, effects of MDMA or of pharmacologically similar but less toxic drugs could be evaluated in patients with social dysfunction where oxytocin is implicated such as autism (Guastella et al., 2010). As expected (Harris et al., 2002), MDMA also increased the plasma levels of cortisol and prolactin. Cortisol and prolactin are primarily markers of hypothalamic–pituitary–adrenal, serotonergic and noradrenergic activity but there are limited data on the role of cortisol in social cognition. In men, high stress-induced cortisol levels were associated with better social cognition (Smeets et al., 2009).
This study has limitations. First, we used only one dose of MDMA. We did not perform a dose–response study because we did not want to expose the mostly drug-naïve subjects to more than two doses of MDMA. Second, we used a relatively high dose of MDMA with obvious subjective effects. Although we used a double-blind design and identical placebo most participants realized which treatment they had been administered over the course of the experimental session. Thus, unblinding the subjective effects of MDMA may have biased task performance. We felt that it is important to use relevant doses of MDMA, which correspond to those typically used in recreational settings (Brunt et al., 2012) or in clinical trials (125 mg plus 62.5 mg after 2 h) (Mithoefer et al., 2010; Mithoefer et al., 2013; Oehen et al., 2013). Lower doses of MDMA and active placebo could be used in future studies. Third, the evaluation of many aspects of social cognition in this study required a relatively large number of statistical comparisons.
In summary, the novel findings are that MDMA increases emotional empathy and prosocial behavior in healthy subjects. The social cognitive effects of MDMA may explain its popularity as a recreational drug and potential beneficial effects of MDMA in the treatment of ‘social disorders’ and post-traumatic stress disorder.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance of A. Fink, N. Meyer, N. Schillinger, C. Bläsi and L. Baselgia in study management, M. Donzelli in the analysis of plasma MDMA levels and M. Arends in editing the manuscript. This work was supported by the Swiss National Science Foundation (grant no. 320030_138481 and 32323B_144996) and the University of Basel (grant no. DPH 2053 and DPH 2064).
REFERENCES
- Anderson IM, Juhasz G, Thomas E, et al. The effect of acute citalopram on face emotion processing in remitted depression: a pharmacoMRI study. European Neuropsychopharmacology. 2011;21:140–8. doi: 10.1016/j.euroneuro.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of ± 3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological Psychiatry. 2010;68:1134–40. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Koeter MW, Niesink RJ, van den Brink W. Linking the pharmacological content of ecstasy tablets to the subjective experiences of drug users. Psychopharmacology. 2012;220:751–62. doi: 10.1007/s00213-011-2529-4. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17433–8. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–26. [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of Psychopharmacology. 2009;23:241–8. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G, Czieschnek D, Weidler F, Berger C, Fast K, Herpertz SC. Recognition of facial affect in Borderline Personality Disorder. Journal of Personality Disorders. 2008;22:135–47. doi: 10.1521/pedi.2008.22.2.135. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dumont GJ, Sweep FC, van der Steen R, et al. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Social Neuroscience. 2009;4:359–66. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) Journal of Autism and Developmental Disorders. 2008;38:464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2010;67:692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Bhagwagar Z, Perrett DI, Vollm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003a;28:148–52. doi: 10.1038/sj.npp.1300004. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hill SA, Taylor MJ, Cowen PJ, Goodwin GM. Toward a neuropsychological theory of antidepressant drug action: increase in positive emotional bias after potentiation of norepinephrine activity. American Journal of Psychiatry. 2003b;160:990–2. doi: 10.1176/appi.ajp.160.5.990. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry. 2004;161:1256–63. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Haruno M, Frith CD. Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nature Neuroscience. 2010;13:160–1. doi: 10.1038/nn.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkelmann K, Dragoi L, Gompf J, et al. Decreased recognition of negative affect after selective serotonin reuptake inhibition is dependent on genotype. Psychiatry Research. 2010;177:354–7. doi: 10.1016/j.psychres.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, et al. Effects of the α2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. Journal of Pharmacology and Experimental Therapeutics. 2012a;340:286–94. doi: 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Domes G, Liechti ME. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012b;222:293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Liechti ME. Effects of MDMA alone and after pretreatement with reboxetine, duloxetine, clonidine, carvedilol, and doxazosin on pupillary light reflex. Psychopharmacology. 2012;224:363–76. doi: 10.1007/s00213-012-2761-6. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Rickli A, et al. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. British Journal of Pharmacology. 2012c;166:2277–88. doi: 10.1111/j.1476-5381.2012.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, et al. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (“ecstasy”) in humans. Clinical Pharmacology and Therapeutics. 2011;90:246–55. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola V, et al. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012d;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke W, Debus G. Die Eigenschaftswörterliste. Göttingen: Hogrefe; 1978. [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. American Journal of Psychiatry. 1998;155:373–9. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biological Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Current Opinion in Neurobiology. 2013;23:11–6. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology. 2001;154:161–8. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology. 2010;209:225–32. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome I, Doblin R. The safety and efficacy of ±3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. Journal of Psychopharmacology. 2010;25:439–52. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, et al. Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: a prospective long-term follow-up study. Journal of Psychopharmacology. 2013;27:28–39. doi: 10.1177/0269881112456611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clinical Chemistry. 2006;52:112–9. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- Murphy RO, Ackermann KA, Handgraaf MJJ. Measuring social value orientation. Judgment Decision Making. 2011;6:771–81. [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochemical Society Transactions. 2007;35:1252–7. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Oehen P, Traber R, Widmer V, Schnyder U. A randomized, controlled pilot study of MDMA (±3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD) Journal of Psychopharmacology. 2013;27:40–52. doi: 10.1177/0269881112464827. [DOI] [PubMed] [Google Scholar]
- Ogeil RP, Rajaratnam SM, Broadbear JH. Male and female ecstasy users: Differences in patterns of use, sleep quality and mental health outcomes. Drug and Alcohol Dependence. 2013;132:223–30. doi: 10.1016/j.drugalcdep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Paulus C. Der Saarbrücker Persönlichkeitsfragebogen SPF (IRI) zur Messung von Empathie: Psychometrische Evaluation der deutschen Version des Interpersonal Reactivity Index. Saarbrücken: Universität des Saarlandes; 2009. [Google Scholar]
- Pringle A, McCabe C, Cowen P, Harmer C. Antidepressant treatment and emotional processing: can we dissociate the roles of serotonin and noradrenaline? Journal of Psychopharmacology. 2013;27:719–31. doi: 10.1177/0269881112474523. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, de Bruin K, et al. Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet. 2001;358:1864–9. doi: 10.1016/S0140-6736(01)06888-X. [DOI] [PubMed] [Google Scholar]
- Rosenson J, Smollin C, Sporer KA, Blanc P, Olson KR. Patterns of ecstasy-associated hyponatremia in California. Annals of Emergency Medicine. 2007;49:164–71. doi: 10.1016/j.annemergmed.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Hysek CM, Liechti ME. Sex differences in the effects of MDMA (ecstasy) on plasma copeptin in healthy subjects. Journal of Clinical Endocrinology and Metabolism. 2011;96:2844–50. doi: 10.1210/jc.2011-1143. [DOI] [PubMed] [Google Scholar]
- Smeets T, Dziobek I, Wolf OT. Social cognition under stress: differential effects of stress-induced cortisol elevations in healthy young men and women. Hormones and Behavior. 2009;55:507–13. doi: 10.1016/j.yhbeh.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Stehouwer JS, et al. Synthesis and evaluation of C-11, F-18 and I-125 small molecule radioligands for detecting oxytocin receptors. Bioorganic and Medicinal Chemistry. 2012;20:2721–38. doi: 10.1016/j.bmc.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–93. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGregor IS. A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146:509–14. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7889–94. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijken GD, Blom RE, Hene RJ, Boer WH. High incidence of mild hyponatraemia in females using ecstasy at a rave party. Nephrology Dialysis Transplantation. 2013;28:277–83. doi: 10.1093/ndt/gft023. [DOI] [PubMed] [Google Scholar]
- Wood RM, Rilling JK, Sanfey AG, Bhagwagar Z, Rogers RD. Effects of tryptophan depletion on the performance of an iterated Prisoner's Dilemma game in healthy adults. Neuropsychopharmacology. 2006;31:1075–84. doi: 10.1038/sj.npp.1300932. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.