Abstract
How does our brain organize knowledge? Traditional theories assume that our knowledge is represented abstractly in an amodal conceptual network of formal logic symbols. The theory of embodied cognition challenges this view and argues that conceptual representations that constitute our knowledge are grounded in sensory and motor experiences. We tested this hypothesis by examining how the concept of social coordination is grounded metaphorically in the tactile sensation of roughness. Participants experienced rough or smooth touch before being asked to judge an ambiguous social interaction. Results revealed that rough touch made social interactions appear more difficult and adversarial, consistent with the rough metaphor. This impact of tactile cues on social impressions was accompanied by a network including primary and secondary somatosensory cortices, amygdala, hippocampus and inferior prefrontal cortex. Thus, the roughness of tactile stimulation affected metaphor-relevant (but not metaphor-irrelevant) behavioral and neural responses. Receiving touch from a rough object seems to trigger the application of associated ontological concepts (or scaffolds) even for unrelated people and situations (but not to unrelated or more general feelings). Since this priming was based on somatosensory brain areas, our results provide support for the theory that sensorimotor grounding is intrinsic to cognitive processes.
Keywords: somatosensory cortex, embodiment, touch, social, fMRI
INTRODUCTION
In the traditional understanding our knowledge is represented abstractly in a supramodal conceptual network of formal logic symbols. The theory of embodied cognition challenges this view and claims that cognitive representations are based in sensory and motor experiences, forming a ‘perceptual symbol system’ (Lakoff and Johnson, 1999; Gallese and Lakoff, 2005; Barsalou, 2008). Following this theory mental processes involve simulations of body-related perceptions and actions. Therefore, the theory argues that the retrieval of conceptual meaning involves a partial re-enactment of sensory and motor experiences. This may be explained by early experiences with the physical world, which structure our later understanding or representation of more abstract concepts (Williams et al., 2009; Ackerman et al., 2010; Meier et al., 2012). Others emphasize that we are ‘evolved from creatures whose neural resources were devoted primarily to perceptual and motor processing’ (Wilson, 2002).
Focusing in particular on the role of metaphors in human thought, numerous behavioral experiments provided support for this theory of embodied cognition (e.g. Zhong and Leonardelli, 2008; Ackerman et al., 2010; Lee and Schwarz, 2010a,b). For example, the abstract concept of importance has been shown to be grounded in bodily experiences of weight. Thus, holding a heavy clipboard made job candidates appear more important (Jostmann et al., 2009; Ackerman et al., 2010). Furthermore, it has been shown that basic tactile sensations have an impact on higher social cognitive processing in dimension- and metaphor-specific ways. For example, roughness is metaphorically associated with the concepts of difficulty and harshness (e.g. ‘having a hard day’). Ackerman et al. (2010) examined the link of this metaphor with sensory processing. Participants were instructed to complete a five-piece-puzzle, either in a version with pieces covered in rough sandpaper (rough condition) or a version with the pieces uncovered (smooth condition). Subsequently, they were asked to read a passage describing an ambiguously valenced social interaction and to answer questions regarding the nature of this interaction. Participants who completed the rough puzzle rated the interaction as less coordinated (more difficult and harsh) than participants who performed the same task but with smooth parts. Hence, the experience of rough objects made social interactions appear more difficult. As there was no effect for a set of questions asking for relationship familiarity, Ackerman et al. (2010) concluded that roughness specially changed evaluations of social coordination consistent with a ‘rough’ metaphor, but did not make the interaction seem more generally impersonal.
Recent advances in neuroimaging now allow testing the hypothesis that our thoughts and feelings are grounded in bodily interaction with the environment in a new way. This study aimed to test the theory of embodied cognition by examining brain responses of participants with functional magnetic resonance imaging (fMRI) in a paradigm on the touch-related metaphor of roughness. Roughness is metaphorically associated with the concepts of difficulty, adversary and harshness. Based on experiments by Ackerman et al. (2010), we hypothesized that tactile experiences with rough objects elicit a ‘haptic mindset’, which may trigger the application of associated concepts such as difficulty and adversary (in contrast to more general feelings) when assessing a social interaction, consistent with the ‘roughness metaphor’. An engagement of the somatosensory cortices in this process would provide support for the assumptions of the embodiment theory.
MATERIALS AND METHODS
Participants
Twenty participants (10 females) with a mean age of 26 years (range 23–39) took part in the study. All participants were right-handed native German volunteers with no neurological or psychiatric history. The participants gave informed consent to the study, which adhered to the Declaration of Helsinki and was approved by the local human subjects’ committee.
Procedure
Participants were explained that the study would involve two separate experiments: an experiment to examine neural correlates of different touch and an experiment to investigate neural correlates of social judgments.
We used a two-factorial experimental design. The first factor described the tactile priming, which was either rough by applying touch with sandpaper, smooth by using a smooth paintbrush, or was omitted (no stimulation). The second factor was the set of questions. One set of questions addressed the social coordination quality, the other set of question was asking for relationship familiarity.
While lying in the scanner the participants received passive tactile stimulation (rough, smooth or no touch) for 10 s. After 5 s participants were prompted with a screen describing an ambiguous valenced social interaction. The presentation of the scenario lasted for 19 s, while the first 5 s overlapped with the tactile stimulation. These social interactions were oriented to the experiments of Ackerman et al. (2010) and included both positive (e.g. kidding around) and negative components (e.g. sharp words), making the whole scenario ambiguous in its meaning (analog to Kay et al., 2004). For example, participants read the following scenario: ‘>>Hello, have you seen the pictures of our last Christmas party? What is your girlfriend thinking of this? Well, she doesn't have to know everything!<< >>Oops, what am I doing there?<< >>Well, you seem to have fun!<< >>That’s what it looks like, he he. Hey, don't show this to someone else!<< >>Don’t worry, I won’t tell her.<< >>Has someone taken these pictures with some intentions behind? What’s all this crap?<< >>Calm down, look, I just deleted them!<<’.
After a break of 4 s we asked the participants about the nature of this interaction. One set of questions addressed the social coordination quality (was the interaction adversarial or friendly, competitive or cooperative, a discussion or an argument), for example: ‘What do you think, was this interaction adversarial or friendly? Friendly: right buttons. Adversarial: left buttons’. The other set of question was asking for relationship familiarity (closeness of relationship, business or casual interaction style, relationship of the agents). The latter set was applied to test if the priming effect would extend to a theoretically unrelated measure. Participants used a key with four buttons (Likert-scale ranging from +2 to −2) to assess the scenarios. Before the experiment they were explained that they could weight their responses from moderate (inner buttons) to extreme (outer buttons). Use of right and left buttons were randomized over the scenarios. They were allowed to spend up to 17 s to respond (earlier responding did not automatically start the next trial). To collect comparable parts of the decision process for each participant, we decided to measure brain activity in a time window around the button press. Thus, condition-related activity was measured using an individual ‘floating’ time window of eight MR images surrounding (four before, one during and three after) each point of response (analog to Greene et al., 2001).
Using the responses of the participants we created a social coordination index (SCI), which included the mean of all three social coordination assessments. Furthermore, we calculated the mean of all three assessments of relationship familiarity, resulting in a relationship familiarity index (RFI).
The scenarios were based on a pre-study to match ambiguity needs. In this pre-study students were asked to evaluate a sample of scenarios with respect to its overall emotional valence by using a nine-point Likert-scale ranging from very positive to very negative. Only those scenarios that were rated as ambiguous were included in the study.
While lying in the scanner, a total of 96 scenarios were presented to each participant. Each scenario was repeated six times. Thus, each scenario was once preceded by a rough, by a smooth or by no tactile stimulation (random order within subjects). In addition, each scenario was once followed by questions from the SCI set and once from the RFI set. There was only one question after each scenario. The order of presentation of the scenarios as well as the order of the subsequent questions was randomized within subjects and between subjects. There were at least five scenarios between repetitions of a scenario.
Tactile stimulation was applied to the right hand. Participants were required to press buttons with their left hand to assess the interactions.
Visual images were back-projected to a screen at the end of the scanner bed close to the subject’s feet. Subjects viewed the images through a mirror mounted on the birdcage of the receiving coil. The experiment consisted out of three runs. Each run included all conditions.
After scanning the participants were probed for suspicions concerning the experimental hypotheses.
This study adopted a paradigm introduced by Ackerman et al. (2010), which described a social psychology experiment. To employ this paradigm within a neuroimaging context, we needed to change it in several points. Thus, since we tried to demonstrate the effect as a within-subjects effect, we used a relatively high number of scenarios for each participant. Furthermore, we applied passive tactile stimulation. In addition, we included a second control condition (no tactile stimulation).
FMRI data acquisition and analysis
Functional scans were acquired by using a 1.5 T scanner [General Electrics Signa LX, USA; gradient echo T2-weighted echo-planar images; repetition time (TR) = 2 s, echo time (TE) = 35 ms, flip angle = 80°, field-of-view (FOV) = 20 mm]. For each subject, data were acquired in three scan runs. Functional volumes consisted of 23 slices. Each volume comprised 5 mm slices (1 mm gap, in plane voxel size 3.125 × 3.125 mm). For anatomical reference, a high-resolution T1-weighted structural image was collected (3D-SPGR, TR = 24 ms, TE = 8 ms).
FMRI data were preprocessed and analyzed using the Statistical Parametric Mapping Software (SPM5, Wellcome Department of Imaging Neuroscience, University College London, London, UK). For each subject, the fMRI scans were realigned to correct for inter-scan movement, using sinc interpolation and subsequently normalized into a standard anatomical space [Montreal Neurological Institute (MNI) template], resulting in isotropic 3 mm voxels. The scans were then smoothed with a Gaussian kernel of 6 mm full-width half maximum.
Statistical parametric maps were calculated using multiple regressions with the hemodynamic response function modeled in SPM5. Data analyses were performed at two levels. We examined data on the individual subject level by using a fixed effects model (all three runs concatenated for each subject). Then, the resulting parameter estimates for each regressor at each voxel were entered into a second-level analysis with the random effects model. To examine brain responses when participants received touch we computed statistical contrasts (t-tests) for rough stimulation (sandpaper) relative to rest, smooth stimulation (paintbrush) relative to rest and both rough and smooth stimulation relative to rest. To reveal brain responses during the judgment process we calculated an ANOVA for repeated measurements with the factors priming (rough, smooth, none) and set of questions (social coordination, relationship familiarity). Subsequently, statistical contrasts (t-tests) were performed to examine cortical activation associated with priming conditions for the different set of questions. Finally, to further investigate if regions that are specific to preprocessing tactile sensations subserve the priming effect, we examined priming effects on the judgments masked with the results of the contrast receiving rough and smooth touch relative to rest.
Scores of participants’ judgments were tested for possible correlations (Pearson) with the parameter estimates for voxels in the somatosensory region of interest [maximum peak in left somatosensory cortex (SI)]. In addition, we tested possible correlations with participants’ judgments for secondary SI (SII), inferior frontal gyrus (IFG), inferior parietal cortex (IPC), hippocampus and amygdala.
We report regions that survived correction for multiple comparisons over the whole brain [at P < 0.05, family wise (FDR) correction]. To describe the anatomical regions, we used the SPM anatomy toolbox (Eickhoff et al., 2005).
RESULTS
Behavioral results
None of the participants reported any suspicions with respect to our experimental hypotheses.
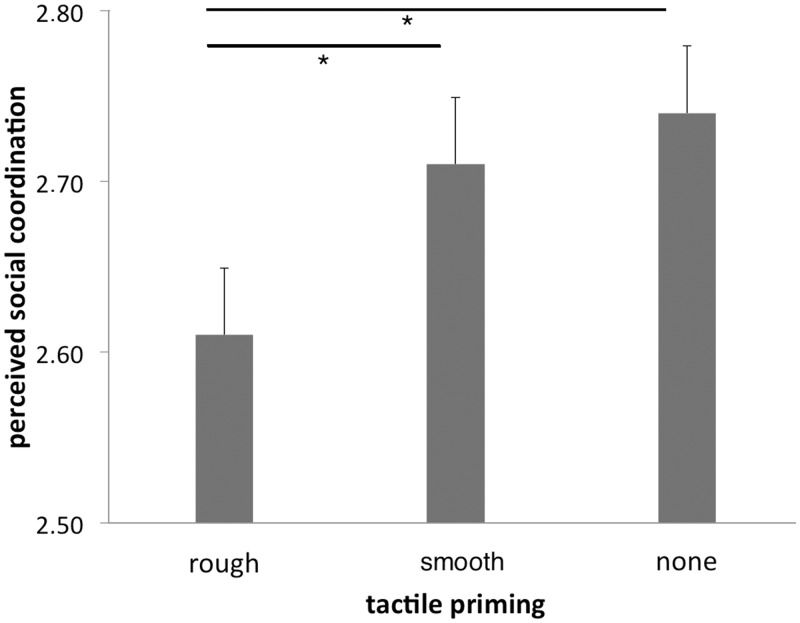
Behavioral results revealed a significant interaction between way of tactile priming and the set of questions [ANOVA interaction with factors condition and set of questions, F(2,38) = 3.77, P < 0.05]. Post hoc t-tests demonstrated effects for tactile priming of the social coordination set. Rough priming revealed significant lower scores on the social coordination scale compared with smooth priming [rough priming of social coordination judgment, mean and s.d.: 2.61 ± 0.39; smooth priming: 2.71 ± 0.39; t(19) = 2.41, P < 0.05, two-sided, Bonferroni corrected for multiple tests] or with no priming {no priming: 2.74 ± 0.39; t(19) = −2.35, P < 0.05; no effect for smooth priming relative to none priming [t(19) = −0.49, P = n.s.]} (Figure 1). Since there were no effects for tactile priming of the relationship familiarity set we concluded that tactile priming with rough objects specifically made social interactions appear less coordinated (more harsh, difficult and adversarial), but did not make the scenario seem more generally impersonal (similar to Ackerman et al., 2010).
Fig. 1.
Effects of tactile priming on social impressions. Perceived social coordination was significantly lower when participants were primed with a rough stimulus (sandpaper) compared with a smooth prime (paintbrush) or no prime.
Analysis of the reaction times revealed no significant effects (social coordination: rough priming: 5.55 ± 1.37 s, smooth: 5.37 ± 1.31 s, none: 5.96 ± 1.73 s; familiarity relationship: rough: 5.42 ± 0.97 s, smooth: 5.46 ± 1.16 s, none: 5.52 ± 1.06 s; ANOVA with factors condition and set of questions: no significant main effects or interactions).
FMRI results: Brain responses while participants received touch
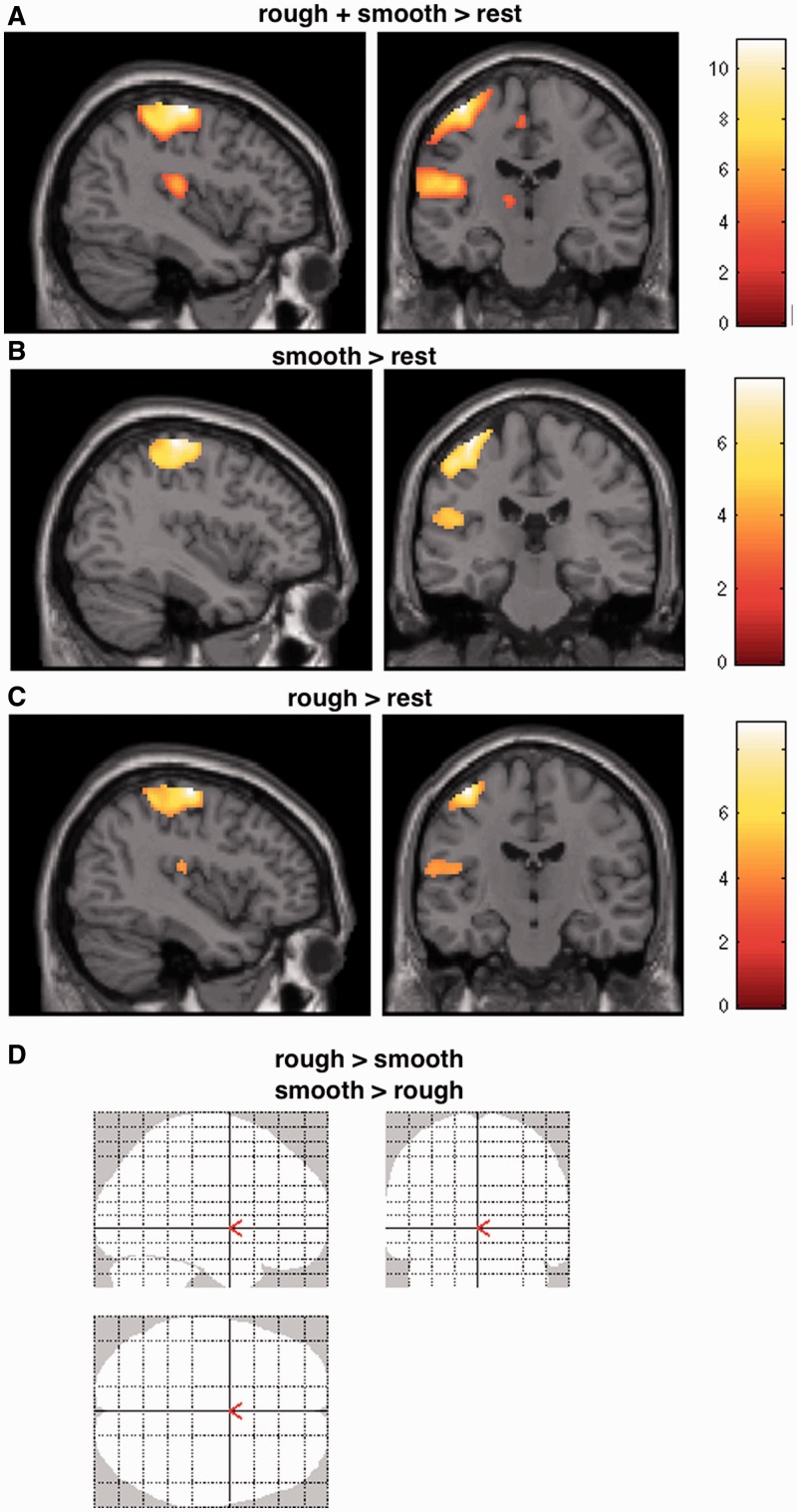
Brain responses during passive touch applied by both a smooth paintbrush and rough sandpaper revealed activation in left primary SI and left SII (rough + smooth touch relative to rest; Figure 2). The contrasts rough touch relative to rest as well as smooth touch relative to rest revealed comparable activation in left SI and left SII. The statistical contrast for rough relative to smooth touch and smooth relative to rough touch failed to reveal any significant activation (at P < 0.05, FDR corrected). Thus, brain responses to rough and smooth touch showed no significant differences in brain activations.
Fig. 2.
Statistical maps showing brain activation while participants were passively touched by a smooth paintbrush and rough sandpaper (A). (B) Displays BOLD responses to touch by a smooth paintbrush alone. (C) Depicts brain activations when participants received touch by sandpaper. (D) Shows that the contrast rough relative to smooth and smooth relative to rough, respectively, failed to show significant activations (at P < 0.05, FDR corrected). Areas of significant fMRI signal change are shown as color overlays on the T1-MNI reference brain. (A–C) demonstrate involvement of left SI and SII.
FMRI results: Brain responses while participants judged the social interactions
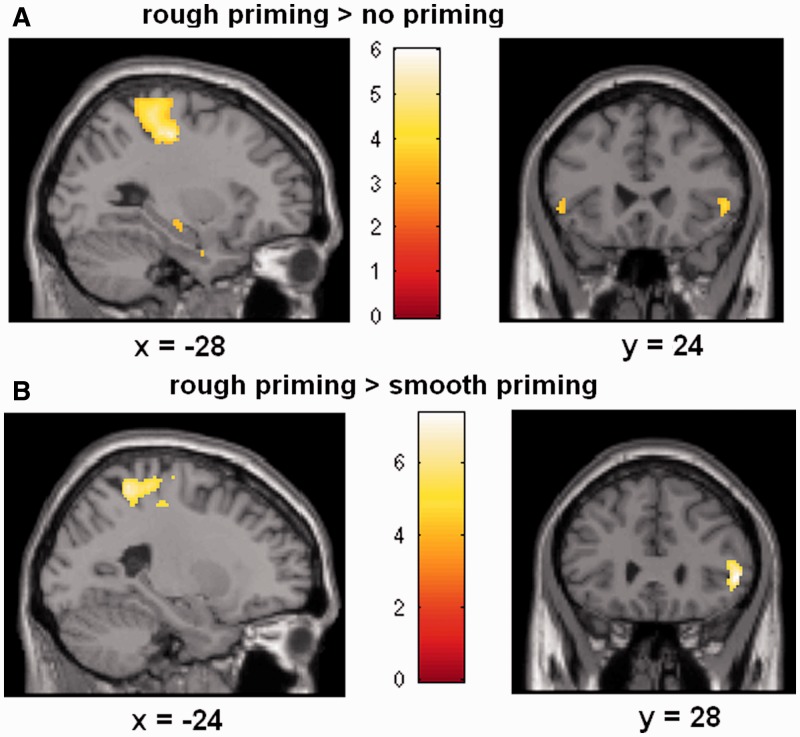
Brain responses during judging interactions revealed involvement of somatosensory brain areas (SI, SII), IFG, hippocampus, amygdala, premotor cortex and IPC (ANOVA interaction with factors condition and set of questions). Post hoc t-tests with respect to social coordination assessments for rough relative to no priming demonstrated involvement of SI, SII, IFG, hippocampus, amygdala, premotor cortex and IPC (rough > no priming, for SCI, FDR corrected; Figure 3 and Table 1). Comparison of rough relative to smooth priming showed engagement of SI, premotor cortex, IFG, hippocampus and amygdala (rough > smooth priming, for SCI, FDR corrected). Smooth relative to rough or relative to no priming failed to reveal significant activations (for SCI; at P < 0.05, FDR corrected; Table 1). Post hoc t-tests with respect to RFI questions (control condition) failed to show significant activations (rough > smooth priming, rough > no priming, smooth > rough priming, smooth > no priming, for RFI; at P < 0.05, FDR corrected).
Fig. 3.
Statistical maps showing brain activation while participants assessed ambiguous social scenarios with respect to social coordination (SCI). Areas of significant fMRI signal change are shown as color overlays on the T1-MNI reference brain. (A) Judging the SCI when primed with rough relative to no touch demonstrated activation in SI (and hippocampus) and IFG. (B) Judging the SCI when primed with rough relative to smooth touch revealed similar areas in SI and IFG.
Table 1.
Results of random effects analysis for social coordination judgments
| Contrast | Brain region | Peak MNI location (x, y, z) | Peak z-value | Number of voxels |
|---|---|---|---|---|
| Rough priming > smooth priming | L SI | −28 −42 58 | 3.88 | 373 |
| Premotor cortex (BA6) | −4 −16 56 | 3.73 | 287 | |
| R IFG (BA45) | 54 28 2 | 4.07 | 122 | |
| L hippocampus/amygdala | −16 0 −10 | 3.56 | 70 | |
| Smooth priming > rough priming | – | – | – | – |
| Rough priming > no priming | L SI | −32 −30 48 | 4.25 | 4549 |
| L SII | −60 −20 27 | 3.16 | ||
| R SI | 18 −41 65 | 4.00 | ||
| R/L premotor cortex (BA6) | 20 −20 50 | 4.49 | ||
| L IPC | −58 −36 24 | 3.07 | ||
| R IFG (BA45) | 52 28 −4 | 3.32 | 76 | |
| L IFG (BA45) | −44 30 −6 | 3.53 | 275 | |
| L hippocampus/amygdala | −18 −8 −14 | 3.80 | 318 | |
| R hippocampus/amygdala | 40 −6 −20 | 3.56 | 168 | |
| R IPC | 66 −36 20 | 4.19 | 120 | |
| Smooth priming > no priming | – | – | – | – |
P < 0.05, FDR corrected. L, left hemisphere; R, right hemisphere.
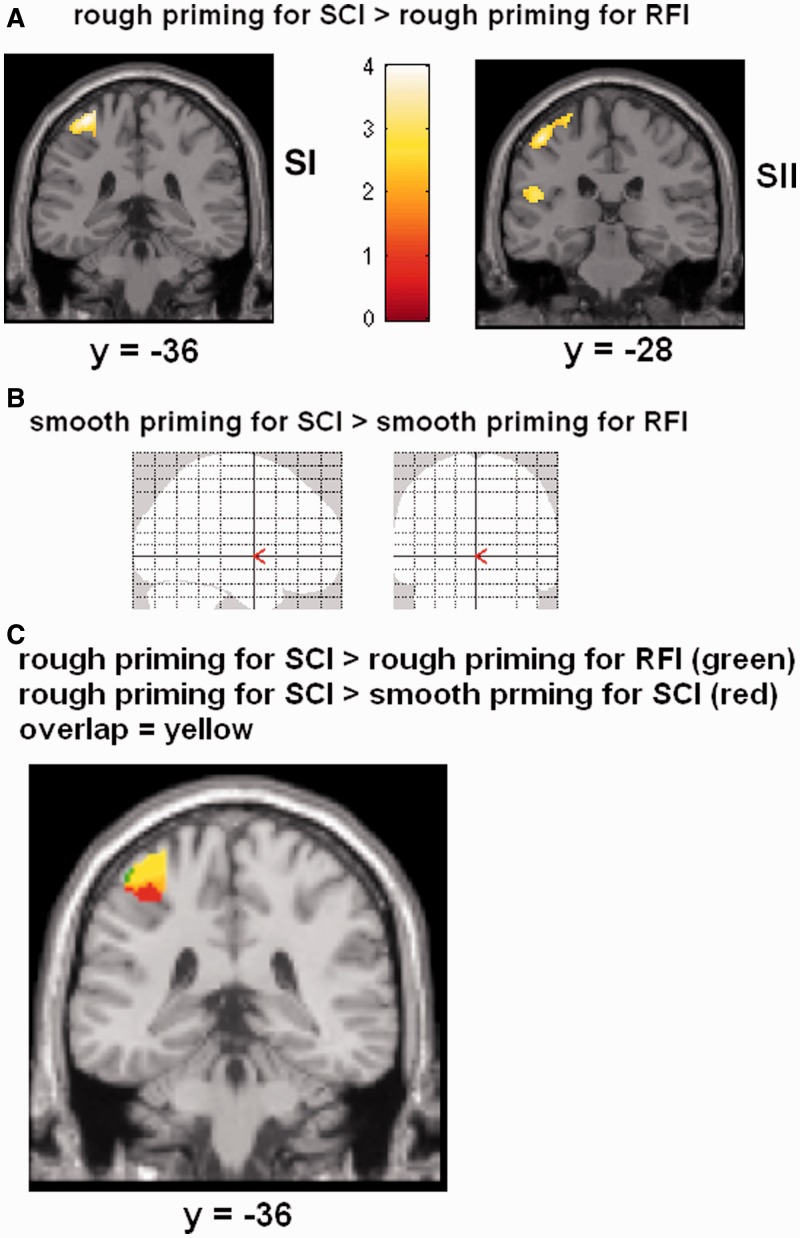
A comparison between rough priming for SCI relative to rough priming for RFI was accompanied by activations of SI, premotor cortex, IPC and IFG (FDR corrected; Table 2). For smooth priming, the analog contrast revealed no significant voxels. Hence, the demonstrated metaphor-specific impact of rough priming on social judgment seems to rely on a network of brain regions including sensorimotor brain regions, IFG, IPC, hippocampus and amygdala.
Table 2.
Results of random effects analysis for social coordination judgments relative to familiarity relationship judgments
| Contrast | Brain region | Peak MNI location (x, y, z) | Peak z-value | Number of voxels |
|---|---|---|---|---|
| Rough priming SCI > rough priming RFI | L SI/premotor cortex (BA6) | −14 −44 70 | 3.88 | 658 |
| R IFG (BA45) | 54 24 0 | 3.56 | 248 | |
| L IFG (BA44) | −58 10 8 | 3.32 | 175 | |
| L IPC | −54 −38 23 | 3.79 | 187 | |
| R IPC | 62 −42 23 | 3.56 | 180 | |
| Rough priming RFI > rough priming SCI | – | – | – | – |
| Smooth priming SCI > smooth priming RFI | – | – | – | – |
| Smooth priming RFI > smooth priming SCI | – | – | – | – |
P < 0.05, FDR corrected.
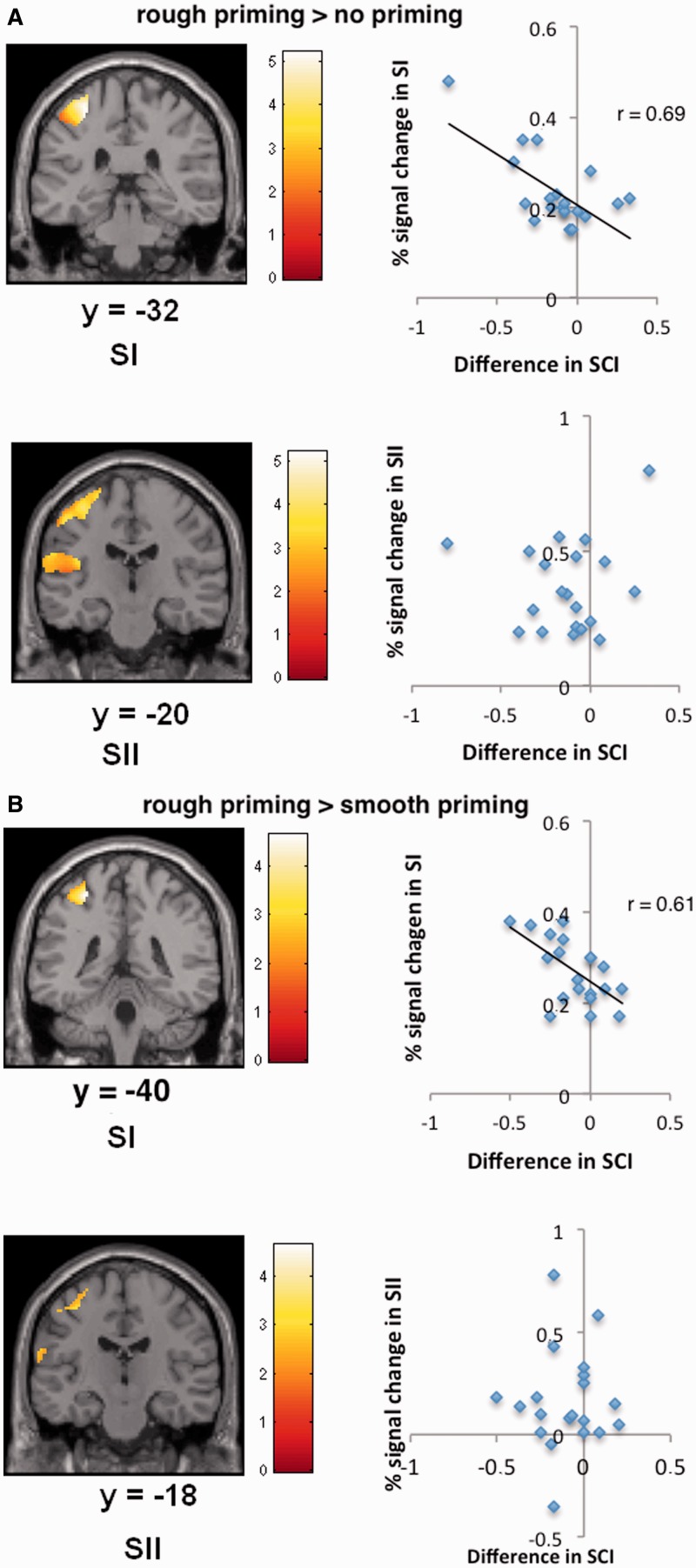
To better understand if regions that are specific to process tactile sensations subserve the tactile priming effect, we masked our analysis with the results of the contrast tactile stimulation (rough + smooth touch) relative to rest (at P < 0.05, FDR corrected). Figure 4 shows for SCI judgments that rough relative to none or relative to smooth priming engaged brain areas that were also involved during receiving the tactile primes (SI and SII). In contrast, Table 3 depicts that control comparisons (smooth relative to rough priming, smooth relative to no priming) failed to reveal any significant activation in SI or SII. Furthermore, Tables 3 and 4 and Figure 5 show that this results accounts only for the SCI judgments, RFI judgments (control condition) did not show any somatosensory activations.
Fig. 4.
Statistical maps showing brain activation in SI and SII while assessing ambiguous social scenarios for the contrast rough relative to no priming (A) and rough relative to smooth priming (B), masked with brain responses when receiving real touch. (A) Activity in left SI could significantly predict reduced social coordination scores. SII failed to show significant activation. (B) Activity in left SI again predicted the reduced social coordination assessments. SII displayed no significant relationships.
Table 3.
Results of random effects analysis, masked with brain responses during real touch
| Contrast | Brain region | Peak MNI location (x, y, z) | Peak z-value | Number of voxels | |
|---|---|---|---|---|---|
| SCI | Rough priming > smooth priming | L SI | −30 −40 58 | 3.78 | 738 |
| L SII | −62 −18 22 | 2.46 | 22 | ||
| Smooth priming > rough priming | – | – | – | – | |
| Rough priming > no priming | L SI | −32 −32 62 | 4.10 | 1196 | |
| L SII | −60 −18 22 | 3.05 | 418 | ||
| Smooth priming > no priming | – | – | – | – | |
| RFI | Rough priming > smooth priming | – | – | – | – |
| Smooth priming > rough priming | – | – | – | ||
| Rough priming > no priming | – | – | – | – | |
| Smooth priming > no priming | – | – | – | – |
P < 0.05, FDR corrected.
Table 4.
Results of random effects analysis for social coordination judgments relative to familiarity relationship judgments, masked with brain responses during real touch
| Contrast | Brain region | Peak MNI location (x, y, z) | Peak z-value | Number of voxels |
|---|---|---|---|---|
| Rough priming SCI > rough priming RFI | L SI | −36 −36 64 | 3.65 | 641 |
| L SII | −50 −30 16 | 3.15 | 161 | |
| Rough priming RFI > rough priming SCI | – | – | – | – |
| Smooth priming SCI > smooth priming RFI | – | – | – | – |
| Smooth priming RFI > smooth priming SCI | – | – | – | – |
P < 0.05, FDR corrected.
Fig. 5.
Statistical maps displaying brain activation when assessing scenarios with respect to social coordination (SCI) relative to relationship familiarity (RFI), masked with brain responses when receiving real touch. (A) When primed with rough touch SI and SII showed activation. (B) When primed with smooth touch no brain areas showed activation (at P < 0.05, FDR corrected). (C) Overlap of brain activation in SI between rough priming for SCI relative to RFI (green) and rough relative to smooth priming for SCI (red). Voxels involved in both contrasts are marked in yellow.
Brain responses in SI for the contrast rough relative to no priming were significantly linked with the bias to assess the interaction less coordinated (r = 0.69, P < 0.005, Pearson, two-sided; Figure 4; correlation between signal change in SI and decreased SCI assessments). Brain responses in SI for the contrast rough relative to smooth priming revealed a similar significant correlation with the behavioral bias to perceive the scenarios less coordinated and more harsh and adversarial (r = 0.61, P < 0.005; Figure 4; correlation between signal change in SI and decreased SCI assessments). Correlations for SII activity failed to show significant relationships with SCI judgments. Furthermore, activation in IFG, IPC, hippocampus and amygdala showed no significant correlations with SCI assessments.
DISCUSSION
This study examined brain responses of participants when being asked to judge ambiguous interactions. Tactile priming with a rough stimulus (compared with smooth or no priming) resulted in a assessing the interaction as more harsh, difficult and adversarial, but did not make the scenario seem more generally impersonal. This bias is in accordance with a rough metaphor of social coordination and was accompanied by an active network of brain responses including sensorimotor brain areas, hippocampus, amygdala, IPC and IFG.
The behavioral responses are in line with the results of Ackerman et al. (2010). Experiencing rough objects made social interactions appear more difficult. Whereas Ackerman et al. (2010) report these results as the outcome of a social psychology experiment, we here demonstrate that this effect can also be operationalized for the purpose of an fMRI study, which usually requires many repetitions and includes less participants.
We hypothesized that the effect originally reported by Ackerman et al. (2010) involves in particular the somatosensory regions, which would provide neurophysiological evidence for the embodiment theory. Our results confirm this hypothesis. Rough tactile priming (relative to smooth or no priming) was associated with activation in somatosensory brain areas, accompanied with an involvement of hippocampus, amygdala, IPC and IFG. Moreover, somatosensory activation correlated highly positive with the degree participants judged the interaction as being harsh, difficult and adversarial. This network was involved when being asked to judge the interactions with respect to its social coordination, but not when participants were instructed to relate the familiarity of the agents. Hence, it was closely associated with the roughness metaphor.
The role for somatosensory brain regions in this metaphor-specific tactile priming of social judgments strongly supports the embodiment theory, which postulates that the retrieval of conceptual meanings (metaphorical knowledge) includes a re-enactment of corresponding sensory experiences. But how may this re-enactment work? It has been demonstrated that cells in the SI may act as a transient storage site for tactile (Harris et al., 2002) and even for visual and acoustic information (Zhou and Fuster, 1996, 2000). Anatomic studies in monkeys revealed that the somatosensory cortices are linked with prefrontal cortices via the medial temporal lobe for long-term encoding (Burton and Sinclair, 2000). The IFG has been related to the evaluation of semantic information (e.g. Heim et al., 2009) and to the storage of rules learned in a social context (Monfardini et al., 2008). In addition, numerous studies relate the IFG with tactile memory (e.g. Romo et al., 1999; Kelly et al., 2007; Auksztulewicz et al., 2012). Together with somatosensory brain areas the IFG seems to code the knowledge (the metaphor) that roughness is associated with the concepts of difficulty and harshness. SI and IFG may be linked via memory structures in the medial temporal lobe (hippocampus, amygdala), which have been suggested to bind distributed activated sites in the neocortex that represent a whole memory (e.g. Squire et al., 1993). The IPC has been related to various social cognition tasks. In particular, the temporo-parietal junction area is known to be related to understanding other people and reasoning about the content of mental states (e.g. Saxe and Kanwisher, 2003; Harenski et al., 2010).
But why are conceptual representations such as ‘roughness’ so closely linked to tactile experiences? From infancy, touch on our hands is always 2-folded: we use it to acquire information, but also to manipulate our environment (Gallace and Spence, 2010). Given that touch may be the first sense to develop, physical touch experiences may establish an ontological scaffold for the development of conceptual and metaphorical knowledge (Williams et al., 2009; Ackerman et al., 2010; Meier et al., 2012). Thus, scaffolding or embodiment might be grounded in the physical substrate of early touch experiences, suggesting that tactile sensations might be crucial for the development of social bonds in early childhood and later application of this then scaffolded or embodied knowledge.
And why it is so easy to manipulate our social perception? According to the theory of embodiment similar areas that were engaged during the creation of the scaffold should be involved in the later application of the concept or metaphor (Wilson, 2002; Meier et al., 2012). Thus, activating similar touch experiences may result in an activation of the metaphor-specific bias. Since our results demonstrated that this embodied knowledge seems to be represented even in primary cortex areas, incidental and unconscious manipulations via tactile experiences appear to be very easy.
Recent studies similarly examining embodied metaphors support our results. Williams and Bargh (2008) demonstrated that experiencing physical warmth, e.g. holding a cup of hot (vs iced) coffee make it likely to judge a person as having a ‘warm’ personality. Kang et al. (2011) employed fMRI to examine the neural underpinnings of this link between physical and social warmth. They demonstrated that insular regions sensitive to physical warm perceptions are also engaged during manipulations of trust in the context of a decision-making game. Thus, physical temperature influenced human decision making associated with an engagement of the insula. Similarly, Eisenberger et al. (2003) reported overlapping activation in anterior cingulate cortex for processing physical and psychological pain.
Both the Kang et al. (2011) as well as our own study demonstrate that embodied metaphor effects are grounded in neurophysiological processes. Whereas Kang et al. (2011) report a functional overlap in the processing of information related to physical and psychological warmth in the left insula, our study shows that the roughness metaphor links physical touch and psychological impressions of a rough conversation in SI. In the traditional view, the map in SI represents physical touch on the body surface. Recent studies in the last two decades challenge this view and suggest a more complex role for SI. For example, an increasing body of evidence demonstrates vicarious activation in SI when witnessing pain or simple touch on other’s bodies in the absence of any real touch (e.g. Keysers et al., 2004; Bufalari et al., 2007; Ebisch et al., 2008). Based on these results, a role for somatosensation in social perception has been suggested, which might point to simulation processes in the observer’s SI (Gallese, 2003, 2005; Keysers et al., 2010). Recent work also link mirror-like responses in SI with interindividual differences in empathy (Gazzola and Keysers, 2008; Schaefer et al., 2012). The results of this study extend the research on mirror-like responses in SI, suggesting that activation in primary somatosensory areas may include higher-order cognitions including psychological concepts such as the roughness metaphor.
Our results provide evidence for tactile priming with rough touch, but fail to demonstrate analog effects for smooth touch. Several reasons might explain this lack of effect. First, the smooth tactile primes we used in the current experiment might not have affected the roughness–smoothness metaphor. Although we used a particular smooth paintbrush another tactile stimulation (e.g. satin) might have been more successful. Second, there may not be a smoothness counterpart of the roughness metaphor. While our study as well as the experiment by Ackerman et al. (2010) provided behavioral evidence for a priming effect of rough tactile experiences on subsequent evaluations of social coordination, no experiment has shown that this effect also works for smooth tactile experiences (Ackerman et al. worked with pieces of a puzzle covered with rough sandpaper relative to pieces uncovered, but there was not a particular smooth condition). Further studies are needed to better understand which haptic experiences influence social impressions.
This study examined brain responses in a time window around a judgment decision. Thus, we did not trace the whole complex decision-making process which may start much earlier. Based on this limitation, it remains unclear if the SI is directly involved in the process of assessing a social interaction, or whether a tactile stimulus can somehow evoke a haptic mindset, triggered by a tactile sensation. However, fMRI has a limited temporal resolution, making it difficult to separate different processing stages of decisions (Hauk and Tschentscher, 2013). Future research is needed to disentangle which aspect in the complex decision-making process are affected by touch sensations.
Several previous studies reported different activation in somatosensory cortices and other brain regions when comparing gentle or smooth with rough or unpleasant touch (e.g. Francis et al., 1999). However, comparing brain responses while experiencing rough relative to smooth touch in our study yielded no significant activation. We speculate that in this study these differences were too small to reach the level of significance.
In sum, our findings demonstrate that basic tactile sensations have an impact on higher social cognitive processing in metaphor-specific ways and that the neural underpinnings of this bias involve SI, IFG and memory-related structures. Thus, physical environment cues may influence people’s judgments and decisions. The results show how an evolutionary physical concept such as touch is functionally related on a neural level to a metaphorically linked psychological concept (Williams and Bargh, 2008; Anderson, 2010; Kang et al., 2011).
Acknowledgments
M.S. was supported by the Deutsche Forschungsgemeinschaft (Scha105/5-1).
REFERENCES
- Ackerman JM, Novera CC, Bargh JA. Incidental haptic sensations influence social judgements and decisions. Science. 2010;328:1712–3. doi: 10.1126/science.1189993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. Neural reuse: a fundamental organizational principle of the brain. Behavioural and Brain Sciences. 2010;33:245–66. doi: 10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- Auksztulewicz R, Spitzer B, Goltz D, Blankenburg F. Impairing somatosensory working memory using rTMS. European Journal of Neuroscience. 2012;34:839–44. doi: 10.1111/j.1460-9568.2011.07797.x. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review Psychology. 2008;59:617–45. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ. Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses. Journal of Clinical Neurophysiology. 2000;17:575–91. doi: 10.1097/00004691-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Perruci MG, Ferretti A, Del Gratta C, Romani GL, Gallese V. The sense of touch: embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. Journal of Cognitive Neuroscience. 2008;20:1–13. doi: 10.1162/jocn.2008.20111. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–9. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Gallace A, Spence C. The science of interpersonal touch: an overview. Neuroscience Biobehavioral Reviews. 2010;34:246–59. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Gallese V. A neuroscientific grasp of concepts: from control to representation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358:1231–40. doi: 10.1098/rstb.2003.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. Embodied simulation: from neurons to phenomenal experience. Phenomenology and the Cognitive Sciences. 2005;4:23–48. [Google Scholar]
- Gallese V, Lakoff G. The Brain's concepts: the role of the Sensory-motor system in conceptual knowledge. Cognitive Neuropsychology. 2005;22:455–79. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed data. Cerebral Cortex. 2008;19:1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Antonenko O, Shane MS, Kiehl KA. A functional imaging investigation of moral deliberation and moral intuition. Neuroimage. 2010;49:2707–16. doi: 10.1016/j.neuroimage.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Miniussi C, Harris IM, Diamond ME. Transient storage of a tactile memory trace in primary somatosensory cortex. Journal of Neuroscience. 2002;22:8720–5. doi: 10.1523/JNEUROSCI.22-19-08720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Tschentscher N. The body of evidence: what can neuroscience tell us about embodied semantics? Frontiers in Psychology. 2013;4:1–14. doi: 10.3389/fpsyg.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan KE, Amunts K. Effective connectivity of the left BA 44 Ba 45 and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Human Brain Mapping. 2009;3:392–402. doi: 10.1002/hbm.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostmann NB, Lakens D, Schubert TW. Weight as an embodiment of importance. Psychological Science. 2009;20:1169–74. doi: 10.1111/j.1467-9280.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- Kang Y, Williams LE, Clark MS, Gray JR, Bargh JA. Physical temperature effects on trust behavior: the role of the insula. Social Cognitive and Affective Neuroscience. 2011;6:507–15. doi: 10.1093/scan/nsq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AC, Wheeler C, Bargh JA, Ross L. Material priming: the influence of mundane physical objects on situational construal and competitive behavioral choice. Organizational Behavior and Human Decision Processes. 2004;95:83–96. [Google Scholar]
- Kelly S, Lloyd D, Nurmikko T, Roberts N. Retrieving autobiographical memories of painful events activates the anterior cingulate cortex and inferior frontal gyrus. Pain. 2007;8:307–14. doi: 10.1016/j.jpain.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Review Neuroscience. 2010;11:417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton J-L, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–46. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenges to Western Thought. New York: Basic Books; 1999. [Google Scholar]
- Lee S, Schwarz N. Of dirty hands and dirty mouths: Embodiment of the moral purity metaphor is specific to the motor modality involved in moral transgression. Psychological Science. 2010a;21:1423–5. doi: 10.1177/0956797610382788. [DOI] [PubMed] [Google Scholar]
- Lee S, Schwarz N. Washing away post-decisional dissonance. Science. 2010b;328:709. doi: 10.1126/science.1186799. [DOI] [PubMed] [Google Scholar]
- Meier BP, Schnall S, Schwarz N, Bargh JA. Embodiment in social psychology. Topics in Cognitive Science. 2012;4:705–16. doi: 10.1111/j.1756-8765.2012.01212.x. [DOI] [PubMed] [Google Scholar]
- Monfardini E, Brovelli A, Boussaoud D, Takerkart S, Wicker B. I learned from what you did: retrieving visuomotor associations learned by observation. Neuroimage. 2008;42:1207–13. doi: 10.1016/j.neuroimage.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernández A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–3. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze H-J, Rotte M. Embodied empathy for tactile events: interindividual differences and vicarious somatosensory responses during touch observation. Neuroimage. 2012;60:952–7. doi: 10.1016/j.neuroimage.2012.01.112. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual Review Psychology. 1993;44:453–95. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Williams LE, Bargh JA. Experiencing physical warmth promotes interpersonal warmth. Science. 2008;22:606–7. doi: 10.1126/science.1162548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Huang JY, Bargh JA. The scaffolded mind: Higher mental processes are grounded in early experience of the physical world. European Journal of Social Psychology. 2009;39:1257–67. doi: 10.1002/ejsp.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. Six views of embodied cognition. Psychonomic Bulletin Review. 2002;9:625–36. doi: 10.3758/bf03196322. [DOI] [PubMed] [Google Scholar]
- Zhong CB, Leonardelli GJ. Cold and lonely: does social exclusion feel literally cold? Psychological Science. 2008;19:838–42. doi: 10.1111/j.1467-9280.2008.02165.x. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Mnemonic neuronal activity in somatosensory cortex. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10533–7. doi: 10.1073/pnas.93.19.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YD, Fuster JM. Visuo-tactile cross-modal associations in cortical somatosensory cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:9777–82. doi: 10.1073/pnas.97.17.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]