Abstract
Although evolution has shaped human infant crying and the corresponding response from caregivers, there is marked variation in paternal involvement and caretaking behavior, highlighting the importance of understanding the neurobiology supporting optimal paternal responses to cries. We explored the neural response to infant cries in fathers of children aged 1–2, and its relationship with hormone levels, variation in the androgen receptor (AR) gene, parental attitudes and parental behavior. Although number of AR CAG trinucleotide repeats was positively correlated with neural activity in brain regions important for empathy (anterior insula and inferior frontal gyrus), restrictive attitudes were inversely correlated with neural activity in these regions and with regions involved with emotion regulation (orbitofrontal cortex). Anterior insula activity had a non-linear relationship with paternal caregiving, such that fathers with intermediate activation were most involved. These results suggest that restrictive attitudes may be associated with decreased empathy and emotion regulation in response to a child in distress, and that moderate anterior insula activity reflects an optimal level of arousal that supports engaged fathering.
Keywords: fathers, empathy, anterior insula, prolactin, fMRI, androgen receptor
INTRODUCTION
In modern western societies, sensitive and responsive fathering improves a child’s social, psychological and educational outcomes (Cabrera et al. 2000; Sarkadi et al., 2008). Nevertheless, there is remarkable variation in paternal involvement (Hrdy, 2009), with father absence increasing over the last half of the 20th century (Cabrera et al., 2000), and marked disparity in quality of parenting among involved fathers (van Ijzendoorn and De Wolff, 1997). Crying is a pre-verbal child’s primary means for eliciting parental care, and evolutionary processes have shaped infant crying to influence parental behavior through negative reinforcement, with parental caretaking behavior leading to reduction of the aversive, cry stimulus, thus leading to increased caretaking over time. While largely effective, crying is also a known trigger of shaken baby syndrome (Lee et al., 2007) and other types of fatal child assault (Cavanagh et al., 2007). Taken as a whole, these data suggest that optimal parental responsiveness to infants requires a sensitive balance of empathy coupled with the ability to regulate strong negative affect, highlighting the importance of identifying neural responses that support this balance.
Although the importance of pregnancy-related hormone changes for maternal caregiving has long been appreciated, several lines of evidence suggest that paternal hormone levels, including oxytocin (OT), prolactin (Prl) and testosterone (T), also influence paternal behavior. First, circulating levels of these three hormones change when human males become fathers, and individual differences in these hormonal changes have been linked with differences in the quantity or quality of paternal investment (Storey et al., 2000; Gordon et al., 2010a,b; Gettler et al., 2011, 2012). Second, all three hormones impact parental responses to cry stimuli. Exogenous administration of OT decreases frustration responses to cry stimuli in nulliparous women (Bakermans-Kranenburg et al., 2012) and enhances neural responses to cries in regions that are thought to be important for empathy [anterior insula (AI) and inferior frontal gyrus (IFG)] (Riem et al., 2011). Prl levels in new and expectant fathers are positively associated with self-reported concern in response to baby cries (Storey et al., 2000) and with more positive responses to infant cries (Fleming et al., 2002). In contrast, T levels are negatively correlated with self-reported sympathy and desire to respond to the crying infant (Fleming et al., 2002), a result that is consistent with T’s more general negative impact on empathic responding (Eisenegger et al., 2011). Together, these studies suggest that OT and Prl may support sensitive responses to infant cries by enhancing activity in neural systems important for empathy and emotion regulation, whereas T may interfere with these systems.
The impact of hormones on paternal behavior and brain activity will depend not only on their circulating levels but also on the brain’s sensitivity to them as reflected in receptor density. For example, the number of CAG trinucleotide repeats in the first exon of the androgen receptor (AR) gene is inversely correlated with expression (Choong et al., 1996), as well as in vitro sensitivity of the receptor (Chamberlain et al., 1994). The number of CAG repeats in the AR gene has an inverse relationship with aggression and with brain activity in response to threatening facial expressions (reviewed in Carré et al., 2011).
In addition to potential hormonal and genetic influences, paternal behavior is also influenced by parenting attitudes and beliefs (Deković et al., 1991). Parental restrictiveness is described as an endorsement of strict and punitive rules and requirements, and it is linked with poorer child outcomes (Smith et al., 2000; Lindhout et al., 2009). In contrast, parental nurturance is described as the willingness of parents to share and accept their child’s feelings and respond to the child’s needs and it is consistently associated with positive child outcomes (e.g. Amato and Fowler, 2002). It is possible that these attitudes modulate a father’s neural response to children, perhaps by influencing the neural systems related to empathy and emotion regulation.
Empathy is defined as an affective reaction similar to, and evoked by, another’s affective state (de Vignemont and Singer, 2006), and the neural systems related to it have been investigated using a variety of paradigms. Consistently, both the perception (audio and visual) and the contemplation of the suffering of another commonly elicit activation in a network of brain regions commonly referred to as a salience network (Seeley et al., 2007), including anterior mid-cingulate cortex, bilateral AI and ventral frontal operculum (Singer et al., 2004; Botvinick et al., 2005; Jackson et al., 2005; Lamm et al., 2010). Activity in the AI, particularly on the right side, may represent a simulated mapping of the observed individual’s body state onto one’s own (Tania Singer et al., 2009), which is foundational for an empathic response. Importantly, activity in AI predicts later helping behavior, suggesting that its activity is related to prosocial emotions and motivation (Batson, 1998; Hein et al., 2010). In the specific realm of parental empathy, the AI is also consistently activated in response to infant cry stimuli (Rilling, 2013).
However, recent evidence suggests that the neural state supporting empathy for another’s suffering may differ from that supporting the compassionate desire to help the sufferer (Klimecki et al., 2013). In fact, extensive evidence from social psychology has shown that empathic over-arousal leads to personal distress, which interferes with helping behavior (Eisenberg, 2000; Batson and Powell, 2003). Empathic over-arousal might also interfere with effectively responsive parenting. For example, maternal intrusiveness during a play session was positively correlated with activity in the AI (Musser et al., 2012). The authors interpreted the finding as suggesting that overly empathic responding led to intrusiveness. Similarly, Strathearn et al. (2009) found that insecure mothers have a stronger AI response to their own infant’s sad facial expression compared with securely attached mothers. One parsimonious interpretation of these data is that there is an optimal level of empathy-related neural responding that supports effective parenting, beyond which leads to maladaptive parenting styles or withdrawal.
The findings reviewed earlier also highlight the importance of emotion regulation for parental caregiving. The orbitofrontal cortex (OFC) has been consistently implicated in emotion regulation (Goldin et al., 2008), and may be particularly important for regulating negative affect in response to aversive cries. Mother’s depression scores are inversely related to activity in the OFC in response to cries (Laurent and Ablow, 2012), and mothers who more strongly activate the lateral OFC in response to their infant’s cries, which the authors interpreted as increased emotion regulation, have less activity of the hypothalamus-pituitary-adrenal (HPA) axis in response to the Strange Situation (Laurent et al., 2011).
Previous functional MRI (fMRI) studies have examined father’s neural response to infant cries (Seifritz et al., 2003). Here, we extend these studies to ask how this neural response relates to hormone levels, variation in the AR gene, paternal attitudes and paternal behavior outside the MRI scanner. Given the role of the AI in empathy, we hypothesized that activity within the right AI would be positively related to plasma levels of OT and Prl, but negatively correlated with plasma T levels. Given the inverse relationship between CAG repeats and AR expression, we hypothesized that the number of CAG repeats would be positively correlated with AI activity. We further expected a negative correlation between AI activity and restrictiveness, and a curvilinear relationship between AI activity and paternal caregiving behavior. We also hypothesized that activity in the prefrontal cortex, a reflection of a father’s ability to regulate a negative emotional reaction to crying, would be negatively correlated with T, AR expression as reflected in CAG repeat number, and positively correlated with the degree of paternal caregiving.
MATERIALS AND METHODS
Subjects
Enrollment in the study required participation by the father, mother and child. The study was approved by the Emory Institutional Review Board, and all participants gave written informed consent. A group of 36 biological fathers of children age 1 or 2 who were currently cohabitating with the child’s mother were recruited using flyers posted around the Emory campus, and at local parks and daycare centers. Fathers had normal or corrected-to-normal (with contact lenses) vision and were screened and excluded for self-reported history of head trauma, seizures or other neurological disorder, psychiatric illness, alcoholism or any other substance abuse, serious medical illness, claustrophobia, and for ferrous metal in any part of body. Fathers were between the age of 21 and 55 (M = 33.0, s.d. = 6.10) and had between 1 and 4 children, with 2 as the modal number (M = 1.94, s.d. = 0.86) (see Table 1).
Table 1.
Descriptive statistics of fathers
| Descriptive statistics | |||||
|---|---|---|---|---|---|
| N | Minimum | Maximum | Mean | s.d. | |
| Age of father (years) | 36 | 21 | 55 | 33.00 | 6.10 |
| Total no. of children | 36 | 1 | 4 | 1.94 | 0.86 |
| Age of child (years) | 36 | 1 | 3 | 1.99 | 0.64 |
| Responsibility | 34 | 41 | 80 | 64.22 | 8.40 |
| Restrictiveness | 34 | 24 | 80 | 56.26 | 11.67 |
| Nurturance | 34 | 48 | 84 | 71.32 | 8.73 |
| Oxytocin (pg/ml) | 34 | 4.61 | 33.36 | 9.82 | 5.06 |
| Prolactin (mIU/l) | 34 | 117.89 | 649.96 | 197.92 | 94.39 |
| Testosterone (ng/dl) | 34 | 173.67 | 745.03 | 407.19 | 136.93 |
| CAG repeats | 32 | 9 | 22 | 17.69 | 3.51 |
Design
First, the mother completed self-report parenting questionnaires (described below). In a separate session, fathers began by completing self-report parenting questionnaires. Next, we acquired a saliva sample for genetic analysis and 16 ml of blood was drawn for hormone assays. Subjects were then positioned in the MRI scanner where they received structural and fMRI scans of their brain.
Parenting questionnaires
To measure caregiving, we used a Parental Responsibility Scale (S.Goodman, personal communication) that combines two scales, the McBride & Mills Parental Responsibility Scale and the Montague & Walker-Andrews Child Care Activity Questionnaire (McBride and Mills, 1993; Montague and Walker-Andrews, 2002). The measure asks the parent to designate who has primary responsibility for 24 tasks along a five-point scale (e.g. ‘Take the baby to preventative health care clinic’), ranging from 1 (mother almost always) to 5 (father almost always). Responsibility is defined for the parent as remembering, planning and scheduling the task. The measure also allows parents to report ‘N/A’ if the item is not applicable to their family (e.g. ‘Pick up baby at day care/sitter’). If ‘N/A’ was chosen for an item, we performed a mean replace using that participant’s mean score. Answers were summed to create a ‘caregiving’ score, such that a higher relative score reflected greater paternal caregiving. If mothers and fathers were equally responsible for all items, the total score would be 72.
To measure parenting attitudes we used the modified Block Child-Rearing Practices Report (CRPR) (Rickel and Biasatti, 1982), which assesses parental attitudes and values using 40 items and a Likert type format. Previous work shows that the items load onto two factors, nurturance (e.g. ‘He usually takes into account our child’s preference when making plans for the family’) and restrictiveness (e.g. ‘He believes that a child should be seen and not heard’), and that it has good reliability and construct validity (Deković et al., 1991). The CRPR was altered in two ways for use in this study. First, the wording was modified so that mothers could evaluate the fathers’ beliefs and values. Second, 12 items were omitted prior to administration because they were not applicable to the age of the children in the study (e.g. ‘He believes children should not have secrets from their parents’). The final CRPR had 11 items from the nurturance subscale and 17 items from the restrictiveness subscale.
Hormone assays
Blood samples were centrifuged at 4°C within 20 min of blood draw. Plasma was collected and frozen at −80°C until assayed. Assays were analyzed in duplicate by the Biomarkers Core Lab of the Yerkes National Primate Research Center at Emory University using coated-tube radioimmunoassay kit (OT: Cat# ADI-901-153, Lot# 10241239A; Prl: Cat# IM103, Lot # PRL 011112; T: Coat-A-Count Total Testosterone, Cat# TKTT1; Siemens, Los Angeles, CA). On the day of the assay, frozen plasma samples were thawed, centrifuged for 30 min at 3000 revolutions per minute, and assayed according to the protocol provided by the manufacturer. The inter-assay CV% was as follows: OT: 7.16% at 26.99 pg/ml; Prl: 6.51% at 328.34 mIU/l; T: 4.05–4.37%. The intra-assay CV% was as follows: OT: 4.41% at 27.30 pg/ml; Prl: 1.62% at 345.20 mIU/l; T: ranged from 2.07% at 136.11 ng/dl to 2.28% at 785.81 ng/dl. The sensitivity of the assays was OT: 15.60–1000.00 pg/ml; Prl: 106.00–3200.00 mIU/l; T: 6.0–1667.00 ng/dl. Prl results from one participant were omitted as his value (650.0 mIU/l) was above what is considered to be in the normal range of a healthy population [mild hyperprolactinemia = 420–735 mIU/l (Corona et al., 2007)] and was 4.8 s.d. greater than the mean of our sample.
Genetic assays
Subjects provided a saliva sample for genotyping analyses using Oragene kits (DNA Genotek). DNA was extracted using automated DNA extraction by Omega-Biotek (Omegabiotek.com). CAG repeat polymorphisms in Exon 1 of the AR were genotyped using methods described previously (Mishra et al., 2005) with some modifications. A PCR reaction was performed using a forward primer labeled with FAM as fluorophore (5′-FAM-CAGAATCTGTTCCAGAGCGTGC) and a reverse primer (5′-AAGGTTGCTGTTCCTCATCCAG-3′) to amplify 10 ng dried DNA/sample in 10 µl final assay volume (1× Buffer, 1.5 mM MgCl2, 0.2 mM dNTP, 0.5 mM each primer, 5% DMSO, 1.25 Units Amplitaq Gold). For cycling, the following touch down conditions were used: 95°C for 5′, 94°C for 1′, annealing temperature starting at 63°C for 1′ and going down to 60°C with 1° decrease, 72°C for 1′ (two cycles for the intermediate temperature and 26 cycles for the final annealean temperature of 60°C); final extension was performed at 72°C for 10′. The PCR products for the AR length polymorphisms were run in ABI Gene Analyzer capillary detection system for fluorescently labeled PCR primers (ABI 3730). All fragments were automatically sized and examined using Applied Biosystems Genemapper 4.0 software. Two independent readers examined all electropherograms to confirm the automatically sized results. For quality control, allele frequencies and distribution were considered. Genetic results from one participant were omitted as his number of repeats (3) fell below what is considered to be in the normal range of a healthy population [8–35 (Hsing et al., 2000)] and was 5 s.d. below the mean of our sample.
Samples with different base pair size were randomly selected for Sanger Sequencing. Briefly, after performing PCR for the CAG repeat as previously described, sample amplifications were evaluated on 2.5% agarose gel and then purified using QIA quick PCR purification kit (Qiagen). Following normalization of the PCR purified-products at 5 ng/µl, samples were cycle sequencing using BigDye terminator plus reverse primer and ran on 3130 ABI Sequencing Machine by Omega-Biotek. Electropherogramms were evaluated using Sequencer 5.1: for each sample, the CAG repeats were manually counted and confirmed by an independent reader. The results of this analysis consistently revealed three additional CAG repeats for each participant compared with GeneMapper values, and a range of 12–25 repeats (M = 20.7; s.d. = 3.51).
Cry stimuli
Cry stimuli were obtained from two infants, aged 3 and 5 months. Stimuli were purchased from an online audio database (www.audionetwork.com), and edited to 10 s clips using online available software (www.audacity.com). Two types of control stimuli were synthesized for each cry using Praat 5.1 (Boersma and Weenink, 2009) and Adobe Audition 3.0 software. For one control, referred to as Con, an emotionally neutral baby vocalization was created to match the duration, intensity, spectral content and amplitude envelope of the cry stimulus. The second control, TCon, was a pure tone that preserved the mean fundamental frequency and amplitude envelop of the cry.
Participants listened to the audio stimuli through Pro Ears Ultra 28 MRI-compatible headphones, and were told prior to the task: ‘You will now hear a series of sounds. You do not have to do anything except listen to them’. The six different sound files (C1, C2, Con1, Con2, TCon1 and TCon2) were presented in pseudorandom order over four blocks such that each sound was presented four times. There was an intertrial interval of 6 s between each stimulus (see Figure 1). Each of the four blocks lasted 126 s and the total duration of the task was 8 min 24 s.
Fig. 1.
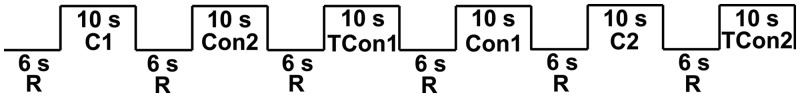
Schematic of block design structure. C, Cry; Con, Control; TCon, Tone Control; R, rest.
Anatomical image acquisition
Subjects were positioned in the Siemens Trio 3 T MRI scanner. Subjects lay motionless in a supine position in the scanner with padded head restraint to minimize head movement during scanning. Each scanning session began with a 15 s scout, followed by a 5 min T1-weighted MPRAGE scan (TR = 2600 ms, TE = 3.02 ms, matrix = 256 × 256, FOV = 256 mm, slice thickness = 1.00 mm, gap = 0 mm).
fMRI image acquisition
Functional scans used an EPI sequence with the following parameters: TR = 2000 ms, TE = 28 ms, matrix =64 × 64, FOV = 224 mm, slice thickness = 2.5 mm, gap thickness = 1.05 mm, 34 axial slices. TE was minimally decreased from the typical value (32 ms) in order to reduce magnetic susceptibility artifact in the orbitofrontal region.
Functional image analysis
Image preprocessing was conducted with Brain Voyager QX (version 2.0.8) software (Brain Innovation, Maastricht, The Netherlands). The first eight volumes of each run were discarded in order to allow the tissue magnetization to equilibrate. Preprocessing involved image realignment by six-parameter 3D motion correction, slice scan time correction using linear interpolation, spatial smoothing with a 8 mm full width at half maximum Gaussian kernel, and temporal smoothing with voxel-wise linear detrending and high-pass filtering of frequencies below three cycles per run length. Images were subsequently normalized into Talairach space (Talairach and Tournoux, 1988).
A separate general linear model (GLM) was defined for each subject that modeled the neural response to the following three regressors: Cry (C), Control (Con) and Tone Control (TCon). Each regressor was convolved with a standardized model of the hemodynamic response. The resulting GLM was corrected for temporal autocorrelation using a first-order autoregressive model. For each subject, contrasts of parameter estimates for various predictors were computed at every voxel of the brain. Each subject's contrast image was entered into a second level, random effects analysis, and one sample t-test were conducted to determine contrast effects. In order to evaluate our hypotheses of interest, we focused on [Cry − Tone Control] to assess fathers’ responses to infant cries. Results were thresholded at P < 0.001, uncorrected.
Given our a priori hypotheses about the role of the AI in paternal empathy, we defined a functional region of interest (ROI) by identifying the local maximum of activity within the AI and including all activated voxels within 10 voxels of the peak in the X, Y and Z directions. Individual subject contrast values from this ROI were explored in bivariate correlation analyses with responsibility, nurturance and restrictiveness. Given our hypothesis that responsibility would have a non-linear relationship with activity in the AI, we also tested for a curvilinear relationship between activity in the AI and responsibility. Finally, we conducted a whole brain exploratory analysis using hormones, CAG repeats, restrictiveness and nurturance as covariates for the contrast [Cry − Tone Control]. Results were thresholded at P < 0.001 uncorrected for multiple comparisons and limited to activations at least 10 functional voxels.
RESULTS
Self-report
Completed measures were acquired from 34 mothers (see Table 1 for descriptive statistics). Post hoc reliability analyses revealed that all measures had good internal reliability (responsibility: α = 0.82, restrictiveness: α = 0.82, nurturance: α = 0.81). There was a significant correlation between age of the child and restrictiveness scores [r(32) = 0.39, P < 0.05], but neither responsibility nor nurturance scores were correlated with the age of the child.
Neuroimaging
Main effect of the cry task (Cry − Tone Control)
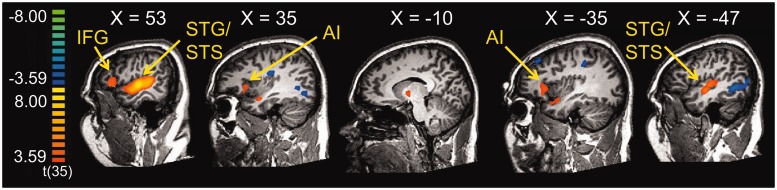
The cry stimuli robustly activated brain regions previously shown to be active in response to infant crying, including bilateral IFG extending into the AI (Lorberbaum et al., 2002; Riem et al., 2011) and bilateral globus pallidus (Kim et al., 2011). We also observed extensive bilateral activity in the auditory cortex of the superior temporal gyrus, likely due to increased attention to the cries compared with the control stimulus, and these activations were right-lateralized as has been reported previously (Lorberbaum et al., 2002). Table 2 lists the results of the contrast [Cry − Tone Control], and the results are displayed in Figures 2 and 3a.
Table 2.
Main effect of the Cry Task [Cry − Tone Control], thresholded at P < 0.001
| Cry − Tone Control (n = 36) | Brodmann’s area | X | Y | Z | Peak t | Voxels |
|---|---|---|---|---|---|---|
| R superior temporal sulcus, extending into superior temporal gyrus | 22 | 56 | −14 | 0 | 9.57 | 778 |
| R IFG, extending into AI and middle frontal gyrus | 45, 13, 9 | 44 | 25 | 6 | 4.99 | 190 |
| L globus pallidus | −10 | −5 | 0 | 4.14 | 14 | |
| L IFG, extending into AI | 47, 45, 13 | −37 | 22 | 3 | 4.57 | 43 |
| L superior temporal sulcus, extending into superior temporal gyrus | 22 | −55 | −23 | 6 | 6.82 | 486 |
Fig. 2.
Main effect of the Cry Task [Cry − Tone Control], thresholded at P < 0.001, uncorrected. STG, superior temporal gyrus; STS, superior temporal sulcus.
Fig. 3.
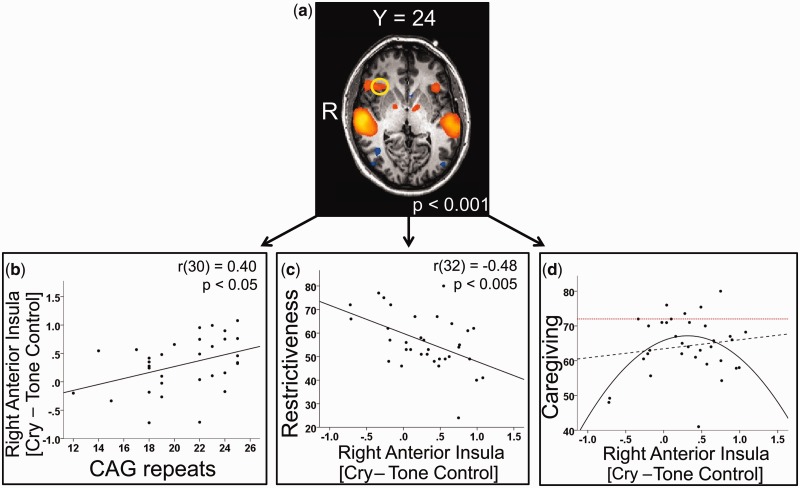
(a) Main effect of the contrast [Cry − Tone Control] thresholded at P < 0.001, uncorrected. The AI ROI was generated by identifying the local maximum of activity within the AI and including all activated voxels within 10 voxels of the peak in the X, Y and Z direction; plot of beta contrast values [Cry − Tone Control] from functionally derived right AI ROI vs (b) number of CAG repeats in exon 1 of AR, (c) restrictiveness, (d) responsibility. Linear and quadratic fits are indicated by the dotted and solid black lines, respectively. Only the quadratic fit is significant [F(31) = 3.49, P < 0.05 for quadratic vs r(32) = 0.15, P = 0.41 for linear]. The dotted red line indicates the score (72) at which mothers and fathers are equally responsible for their child’s daily care. Scores below 72 imply that the mother does more than the father and scores above 72 imply the opposite.
Relationship between brain activity and covariates
ROI analysis
Individual subjects’ contrast values from the functionally derived ROI in the right AI were positively correlated with number of CAG repeats [r(30) = 0.40, P < 0.05], and negatively correlated with restrictiveness [r(32) = −0.48, P < 0.005] (Figure 3b and c). The correlation between the number of AR CAG repeats and AI activity was not driven by differences in ethnicity, as the correlation remained when only the Caucasian subset of the participants was analyzed (n = 21) [r(19) = 0.46, P < 0.05]. Activity in the AI was not linearly related to OT, T, Prl, responsibility, or nurturance scores. However, there was a significant curvilinear relationship between AI activity and responsibility: F(31) = 3.49, P < 0.05 (see Figure 3d), indicating that fathers with low and high levels of AI activity participated less in childcare. Fathers with moderate levels of AI activity had the highest levels of paternal caregiving behavior, as reported by the mother.
Whole brain exploratory analysis
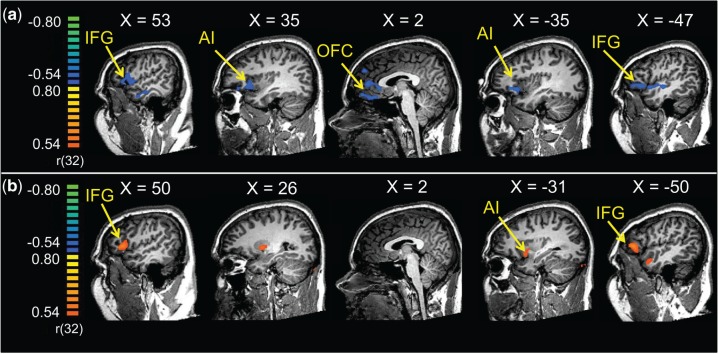
The results of a whole brain exploratory analysis using OT, Prl, T, CAG repeats, restrictiveness, nurturance and responsibility as covariates for the contrast [Cry − Tone Control] are listed in Table 3 and shown in Figure 4a and b. The analysis revealed multiple regions that were negatively correlated with restrictiveness, including the bilateral IFG stretching into the AI, anterior cingulate cortex (ACC) and medial orbitofrontal cortex (Figure 4a). Activity in bilateral IFG and left insula was correlated with the number of CAG repeats (Figure 4b). There were no regions in which activity correlated with OT, T, Prl, nurturance, or responsibility.
Table 3.
Results of whole-brain analysis [Cry − Tone Control] using restrictiveness and CAG repeats as covariates, thresholded at P < 0.001
| Brodmann’s area | X | Y | Z | Peak r | Voxels | |
|---|---|---|---|---|---|---|
| Cry − Tone Control: Restrictiveness (n = 34) | ||||||
| R inferior temporal gyrus | 21 | 59 | −11 | −15 | −0.63 | 29 |
| R IFG | 47 | 44 | 28 | 0 | −0.67 | 202 |
| R insula, extending into planum polare and superior temporal gyrus | 13, 22 | 44 | −5 | 0 | −0.59 | 29 |
| R cerebellum | 23 | −65 | −36 | −0.62 | 13 | |
| Medial orbital gyrus | 10, 11 | −10 | 46 | −9 | −0.66 | 138 |
| Superior frontal gyrus | 9 | 5 | 55 | 33 | −0.59 | 23 |
| ACC | 24, 32 | 2 | 28 | 3 | −0.62 | 46 |
| L IFG | 47 | −28 | 28 | −3 | −0.63 | 112 |
| L superior temporal gyrus, extending into superior temporal sulcus and IFG | 22, 47 | −52 | −14 | −3 | −0.63 | 124 |
| Cry − Tone Control: CAG repeats (n = 34) | ||||||
| R IFG | 45 | 50 | 25 | 6 | 0.64 | 46 |
| R putamen | 26 | 1 | 3 | 0.61 | 28 | |
| L claustrum stretching into insular gyrus | 13 | −28 | 7 | −6 | 0.61 | 22 |
| L IFG | 45 | −52 | 37 | 6 | 0.67 | 65 |
| L middle temporal gyrus | 21 | −52 | 7 | −21 | 0.60 | 16 |
Fig. 4.
Results from a whole brain exploratory analysis using (a) restrictiveness and (b) CAG repeats as covariates for the contrast [Cry − Tone Control]. All results are thresholded at P < 0.001, uncorrected.
DISCUSSION
The purpose of this study was to investigate the neural systems that correlate with paternal attitudes and support paternal caregiving as well as their relationship with hormones and genetic polymorphisms thought to be important for parenting. The cry task elicited a robust pattern of neural responses consistent with previous studies of parental responses to infant cries (Rilling, 2013). Some regions thought to be important for maternal responses to cries were not significantly active in this study, including the amygdala and the ventral tegmental area (VTA) (Lorberbaum et al., 2002; Riem et al., 2011), raising the possibility that this reflects a genuine difference between mothers and fathers. The AI response was positively correlated with number of CAG repeats and negatively correlated with restrictive attitudes. Activity in the AI had a non-linear relationship with paternal caregiving that followed an inverted u-shaped curve.
Genetics
There was a significant relationship between AR CAG repeat number and neural responses, which suggested that fathers who are less sensitive to T have increased neural responses in regions important for empathy, including in the AI and bilateral IFG. The fact that neural activity was related to genetic polymorphisms in the AR but not to actual hormone levels may suggest that differential responses are related to organizational effects that occurred early in development rather than activation effects. Related to this, relationships between AR CAG repeat number and brain activity were not seen in primate (rhesus macaque) brain regions known to be rich in AR receptors, such as the hypothalamus, lateral septal nuclei, bed nucleus of the stria terminalis and amygdala (Clark et al., 1988; Michael et al., 1989), even at a liberal statistical threshold of P < 0.01. However, many of these regions are connected with the AI where we do find effects of AR CAG repeat number (Mufson et al., 1981). Thus, we may be imaging downstream effects of AR binding.
Hormones
Contrary to expectations, T, Prl and OT levels were unrelated to neural responses to cries. With respect to OT, these null results may be due to potential limitations of plasma measures, which may not accurately reflect OT levels in the brain (Churchland and Winkielman, 2012; Kagerbauer et al., 2013). However, plasma OT levels have been linked with parenting behavior (Gordon et al., 2010a,b), and it may be that we were underpowered to find a relationship between plasma OT and brain function. Alternatively, OT, Prl and T may influence paternal brain function in response to child stimuli different from the unknown infant cries used here, and may influence paternal behavior in other ways, for example by modulating empathy in other contexts. It is also worth noting that when we include the extreme Prl data point, Prl levels were significantly related to BOLD activity in the AI ROI, and in a whole brain analysis, were significantly related to activity in the IFG and in regions important for processing and attending to auditory prosodic information, including the primary auditory cortex and the right medial geniculate nucleus (see Supplementary Figures S1 and S2 and Table S1). These data are consistent with previous studies linking Prl levels with attention to, and empathy for infant cries (Fleming et al., 2002), and with monkey tract-tracing studies that link the insula and auditory cortex (Mesulam and Mufson, 1982), and they indicate that future investigations using a larger sample with more variance in Prl levels are warranted. Furthermore, it is important to note that the studies showing effects of T and OT on neural responses to child cries have relied on exogenous administration of hormones in women (Bos et al., 2010; Riem et al., 2011), and an important next step will be to investigate the effects of exogenously administered hormones on fathers (e.g. Wittfoth-Schardt et al., 2012).
Attitudes and behavior
The significant non-linear relationship between activity in the right AI and paternal responsibility scores supports our hypothesis that moderate levels of activity in this region would predict optimal caregiving. Only one other study has attempted to relate paternal fMRI activity to paternal behavior outside the scanner, with the finding that fathers who exhibited more sensitive caregiving during a laboratory interaction with their child had less activity in the OFC in response to their own child (Kuo et al., 2012). This finding is difficult to interpret within existing models of the neurobiology of parental care, which link maternal attachment with OFC activation in response to one’s own child (Nitschke et al., 2004; Minagawa-Kawai et al., 2009; Laurent and Ablow, 2012). In addition, the aforementioned study used video stimuli to elicit paternal empathy, and it is likely that different neural systems are engaged by infant cries and may differentially predict behavior. Consistent with this idea, we have found that for visual picture stimuli activity in the VTA predicted paternal caregiving (Mascaro et al., 2013).
These results are also consistent with the complex picture emerging from research on the AI. Despite its role in empathy (Lamm et al., 2010; Fan et al., 2011) and in emotion understanding (Zaki et al., 2012), hyperactivity in the AI has long been implicated in anxiety and anxiety disorders (Critchley et al., 2004; Simmons et al., 2006, 2008, 2011). With respect to the data presented here, it appears that moderate levels of activity in response to cry stimuli are related to high levels of instrumental support. It may be the case that fathers with overactive AI have an excessive autonomic response to the cry stimuli, which then leads to an increased response by the AI. Alternatively, these fathers may focus excessively on interoceptive feedback from the body (Barrett et al., 2004; Terasawa et al., 2011), a bias in processing thought to be predictive of anxiety-related pathology (Paulus and Stein, 2006; Paulus and Stein, 2010). One prominent account of the insula’s role in anxiety is that top-down cognitive appraisal exaggerates the threat-value of a stimulus (in this case, a cry), leading to an augmented interoceptive signal from the AI that can lead to behavioral avoidance (Paulus and Stein, 2006). A testable hypothesis that emerges from this theory and from the present data is that fathers who have an overly negative or catastrophizing cognitive appraisal of cries (e.g. ‘I am a terrible father’ or ‘This baby is turning into a brat’) have enhanced interoceptive responses in the AI that lead to distress, and as a result, find it more aversive to engage with their child. Future studies coupling psychophysiological responses to cries with functional imaging will be necessary to determine which interpretation is most likely. Relatedly, the acquisition of self-reported levels of anxiety and distress would help explain the role of arousal and interoceptive processes in fathers’ responses to infant cries.
In addition, activity in AI was negatively related with restrictiveness scores, and a whole-brain analysis revealed multiple additional regions of activity that were inversely correlated with restrictiveness, including regions important for empathy (bilateral IFG) and for emotion processing (Roy et al., 2012) and regulation (Diekhof et al., 2011) (OFC). In general, studies have shown that restrictive parenting attitudes, as assessed using the CRPR, predict poorer child social and cognitive outcomes (Sommerfelt et al., 1995; Smith et al., 2000; Lindhout et al., 2009). Parents who heavily endorse restrictiveness attitudes are characterized as more controlling of children’s behaviors and feelings and tend to be more reliant on punishment (Rickel and Biasatti, 1982). We interpret this result to suggest that restrictive fathers are less empathic and more punitive, and this may be reflected by decreased activity in the AI, IFG and OFC in response to cries. Related, it may be that hypoactivity in these regions that are known to be important for empathy and emotion regulation is causal in its relationship with restrictive and punitive attitudes.
Neither are very low levels of restrictiveness expected to be ideal as the least restrictive fathers in our sample have the highest AI activity, which is related to decreased responsibility. There is evidence to support the notion that moderate levels of restrictiveness are more predictive of positive child outcomes. First, one longitudinal study parsed mothers into groups based on their child’s social and cognitive outcomes and found that although the most successful mothers were those with high levels of warmth and low levels of restrictiveness over the first 24 months of their child’s life, restrictiveness increased in these mothers as their children moved into the second year of life (Smith et al., 2000). The authors interpreted this to suggest that the most optimal maternal attitudes left latitude for a moderate amount of restriction. Second, extensive literature on parenting styles suggests that the style most predictive of good child outcomes is the authoritative style, which couples high warmth with boundary-setting and enforcement of rules (Steinberg et al., 1992; Darling and Steinberg, 1993).
LIMITATIONS AND FUTURE STUDIES
The interpretations outlined earlier hinge on the supposition that activity in the AI is causal in its relationship with paternal behavior and attitudes. However, it could also be the case that men who engage less with their children are unaccustomed to hearing a child in distress, and as a result, find it more aversive. These models are admittedly speculative, but they can be further tested with longitudinal studies that would allow researchers to more definitively test whether optimal AI responding to infant cries predicts future paternal engagement. Such a study would also mitigate another potential limitation of this study, which is that fathers’ children were older than the infants whose cries were used as stimuli. A longitudinal study would allow researchers to test the possibility that paternal responses to cries depend on the age of the father’s child.
Our modest sample size gives rise to a number of limitations: (i) although paternal AI activation explains significant variance in caregiving, the proportion of explained variance is small (R2 = 0.18), and the significance could be driven by a small number of influential data points; (ii) although the primary aim of this study was to investigate biological and behavioral predictors of the neural response to cries, our findings point to potential mediation models by which genes act on the brain to influence paternal behavior and attitudes. Although AR CAG repeat number explains a small but significant amount of variance in paternal brain function, which in turn explains a small but significant amount of the variance in paternal restrictiveness and caregiving behavior, there is not a significant relationship between AR CAG repeat numbers and restrictiveness or caregiving. Thus, we cannot test mediation models with the current data set. It may be that we were underpowered to find such associations, and future studies might be designed to test the models suggested by the data presented here.
Although we have no reason to believe that the fathers recruited for this study have a history of abuse or maltreatment, the findings from this presumably healthy population nevertheless highlight the importance of interventions aimed at optimizing the paternal response to infant cries, particularly for abusive fathers and fathers with post-partum depression. Given that infant crying is a common trigger for paternal abuse, fathers should be educated about infant crying. One ongoing program, the PURPLE Crying program, teaches parents that inconsolable infant crying is normal and understandably frustrating, and that it does not reflect bad parenting (Barr, 2012). In addition, meditation interventions have shown promise for reducing stress and negative affect in new moms (Duncan and Bardacke, 2010), and may be an effective intervention for decreasing aggression and increasing compassion in fathers. Finally, pharmacological interventions, such as intranasal OT (Naber et al., 2010, 2013), may help to reduce frustration and enhance empathy in at-risk fathers. Neuroimaging could be used to evaluate the effectiveness of these interventions, and the prediction arising from the data presented here is that efficacy will be related to augmenting the neural response in the AI in fathers suffering from empathy deficits, while reducing it in fathers prone to frustration and distress.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by a Positive Neuroscience Award from the John Templeton Foundation. The authors have no conflicts of interest to disclose. The authors thank Sherryl Goodman for guidance regarding the measurement of paternal behavior. Assay services were provided by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center. This facility is supported by the Yerkes National Primate Research Center Base Grant 2P51RR000165-51. Blood draws were provided by the Atlanta Clinical and Translational Science Institute’s Clinical Research Network, supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Amato PR, Fowler F. Parenting practices, child adjustment, and family diversity. Journal of Marriage and Family. 2002;64(3):703–16. [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Riem MME, Tops M, Alink LRA. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Social Cognitive and Affective Neuroscience. 2012;7(8):951–7. doi: 10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG. Preventing abusive head trauma resulting from a failure of normal interaction between infants and their caregivers. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl. 2):17294–301. doi: 10.1073/pnas.1121267109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, Bliss-Moreau E, Aronson KR. Interoceptive sensitivity and self-reports of emotional experience. Journal of Personality and Social Psychology. 2004;87(5):684. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD. Altruism and prosocial behavior. In: Gilbert D, Fiske S, Lindzey G, editors. The Handbook of Social Psychology. Boston: McGraw-Hill; 1998. pp. 282–316. [Google Scholar]
- Batson CD, Powell AA. Altruism and prosocial behavior. In: Millon T, Lerner M, editors. Handbook of psychology. New Jersey: John Wiley & Sons, Inc.; 2003. pp. 463–84. [Google Scholar]
- Boersma P, Weenink D. Praat: doing phonetics by computer (Version 5.1. 05)[Computer program] 2009. Retrieved May 1, 2009. [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35(1):114–21. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25(1):312–9. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Cabrera NJ, Tamis-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twenty-first century. Child Development. 2000;71(1):127–36. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM, Hariri AR. The social neuroendocrinology of human aggression. Psychoneuroendocrinology. 2011;36(7):935–44. doi: 10.1016/j.psyneuen.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Cavanagh K, Dobash RE, Dobash RP. The murder of children by fathers in the context of child abuse. Child Abuse & Neglect. 2007;31(7):731–46. doi: 10.1016/j.chiabu.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Research. 1994;22(15):3181–6. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong CS, Kemppainen JA, Zhou Z, Wilson EM. Reduced androgen receptor gene expression with first exon CAG repeat expansion. Molecular Endocrinology. 1996;10(12):1527–35. doi: 10.1210/mend.10.12.8961263. [DOI] [PubMed] [Google Scholar]
- Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Hormones and Behavior. 2012;61(3):392–9. doi: 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen binding and metabolism in the cerebral cortex of the developing rhesus monkey. Endocrinology. 1988;123(2):932–40. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- Corona G, Mannucci E, Fisher AD, et al. Effect of hyperprolactinemia in male patients consulting for sexual dysfunction. Journal of Sexual Medicine. 2007;4(5):1485–93. doi: 10.1111/j.1743-6109.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Darling N, Steinberg L. Parenting style as context: an integrative model. Psychological Bulletin. 1993;113(3):487. [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends in Cognitive Sciences. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Deković M, Janssens JM, Gerris JR. Factor structure and construct validity of the Block Child Rearing Practices Report (CRPR) Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3(2):182–7. [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58(1):275–85. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Duncan L, Bardacke N. Mindfulness-based childbirth and parenting education: promoting family mindfulness during the perinatal period. Journal of Child and Family Studies. 2010;19(2):190–202. doi: 10.1007/s10826-009-9313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annual Review of Psychology. 2000;51(1):665–97. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends in Cognitive Sciences. 2011;15(6):263–71. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Hormones and Behavior. 2002;42(4):399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(39):16194–9. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Prolactin, fatherhood, and reproductive behavior in human males. American Journal of Physical Anthropology. 2012;148(3):362–70. doi: 10.1002/ajpa.22058. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010a;68(4):377–82. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Prolactin, oxytocin, and the development of paternal behavior across the first six months of fatherhood. Hormones and Behavior. 2010b;58(3):513–8. doi: 10.1016/j.yhbeh.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, Singer T. Neural responses to ingroup and outgroup members' suffering predict individual differences in costly helping. Neuron. 2010;68(1):149–60. doi: 10.1016/j.neuron.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge: Belknap Press; 2009. [Google Scholar]
- Hsing AW, Gao Y-T, Wu G, et al. Polymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in China. Cancer Research. 2000;60(18):5111–6. [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–9. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. Journal of Neuroendocrinology. 2013;25(7):668–73. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, et al. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011;52(8):907–15. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T. Functional neural plasticity and associated changes in positive affect after compassion training. Cerebral Cortex. 2013;23(7):1552–61. doi: 10.1093/cercor/bhs142. [DOI] [PubMed] [Google Scholar]
- Kuo PX, Carp J, Light KC, Grewen KM. Neural responses to infants linked with behavioral interactions and testosterone in fathers. Biological Psychiatry. 2012;91(2):302–6. doi: 10.1016/j.biopsycho.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2010;54(3):2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience. 2012;7(2):125–34. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, Ablow JC. Neural correlates of hypothalamic-pituitary-adrenal regulation of mothers with their infants. Biological Psychiatry. 2011;70(9):826–32. doi: 10.1016/j.biopsych.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Barr RG, Catherine N, Wicks A. Age-related incidence of publicly reported shaken baby syndrome cases: is crying a trigger for shaking? Journal of Developmental and Behavioral Pediatrics. 2007;28(4):288–93. doi: 10.1097/DBP.0b013e3180327b55. [DOI] [PubMed] [Google Scholar]
- Lindhout IE, Markus MT, Hoogendijk TH, Boer F. Temperament and parental child-rearing style: unique contributions to clinical anxiety disorders in childhood. European Child & Adolescent Psychiatry. 2009;18(7):439–46. doi: 10.1007/s00787-009-0753-9. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51(6):431–45. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Mascaro JS, Hackett PD, Rilling JK. Testicular volume is inversely correlated with nurturing-related brain activity in human fathers. Proceedings of the National Academy of Sciences. 2013;110(39):15746–51. doi: 10.1073/pnas.1305579110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride BA, Mills G. A comparison of mother and father involvement with their preschool age children. Early Childhood Research Quarterly. 1993;8(4):457–77. [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of Comparative Neurology. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Michael RP, Rees HD, Bonsall RW. Sites in the male primate brain at which testosterone acts as an androgen. Brain Research. 1989;502(1):11–20. doi: 10.1016/0006-8993(89)90456-3. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Matsuoka S, Dan I, Naoi N, Nakamura K, Kojima S. Prefrontal activation associated with social attachment: facial-emotion recognition in mothers and infants. Cerebral Cortex. 2009;19(2):284–92. doi: 10.1093/cercor/bhn081. [DOI] [PubMed] [Google Scholar]
- Mishra D, Thangaraj K, Mandhani A, Kumar A, Mittal R. Is reduced CAG repeat length in androgen receptor gene associated with risk of prostate cancer in Indian population? Clinical Genetics. 2005;68(1):55–60. doi: 10.1111/j.1399-0004.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- Montague DPF, Walker-Andrews AS. Mothers, fathers, and infants: the role of person familiarity and parental involvement in infants' perception of emotion expressions. Child Development. 2002;73(5):1339–52. doi: 10.1111/1467-8624.00475. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6(7):1231–48. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, Ablow JC. The neural correlates of maternal sensitivity: an fMRI study. Developmental Cognitive Neuroscience. 2012;2(4):428–36. doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber F, van Ijzendoorn MH, Deschamps P, van Engeland H, Bakermans-Kranenburg MJ. Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: a double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35(10):1583–6. doi: 10.1016/j.psyneuen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Naber FB, Poslawsky IE, van IJzendoorn MH, Van Engeland H, Bakermans-Kranenburg MJ. Brief report: oxytocin enhances paternal sensitivity to a child with autism: a double-blind within-subject experiment with intranasally administered oxytocin. Journal of Autism and Developmental Disorders. 2013;43(1):224–9. doi: 10.1007/s10803-012-1536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–92. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biological Psychiatry. 2006;60(4):383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure and Function. 2010;214(5):451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickel AU, Biasatti LL. Modification of the block child rearing practices report. Journal of Clinical Psychology. 1982;38(1):129–34. [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Pieper S, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry. 2011;70(3):291–7. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51(4):731–47. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16(3):147–56. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S. Fathers' involvement and children's developmental outcomes: a systematic review of longitudinal studies. Acta Paediatrica. 2008;97(2):153–8. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry. 2003;54(12):1367–75. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biological Psychiatry. 2006;60(4):402–9. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biological Psychiatry. 2008;64(8):681–90. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32(11):1836–46. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Landry SH, Swank PR. The influence of early patterns of positive parenting on children's preschool outcomes. Early Education and Development. 2000;11(2):147–69. [Google Scholar]
- Sommerfelt K, Ellertsen B, Markestad T. Parental factors in cognitive outcome of non-handicapped low birthweight infants. Archives of Disease in Childhood-Fetal and Neonatal Edition. 1995;73(3):F135–42. doi: 10.1136/fn.73.3.f135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Lamborn SD, Dornbusch SM, Darling N. Impact of parenting practices on adolescent achievement: authoritative parenting, school involvement, and encouragement to succeed. Child Development. 1992;63(5):1266–81. doi: 10.1111/j.1467-8624.1992.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evolution and Human Behavior. 2000;21(2):79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- Terasawa Y, Fukushima H, Umeda S. How does interoceptive awareness interact with the subjective experience of emotion? An fMRI Study. Human Brain Mapping. 2011;34(3):598–612. doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn MH, De Wolff MS. In search of the absent father—meta-analyses of infant-father attachment: a rejoinder to our discussants. Child Development. 1997;68(4):604–9. [PubMed] [Google Scholar]
- Wittfoth-Schardt D, Gründing J, Wittfoth M, et al. Oxytocin modulates neural reactivity to children's faces as a function of social salience. Neuropsychopharmacology. 2012;37(8):1799–807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62(1):493–9. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.