Abstract
Self-concept is deeply affected in schizophrenia. Positive symptoms in particular are related to disturbed self/other distinctions. The neural networks underlying self-evaluation in schizophrenia have barely been investigated. The study reported here involved 13 patients with schizophrenia and 13 matched controls. During functional MRI, participants decided in three conditions whether the presented positive and negative personality traits characterized themselves, an intimate person, or included a certain letter. Based on the responses, each experimental condition was designed using a flexible factorial model. Controls and patients showed a similar behavioral pattern during self-evaluation, with group comparison revealing decreased activation in patients in the left inferior temporal gyrus and both temporal poles during self-ascription of traits, and in the anterior medial prefrontal cortex during evaluation of an intimate person. In patients, positive symptoms correlated positively with brain activation in the left parahippocampus during trait self-ascription. Hence, while evaluating themselves, schizophrenia patients revealed decreased activation in areas related to self-awareness overlapping with networks involved in theory of mind, empathy and social knowledge. Moreover, patients’ brain activation during self-reflection was affected by the current positive symptomatology. The close interaction between self and other highlights the clinical and social relevance of self-processing deficits in schizophrenia.
Keywords: self, schizophrenia, temporal dysfunctions, social cognition, fMRI
INTRODUCTION
Schizophrenia constitutes a severe mental illness affecting the innermost self. Monitoring deficits regarding the source of self-generated information are thought to be related to positive symptoms, such as thought insertion or hallucinations (Keefe et al., 2002). Moreover, in contrast to healthy subjects, who consistently reveal positive self-perception (Blackwood et al., 2003; Beer et al., 2010; Seidel et al., 2010), schizophrenia patients may exhibit a negative bias in self-evaluation (Barrowclough et al., 2003), e.g. self-ascribing more negative and fewer positive personality traits compared with their healthy counterparts (Pauly et al., 2011). The neural correlates underlying self-evaluation in schizophrenia are yet to be fully understood.
In healthy subjects, the evaluation of one’s personality traits results in activation in the cortical midline structures, mainly in the anterior medial prefrontal cortex (mPFC) and the posterior cingulate cortex as compared with various baseline conditions, such as lexical (letter) tasks, semantic demands, e.g. the evaluation of social desirability or positivity of traits or the correctness of a statement (Johnson et al., 2002; Fossati et al., 2003; Schmitz et al., 2004; see also Northoff and Bermpohl, 2004; Ochsner et al., 2005; Northoff et al., 2006), or the evaluation of a famous person (Kelley et al., 2002). However, a comparison between self-evaluation and a similar task, like the evaluation of a close person, revealed largely overlapping clusters (Schmitz et al., 2004; Ochsner et al., 2005; Vanderwal et al., 2008; Grigg and Grady, 2010) with only subtle differences, such as stronger activation during the self-referential condition in the anterior or inferior mPFC (Heatherton et al., 2006; Pauly et al., 2013), the dorsolateral prefrontal cortex (Schmitz et al., 2004; Vanderwal et al., 2008; Pauly et al., 2013) or the cingulate gyrus (Grigg and Grady, 2010).
Self-ascription of positive and negative traits is often emotion-related, which may also be reflected in the underlying brain networks. For example, although the mPFC responds to self-descriptive material independent of the valence (Moran et al., 2006), the temporal poles (Blackwood et al., 2004; Pauly et al., 2013) or the middle temporal gyrus (Pauly et al., 2013) are related to self-evaluation and resultant feelings such as pride (Takahashi et al., 2008) or embarrassment (Takahashi et al., 2004).
Cerebral dysfunctions in patients with schizophrenia during self-evaluative tasks have hardly been investigated. The few studies that have been carried out so far have shown an inconsistent picture implying alterations in the networks involved in other/self differentiation, such as the superior temporal pole, the inferior temporal and parietal gyri (Murphy et al., 2010), or decreased activation in the dorsal superior mPFC (Bedford et al., 2012). Also, an anterior to posterior shift in the cortical midline structures has been proposed, i.e. decreased mPFC (Holt et al., 2011) or rostral–ventral anterior cingulate cortex (ACC) activation (Blackwood et al., 2004) together with an increase in activation in the posterior cingulate gyrus as compared with healthy subjects (Blackwood et al., 2004; Holt et al., 2011). Taking the symptoms into account, the first results indicate a relationship between brain activation during self-evaluation and illness awareness (Bedford et al., 2012; Van der Meer et al., 2013). However, to what extent activation changes are related to positive symptomatology is still unknown. Moreover, most studies so far have referred to brain activation during certain self-related conditions (in block designs) without taking the actual behavioral responses into account.
In this study, we sought to explore the neural networks underlying self-ascription of personality traits in a response-dependent design in healthy subjects and patients with schizophrenia. We hypothesized dysfunction in the core areas of self-related brain networks, mainly in the cortical midline structures and temporal areas, during self-evaluation in schizophrenia patients. Due to the evaluative character of the task and the valenced connotation of personality traits, we also assumed altered activation in the emotion-related areas, such as the amygdala–parahippocampal complex, in which dysfunction has been consistently detected in schizophrenia patients (e.g. Habel et al., 2010; Pauly and Habel, 2011). We further wanted to explore the extent to which networks were comparable to the observed activation during evaluation of an intimate other person. Finally, we expected dysfunctions to increase with increasing positive symptomatology.
The investigation of self-evaluation is of high clinical relevance as the way patients evaluate themselves may not only be associated with positive symptoms (Erickson and Lysaker, 2012; Guerrero and Lysaker, 2013) but it can also affect their personal well-being (e.g. Weinberg et al., 2012; Mashiach-Eizenberg et al., 2013). Moreover, impairments in the perception of self and other are related to (social) functional disability (Fett et al., 2011).
MATERIALS AND METHODS
The study was performed in accordance with the standards of the Declaration of Helsinki. The Institutional Review Board of the Medical Faculty of the RWTH Aachen University approved the protocol. After complete description of the study to the subjects, written informed consent was obtained.
Participants
A total of 13 patients with schizophrenia and 13 controls (seven men and six women in each group), all right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971), took part in this study. Patients were recruited from the inpatient ward and outpatient clinic of the Department of Psychiatry, Psychotherapy, and Psychosomatic Medicine, RWTH Aachen University. Groups were matched for age, (parental) education and IQ (Table 1). Patients were diagnosed with the paranoid type of schizophrenia (one remitted, nine partly remitted, three chronically ill) using the Structured Clinical Interview for DSM-IV (SCID-I, German version: Wittchen et al., 1997). On an average, patients had been ill for 12 years (s.d. = 6.93) with 3.21 (s.d. = 1.97) illness episodes.
Table 1.
Demographical and neuropsychological data for patients with schizophrenia and healthy subjects [two-sample t-test: mean (±s.d.), t-scores, df and P-values]
| Sz patients mean (±s.d.) | Healthy subjects mean (±s.d.) | t | df | P-value | |
|---|---|---|---|---|---|
| Age (in years) | 36.23 (±9.46) | 34.46 (±8.58) | −0.50 | 24 | 0.622 |
| Parental education (in years) | 10.23 (±3.59) | 10.42 (±3.95) | 0.13 | 24 | 0.898 |
| Own education (in years) | 12.69 (±3.07) | 13.31 (±3.20) | 0.50 | 24 | 0.621 |
| MWT-B (IQ) | 111.38 (±10.95) | 109.62 (±14.64) | −0.35 | 24 | 0.730 |
| TMT-A (in s) | 29.74 (±5.72) | 22.79 (±6.48) | −2.90 | 24 | 0.008* |
| TMT-B (in s) | 85.49 (±31.67) | 50.45 (±19.49) | −3.40 | 24 | 0.002** |
| PERT (% correct) | 77.88 (±10.30) | 79.04 (±8.39) | 0.31 | 24 | 0.757 |
MWT-B, Mehrfachwahl-Wortschatz-Intelligenztest—Version B (German multiple choice vocabulary test for crystalline intelligence estimation).
*P < 0.05; **P < 0.006 (Bonferroni corrected).
Subjects with medical or neurological conditions that could potentially impact cerebral metabolism were excluded. The application of the SCID-I allowed for the exclusion of patients with psychiatric comorbidities (other than lifetime substance abuse) or controls with mental illness. Controls with mentally ill first-degree relatives were also excluded. Urine drug tests affirmed current drug abstinence. All patients received atypical antipsychotic medication.
In patients, the sum of the four global scores of the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) within the last week averaged 4.31 (s.d. = 4.21). The average sum of the five global scores of the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) was 7.00 (s.d. = 4.28). The Global Assessment of Functioning scale (American Psychiatric Association, 1994) yielded a mean score of 46.15 (s.d. = 14.46). The Hamilton Depression Scale (Hamilton, 1960) reflected no clinically relevant depressive symptomatology given a mean score of 6.15 (s.d. = 4.76).
Functional MRI paradigm and data acquisition
Functional images were collected from the whole brain by means of echo-planar imaging (T2*, TR = 2.4 s, TE = 30 ms, 64 × 64 matrix, α = 90°; FoV: 211 × 211 mm2, voxel size: 3.3 × 3.3 × 3 mm3, gap: 0.3 mm, 37 slices) on a Phillips 3 T MR scanner. A scanning run of 360 volumes was collected for each subject.
Stimuli consisted of 126 positive (50%) and negative (50%) personality traits not differing in length, frequency, imagery or concreteness [see Pauly et al. (2011) for more details]. The adjectives were presented via PRESENTATION software package for 2 s followed by a fixation cross jittered between 1.5 and 5.5 s in three recurring conditions in pseudo-randomized order: participants were required to decide, by pushing a button, whether the traits (i) characterized themselves (self-evaluation), (ii) characterized an intimate person of personal choice (other-evaluation) or (iii) whether the word included the letter ‘r’ (lexical task). This design facilitated a comparison between self-ascription of traits and a similar task such as the evaluation of a close person. On the other hand, by means of objective performance measures a further lexical baseline led to the conclusion that the traits had been processed (correctly) while controlling for unwanted influences such as visual input and implicit valence processing, reading or motor reactions. Thus, stimulus material and response condition were kept constant across all three tasks.
Three words of each of the six balanced sub-conditions (self-, other- and lexical task with positive and negative adjectives, respectively) were presented in mini-blocks. Short (5 s) instructions indicated the next task prior to each block. All subjects practiced the task outside the scanner. The evaluation paradigm was followed by an unannounced recognition task reported elsewhere.
After the measurement, each participant was asked to rate the person he or she had thought of during the ‘other’ condition on a bipolar 8-point scale [−4 (very negative), … , 4 (very positive)]. The relationship with the intimate other person was not predefined to account for interindividual differences pertaining to family background.
Neuropsychological data
Neuropsychological testing entailed crystalline intelligence estimation (Mehrfachwahl-Wortschatz-Intelligenztest—Version B; Lehrl, 1989) to examine the corresponding comparability of the groups and to characterize patients’ cognitive status. Emotion discrimination ability was assessed by means of the Penn Emotion Recognition Test (PERT; Kohler et al., 2004). Finally, the Trail-Making Test (TMT) A and B (Reitan, 1958) analyzed potential group differences in visuomotor information processing performance and cognitive flexibility.
Data analysis
Behavioral data
All behavioral data were calculated by means of SPSS 18.0 for Windows. Evaluation patterns were assessed by the mean percentage of affirmed positive and negative personality traits. As responses to negative self- and other-referred traits as well as the amount of misses (Supplementary Data) in each sub-condition contradicted normal distribution (Kolmogorov–Smirnov test), the effects of the within-subject factors reference (self, other) and emotion (positive, negative) and the between-subjects factor group were analyzed non-parametrically by Mann–Whitney tests for independent and Wilcoxon tests for dependent samples. The same held true for the percentage of correct answers during the lexical condition with the independent factors emotion and group. Evaluation response patterns were further correlated (Spearman Rho) with the SAPS and SANS scores.
Reaction times for all sub-conditions were normally distributed and analyzed in a 3 × 2 × 2 repeated measures ANOVA with the factors condition (self, other, lexical), emotion and group.
A correction of the degrees of freedom was undertaken if Levene’s test for equality of variances (post hoc t-tests) or the Mauchly test on sphericity (ANOVA) revealed significance.
Functional MRI data
Functional magnetic resonance imaging (fMRI) data analysis was performed with SPM5. After realignment and stereotaxic normalization (3 × 3 × 3 mm3), smoothing was conducted with an 8 mm full-width-at-half-maximum Gaussian blurring kernel. A 1/128 Hz high-pass filter removed low-frequency noise. Realignment parameters proved that none of the participants moved more than one voxel.
In an event-related design relying on the individual button presses, we determined six trial types for personality traits ascribed and rejected during the self- and other-reference conditions, and for correctly affirmed and correctly rejected stimuli of the lexical task. A seventh regressor modeled the instruction onsets and the onset of a previous standardized button test.
On a second level, contrasts were entered into a flexible factorial design (random-effects analysis) with the individual reaction times acting as covariate. We contrasted self-ascribed traits (SELF) and traits ascribed to a well-known person (OTHER) with lexically correctly classified traits (baseline, BL) and SELF with OTHER, directly. Furthermore, SELF vs BL and OTHER vs BL were combined in a conjunctional analysis investigating the overlapping networks of both evaluative tasks. The corresponding results are reported for patients and controls separately. Moreover, between-groups comparisons for SELF vs BL, OTHER vs BL and SELF vs OTHER are reported. To control for unwanted effects of the subtractions of negative values (resulting in positive findings), we further compared the SELF conditions of both groups exclusively masking (P < 0.001) the OTHER group contrast and plotting the corresponding parameter estimates for all conditions.
Identifying the networks specific for self-evaluation as compared with other-evaluation, we further sought to investigate whether activation found in these areas in healthy subjects (Table 2) varied with the degree of symptomatology in patients, i.e. whether increasing dysfunctions were found in patients with higher symptom scores. Two separate correlation analyses were performed analyzing the networks underlying SELF in relation to positive and negative symptomatology in patients (as assessed by the sum of the SAPS global score and the sum of the SANS global score, respectively) in the three regions of interest [ROI, P < 0.05 family-wise error (FWE) corrected], namely the superior temporal poles, the gyrus rectus and the parahippocampi (as defined via the WFUPickatlas; Maldjian et al., 2003). Most patients revealed a partially remitted clinical condition. Therefore, low SAPS scores for hallucinations and self-related delusions alone did not vary sufficiently for inclusion into the correlation analyses.
Table 2.
Brain activation during (a) self-ascription (SELF) vs lexical processing of positive and negative personality traits, (b) ascription of positive and negative personality characteristics to the intimate other (OTHER) vs lexical processing, (c) the conjunction of SELF vs lexical processing and OTHER vs lexical processing, and (d) SELF vs OTHER in healthy subjects and schizophrenia patients with no activation for the opposite contrast (OTHER vs SELF) in both groups (flexible factorial analysis, P < 0.05 Monte Carlo corrected for multiple comparisons, MNI coordinates)
| Region | Side | x | y | z | kE | t |
|---|---|---|---|---|---|---|
| (a) SELF > lexical | ||||||
| Controls | ||||||
| Superior medial prefrontal cortex | L | −3 | 59 | 20 | 471 | 5.54 |
| Inferior orbitofrontal gyrus | L | −43 | 30 | −17 | 353 | 5.11 |
| Superior temporal pole (BA38) | R | 40 | 20 | −33 | 114 | 4.41 |
| Precuneus, posterior cingulate gyrus (BA30) | L | −3 | −53 | 20 | 79 | 4.47 |
| Middle temporal gyrus | L | −50 | −10 | −20 | 16 | 3.97 |
| Schizophrenia patients | ||||||
| Superior medial prefrontal gyrus | L | −13 | 46 | 46 | 413 | 7.37 |
| Inferior orbitofrontal gyrus | L | −46 | 30 | −17 | 108 | 7.24 |
| Precuneus, posterior cingulate gyrus (BA31) | L | −3 | −56 | 30 | 51 | 4.32 |
| Lingual gyrus (BA19) | L | −10 | −59 | −3 | 33 | 3.91 |
| (b) OTHER > lexical | ||||||
| Controls | ||||||
| Superior medial prefrontal cortex | L | −3 | 56 | 33 | 477 | 5.31 |
| Inferior orbitofrontal gyrus | L | −50 | 30 | −13 | 98 | 4.59 |
| Precuneus, posterior cingulate gyrus (BA30) | L | −3 | −53 | 20 | 137 | 4.72 |
| Schizophrenia patients | ||||||
| Superior prefrontal gyrus (BA8) | L | −13 | 46 | 49 | 140 | 6.29 |
| Inferior orbitofrontal gyrus | L | −43 | 30 | −17 | 56 | 4.55 |
| (c) Conjunction of SELF > lexical and OTHER > lexical | ||||||
| Controls | ||||||
| Superior medial prefrontal cortex | 0 | 56 | 20 | 374 | 5.25 | |
| Inferior orbitofrontal gyrus | L | −50 | 30 | −13 | 93 | 4.59 |
| Precuneus (BA30) | L | −3 | −53 | 20 | 59 | 4.47 |
| Schizophrenia patients | ||||||
| Superior prefrontal gyrus (BA8) | L | −13 | 46 | 49 | 140 | 6.29 |
| Inferior orbitofrontal gyrus | L | −43 | 30 | −17 | 55 | 4.55 |
| (d) SELF > OTHER | ||||||
| Controls | ||||||
| Superior temporal pole (BA38) | R | 46 | 20 | −30 | 23 | 4.13 |
| Gyrus rectus (BA25), parahippocampus | L | −3 | 20 | −20 | 23 | 3.79 |
| Parahippocampus | L | −20 | −10 | −33 | 16 | 3.66 |
| Schizophrenia patients | ||||||
| Superior medial prefrontal cortex (BA10) | L | −10 | 53 | 16 | 39 | 3.88 |
| Medial prefrontal cortex | R | 23 | 36 | 20 | 17 | 3.53 |
| Middle frontal gyrus | R | 43 | 30 | 20 | 32 | 3.73 |
| Precentral gyrus | R | 46 | −7 | 46 | 352 | 4.28 |
| Cuneus | L | −10 | −89 | 33 | 24 | 4.12 |
We sought to eliminate medication effects by correlating patients’ individual chlorpromazine equivalents with brain activation during SELF and OTHER vs BL, respectively (Supplementary Data). Further exploratory correlation analyses between self-evaluative networks and cognitive flexibility (TMT) are reported in the Supplementary Data.
A Monte Carlo corrected error probability was adopted for all whole brain fMRI analyses. Monte Carlo simulations were computed using AlphaSim (Ward, 2000) implemented in AFNI 2011. Entering the mask headers of the analyses (including information on voxel size and mask extension) and defining a per voxel probability threshold of P = 0.001, after 1000 simulations a cluster size of 16 contiguous resampled voxels was indicated to correct for multiple comparisons at P < 0.05.
RESULTS
Behavioral data
Patients and controls neither differed regarding their ability to differentiate emotions (PERT, Supplementary Table S1) nor concerning the percentage of affirmed positive (with 79% vs 80%, respectively; Z = −0.10; P = 0.918) or negative (16% vs 5%, Z = −1.08; P = 0.282) personality traits during self-evaluation or the evaluation of the intimate other (positive: 75% in patients vs 81% in controls; Z = −0.75; P = 0.456; negative: 17% vs 9%, respectively; Z = −0.55; P = 0.582). Overall, significantly more positive than negative personality traits have been affirmed when referred to one’s self (Z = −4.46; P < 0.001) or the close other (Z = −4.43; P < 0.001). No significant effects were found for the evaluation condition, i.e. neither the amount of affirmed positive (Z = −0.18; P = 0.859) nor of the affirmed negative (Z = −1.42; P = 0.155) traits differed between self- and other-evaluation. There was no correlation with the SAPS or SANS scores (all P > 0.140). Correct answers during the lexical task revealed no significant effects for group (Z = −1.76; P = 0.079) or emotion (Z = −0.43; P = 0.670).
The ANOVA for reaction times yielded significant main effects for group (F = 4.77, df = 1, 24; P = 0.039) and condition (F = 22.21, df = 1.30, 31.30; P < 0.001) as well as a significant interaction effect for condition × emotion (F = 5.35, df = 2, 48; P = 0.008). No significant effects were found for emotion, group × condition, group × emotion or the triple interaction group × emotion × condition (all P > 0.188). Post hoc t-tests revealed overall slower reactions in patients (t = −2.23, df = 24; P = 0.036). Across both groups, the evaluation of oneself (t = 5.27, df = 25; P < 0.001) and the intimate other (t = 4.48, df = 25; P < 0.001) took longer than the lexical processing of traits, with no significant difference between self- and other-evaluation (t = 1.36, df = 25; P = 0.187). However, although there was no difference in reaction times with regard to emotion during self-evaluation (t = 0.53, df = 25; P = 0.958) and other-evaluation (t = 1.00, df = 25; P = 0.326), lexical decision for negative traits took longer than reactions to positive traits (t = −3.17, df = 25; P = 0.004).
Also, reaction times during the TMT differed between the groups (Supplementary Table S1).
Patients and controls chose their life partner (n = 2 and n = 6, respectively), a close relative (n = 8/4) or an intimate friend as the intimate other (n = 3 in each group), with no significant group differences in the relative frequencies (χ2 = 3.33, df = 2; P = 0.189). Patients and controls did not differ in their positive–negative rating of this intimate other person (median: 3.00 in both groups, Z = −1.47; P = 0.143).
Functional MRI
One-sample analyses
Self-evaluation
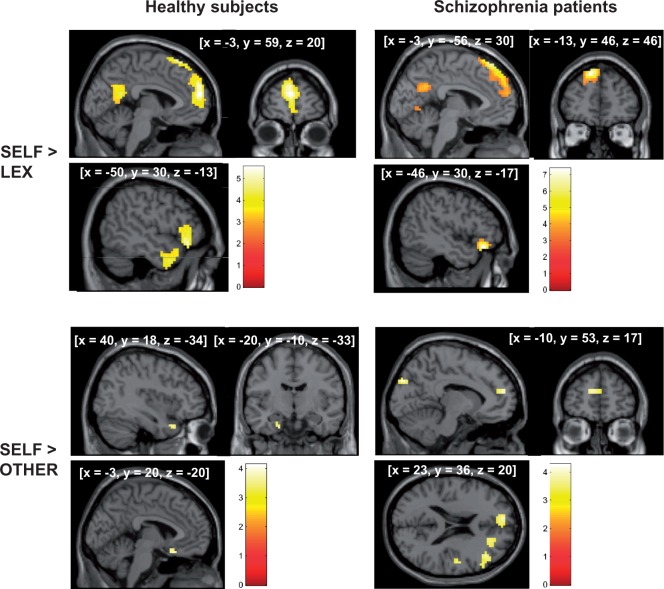
In controls, SELF vs BL revealed activation in the left superior mPFC, the left inferior OFC, the left middle temporal gyrus, right superior temporal pole (BA38) and the precuneus extending to the posterior cingulate gyrus (BA30).
In patients, activation was found in the left superior mPFC, left inferior OFC, left lingual gyrus (BA19) and the posterior cingulate gyrus extending to the precuneus (BA31; Table 2).
Other-evaluation
In healthy subjects, the comparison between OTHER and BL reflected activation in the left superior MPFC, the precuneus and the left inferior OFC. Patients showed activation in the left superior prefrontal and inferior OFC (Table 2).
Conjunction of self- and other-evaluation
In controls, common activation for SELF and OTHER (vs BL, respectively) encompassed the superior mPFC, the left inferior OFC and the precuneus extending to the posterior cingulate gyrus.
In patients, the conjunction yielded activation in the left superior prefrontal gyrus (BA8) and the inferior OFC (Table 2).
Self- vs other-evaluation
The comparison of traits ascribed to oneself vs the other revealed stronger activation for the self-condition, whereas the reversed contrast (OTHER vs SELF) failed to show significant effects in either group. Activated areas comprised the right superior temporal pole (BA38), the left rectal gyrus (BA25) and the parahippocampus in controls, and the left superior medial (BA10), right medial and right middle PFC, the right precentral gyrus and the left cuneus in schizophrenia patients (Table 2; Figure 1).
Fig. 1.
Brain activation during self-ascription of positive and negative personality traits (SELF) (a) vs correct lexical processing (LEX) of trait adjectives (upper row) and (b) vs reference to an intimate person (OTHER; lower row) in healthy controls (on the left) and patients with schizophrenia (on the right; P < 0.05 Monte Carlo corrected for multiple comparisons).
Group comparisons
Self-evaluation
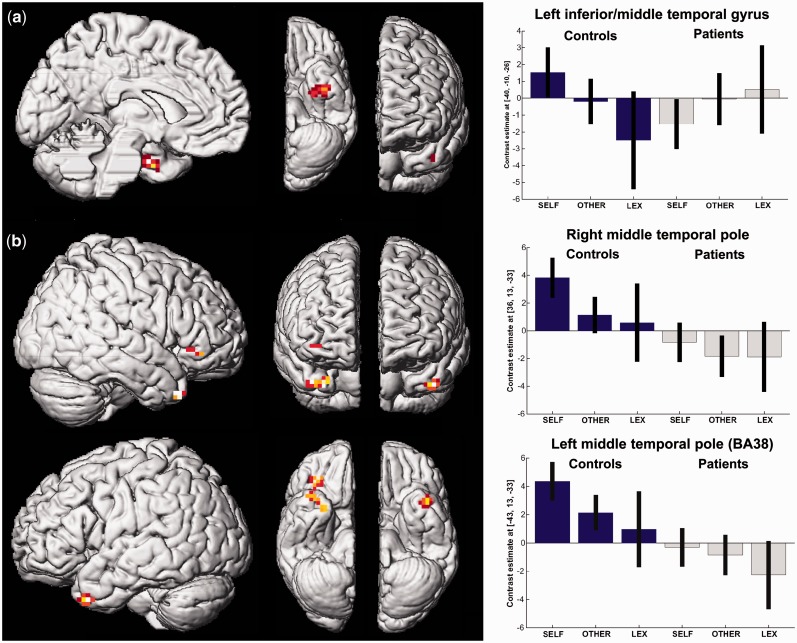
Although schizophrenia patients revealed no activation ‘increase’ as compared with controls when ascribing traits to themselves (compared with BL), ‘decreased’ activation was found in the left inferior extending to the middle temporal gyrus.
The comparison of SELF vs OTHER only revealed stronger right precentral activation in patients.
Finally, exclusively masking group differences during SELF condition with activation differences also found during OTHER (avoiding the subtractions of negative values) yielded stronger activation in healthy subjects in both temporal poles as well as in the right anterior insula but no stronger activation in patients (Table 3; Figure 2).
Table 3.
Brain activation differences between patients with schizophrenia and healthy subjects during (a) self-ascription of positive and negative personality traits (SELF) vs lexical processing, (b) ascription of positive and negative personality characteristics to the intimate other (OTHER) vs lexical processing, (c) the direct comparison of SELF and OTHER and (d) SELF exclusively masked with OTHER (flexible factorial analysis, P < 0.05 Monte Carlo corrected for multiple comparisons, MNI coordinates)
| Region | Side | x | y | z | kE | t |
|---|---|---|---|---|---|---|
| (a) SELF > lexical | ||||||
| Controls > patients | ||||||
| Inferior/middle temporal gyrus | L | −40 | −10 | −26 | 34 | 3.91 |
| Patients > controls | ||||||
| — | ||||||
| (b) OTHER > lexical | ||||||
| Controls > patients | ||||||
| Medial prefrontal cortex | 0 | 56 | 16 | 97 | 4.48 | |
| ACC | R | 16 | 33 | 16 | 24 | 3.54 |
| Insula | R | 33 | 20 | 13 | 18 | 3.53 |
| Insula | L | −43 | 3 | 10 | 16 | 3.45 |
| Cuneus | L | −7 | −76 | 26 | 18 | 3.91 |
| Patients > controls | ||||||
| — | ||||||
| (c) SELF > OTHER | ||||||
| Controls > patients | ||||||
| — | ||||||
| Patients > controls | ||||||
| Precentral gyrus | R | 49 | −7 | 46 | 17 | 3.65 |
| Precentral gyrus | R | 36 | −20 | 43 | 21 | 3.69 |
| (d) SELF masked with OTHER | ||||||
| Controls > patients | ||||||
| Insula | R | 33 | 30 | 0 | 28 | 3.65 |
| Middle temporal pole (BA38) | L | −43 | 13 | −33 | 17 | 3.95 |
| Middle temporal pole | R | 36 | 13 | −33 | 24 | 3.77 |
| Patients > controls | ||||||
| — |
Fig. 2.
Decreased brain activation in patients with schizophrenia (gray) as compared with healthy subjects (blue) for (a) self-ascribed personality traits (SELF) vs lexically processed traits in the left inferior/middle temporal gyrus and (b) for SELF exclusively masked with the other-evaluation condition in the temporal poles and the insula (P < 0.05 Monte Carlo corrected for multiple comparisons).
Other-evaluation
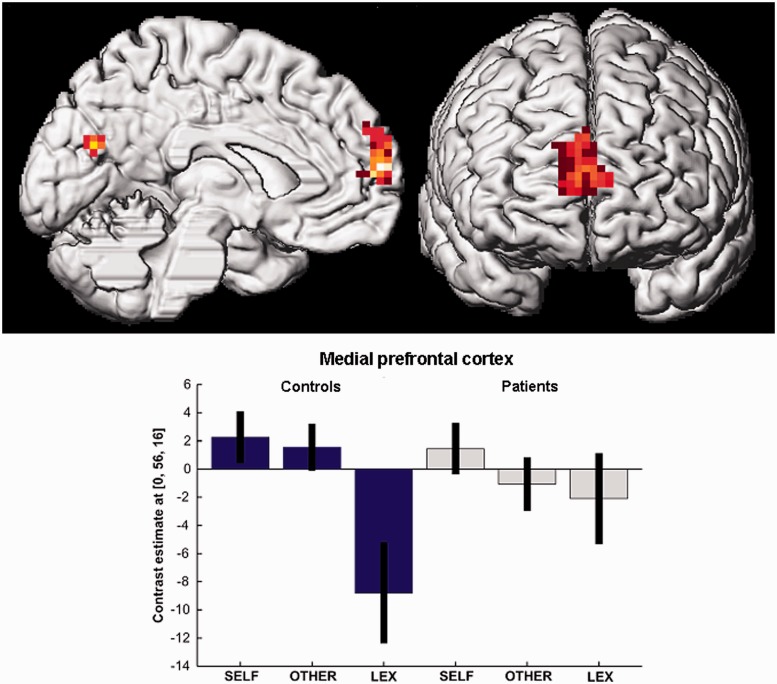
The group contrast of OTHER and BL yielded hypoactivation (but no hyperactivation) in patients in the anterior mPFC, right ACC, both insulae and the left cuneus (Table 3; Figure 3).
Fig. 3.
Decreased brain activation in patients with schizophrenia (gray) as compared with healthy subjects (blue) for personality traits ascribed to an intimate other person vs traits processed lexically in the anterior medial prefrontal cortex (P < 0.05 Monte Carlo corrected for multiple comparisons).
None of the activation differences was explainable in terms of medication effects (Supplementary Data).
Correlations
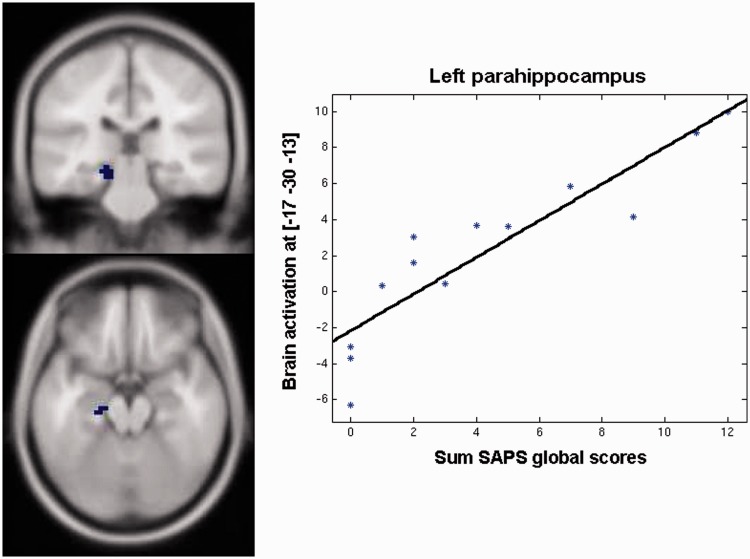
SANS scores did not correlate with brain activation during SELF (vs BL). However, positive symptomatology (SAPS) correlated positively with brain activation in the left parahippocampus ROI (x = −17, y = −30, z = −13, k = 24, t = 7.08; Figure 4). To test the specificity of this result, SAPS scores were further correlated with the activation during the evaluation of the intimate other (vs BL). However, no further correlations were found.
Fig. 4.
Positive correlation between the patients’ sum of the SAPS global scores and brain activation during self-ascription (vs lexical processing) of personality traits in the left parahippocampus (ROI analysis, P < 0.05 FWE corrected).
DISCUSSION
Investigating the neural basis of self-evaluation in schizophrenia patients, we found dysfunctions in networks associated with self-awareness and social knowledge, highlighting the close relationship between self-related and social processes. A positive correlation between positive symptoms and activation in the parahippocampus might indicate an increase in emotion-related responses with increasing psychopathology.
Self-evaluation
Patients with schizophrenia and healthy subjects revealed a comparable positive behavioral evaluation pattern concerning themselves and the intimate other. Accordingly, relative negative biases in self-evaluation (e.g. Barrowclough et al., 2003; Pauly et al., 2011) might represent state markers found in more severely impaired patients with florid symptomatology.
The brain networks involved in self-evaluation (vs baseline) were also largely comparable between the groups. However, a group-wise comparison between the activation of self-reflection and other-evaluation indicated different networks which might reflect different strategies applied.
Although controls revealed stronger activation mainly in the temporal areas, namely the right temporal pole and the left parahippocampus, as well as in the rectal gyrus, patients showed activation in the (superior) mPFC and the right middle prefrontal gyrus. Thus, healthy subjects might have relied on areas involved not only in self-referential thoughts (D’Argembeau et al., 2005; Lombardo et al., 2009), such as the ventral medial frontal gyrus, but also on the temporal networks involved in social knowledge (see also D’Argembeau et al., 2005; Moriguchi et al., 2006; Völlm et al., 2006) and emotion processing (Royet et al., 2000; Takahashi et al., 2004; Ochsner et al., 2005; Pauly et al., 2013), indicating a greater emotional involvement for self-evaluation vs evaluation of another person. Patients with schizophrenia, on the other hand, seem to have relied on areas responsible for higher cognition and self-knowledge (e.g. Schmitz et al., 2004; Ochsner et al., 2005).
A direct comparison of the groups underlined a greater temporal involvement in controls. Healthy subjects revealed stronger activation than patients in the left inferior extending to the middle temporal gyrus during the self-ascription of traits (as compared with the lexical baseline task) as well as in both temporal poles and the anterior insula (when exclusively masking activation differences also found during the other-evaluation). A closer inspection of the parameter estimates revealed a continuum in controls between self-evaluation and lexical processing with other-evaluation in between, whereas patients did not reveal comparable activation patterns (Figure 2).
Both temporal areas were found to be active during the evaluation of one’s own personality traits (D’Argembeau et al., 2005; Pauly et al., 2013), those of famous persons, or when reflecting on social issues (D’Argembeau et al., 2005). Moreover, in addition to the cortical midline structures, the temporal poles are considered to be part of a network involved in self-referential memory with decreasing activation in lateral temporal areas with decreasing self-relevance (self vs best friend vs famous person; Lou et al., 2004). They are involved in knowledge of social scripts and comprehension of the state of mind of others by relying on personal experiences (Frith and Frith, 2003; D’Argembeau et al., 2005). In this context, it has been hypothesized that self-reassurance involves similar regions as processes of empathy and compassion towards others, namely the left temporal pole and insula (whereas the middle temporal gyri were involved in self-criticism; Longe et al., 2010). This is in line with the simulation theory of emotion and empathy implying that humans anticipate the behavior of others by relying on their own mental states, resulting in overlapping neural implementations of mentalizing about self and other (e.g. Gallese et al., 2004; Lombardo et al., 2009). Self-conscious emotions may partly arise from concerns about others’ opinions of oneself and reflection on the mental state of individuals around one (Takahashi et al., 2004). Accordingly, activation in the temporal pole and the middle temporal gyrus (but also in the mPFC) was found in controls during theory-of-mind (ToM) tasks (Moriguchi et al., 2006; Völlm et al., 2006) and empathy (Völlm et al., 2006).
From a clinical point of view, this is of special interest as related deficits might partly act as phenotypes for schizophrenia (e.g. Montag et al., 2012). In a healthy sample, decreased activation of the lateral temporal cortex was associated with an increase in delusional thinking during the perception of self and others (Brent et al., in press). Temporal pole gray matter volume reductions in schizophrenia patients have been repeatedly reported (e.g. Kasai et al., 2003; Witthaus et al., 2009; Schultz et al., 2010), indicating dysfunctions in the limbic circuit, decreased regional intercorrelations between the temporal pole and other temporal regions (Mitelman et al., 2005) as well as longer pathway lengths to other nodes of the brain reflecting decreased efficiency (Van den Heuvel et al., 2010). Behaviorally, poor ToM performance in schizophrenia patients was correlated with gray matter density reduction in the temporal pole and the OFC (Herold et al., 2009). Moreover, decreased activation (Benedetti et al., 2009) and a lack of discriminating activation in the temporal pole in schizophrenia in response to different contextual information (Park et al., 2011) were found in ToM paradigms. In empathy-related tasks, both decreases (Lee et al., 2010) and increases (Benedetti et al., 2009) in activation were observed in the temporal pole.
Contrary to our findings, Murphy et al. (2010) reported hypoactivation in the inferior temporal gyrus and the temporal pole (as well as in the right inferior parietal lobe) in patients with schizophrenia for the opposite direction, i.e. when comparing the evaluation of another person with self-evaluation, implicating that those temporal dysfunctions might be more generally involved in altered self/other-differentiation. In line with this, a disturbed modulation of self-other processes in patients with schizophrenia was also reflected in reduced BOLD amplitude differences during an implicit self-other distinction task using voices (Jardri et al., 2011).
Also, insula activation (found to be reduced in our sample of schizophrenia patients during self- and other-reflection) has been linked to a sense of self (Kircher et al., 2000; Palaniyappan et al., 2011), self-reflection (Modinos et al., 2011), awareness (Craig, 2009) and the relationship between insight and self-reflection (Van der Meer et al., 2013). The morphometry of insula volume was inversely related to the severity of positive and negative symptoms in patients with schizophrenia over time (Takahashi et al., 2009). Functionally, insula activation was correlated with passivity symptom scores of the SAPS (Schnell et al., 2008) and was detected with the artificial induction (hypnosis) of delusions of control in healthy subjects (Blakemore et al., 2003). Accordingly, in schizophrenia, dysfunctions in the insula might reflect a diminished self/other boundary, e.g. deficits in the ability to differentiate between one’s own thoughts and the voices of others (e.g. Leube and Pauly, 2008).
Overlap between self- and other-evaluation
A close overlap between self-reflection and reflecting on others (see also Van der Meer et al., 2013), which (if unimpaired) might facilitate our understanding of other people, was also revealed by common activation in the superior mPFC, the OFC and the precuneus extending to the posterior cingulate cortex in a conjunction of self- and other-evaluation in healthy subjects. However, although patients with schizophrenia showed activation in the left superior prefrontal gyrus and the OFC in both conditions, no common activation was found in typical cortical midline structures, which might reflect a higher inter-individual variability during self- and other-related processes and a smaller overlap of both networks in schizophrenia.
Common networks for self-evaluation and the attribution of traits to others suggest that we may draw conclusions concerning the minds of others based partly on our own mental states (Happé, 2003). In this context, activation in the anterior PFC not only correlated with self-referential thoughts (D’Argembeau et al., 2005) but also with perceived similarities between oneself and another person when evaluating the traits of the other (Benoit et al., 2010) or when mentalizing the feelings of the counterpart (Mitchell et al., 2005). This may explain why activation differences in the MPFC become more obvious when comparing oneself not with an intimate other but famous persons or ‘most people’ (e.g. Kelley et al., 2002; D’Argembeau et al., 2005; Ochsner et al., 2005).
Interestingly, we found no activation differences in the cortical midline structures during self-ascription of traits in patients but decreased anterior mPFC and ACC activation for the other-evaluation contrast. However, decreased mPFC activation resulted from a diminished differentiation between other-evaluation and lexical processing in schizophrenia patients as compared with healthy subjects, who showed mPFC activation for both, SELF and OTHER, but strong deactivation during the lexical baseline (Figure 3). This corroborates the possibility of more general changes in brain networks underlying self/other-distinction in schizophrenia.
Positive symptoms
Generally, impairments in self/other-distinction in schizophrenia are related to positive symptomatology. Indeed, patients’ SAPS scores correlated with left parahippocampus activation (which was also found in controls but not in patients for the self- vs other-evaluation contrast). Interestingly, activation ‘increased’ with increasing symptomatology. Based on this finding, we can speculate that an increase in parahippocampus activation was related to compensatory efforts in more impaired patients, who, however, still managed to perform like healthy controls during the emotionally valenced scanner task (as well as in emotion discrimination during neuropsychological testing; see also Li et al., 2010). Parahippocampal dysfunctions during emotional tasks are well-known in schizophrenia (e.g. Habel et al., 2010; Li et al., 2010) and increased responses in the medial temporal and other limbic areas have been linked to enhanced emotional responses and the assignment of emotional content (Holt et al., 2006; Satterthwaite et al., 2010). However, increases in parahippocampal activation have also been found in the default mode network (closely related to self-referential activity) and are likely the result of disturbed connectivity (Garrity et al., 2007). Alternatively, increased parahippocampal activation may be linked to increased effort demanded by (episodic) autobiographical memory performance (e.g. Vandekerckhove et al., 2005; Gardini et al., 2006; Svoboda et al., 2006; Rabin et al., 2009) during self-ascription of ‘typical’ personality traits.
CONCLUSION
As a limitation, we acknowledge the problem of the small sample size, especially in comparison to behavioral studies. Correspondingly, some weaker effects might have reached significance given the larger statistical power.
In sum, during self-evaluation, schizophrenia patients revealed decreased activation in a brain network involved in self-referential tasks and overlapping with areas implicated in empathy and ToM. Mapping activation in the associated areas by means of parameter estimates revealed a continuum in controls, with the highest activation during self-evaluation, lexical processing at the other end of the continuum, and other-evaluation in between. However, a disturbed modulation (unrelated to medication) was found in patients with schizophrenia. Dysfunctions in a network underlying self-other reflections may act as endophenotypes and mirror a certain vulnerability to psychosis, in addition to impeding the well-being of schizophrenia patients, calling for specific psychotherapeutic interventions. A better understanding of the underlying neural correlates may help overcome this burden in the future.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Georg Eder, Andreas Finkelmeyer and Eugene Datta for their support, and all the participants whose collaboration has made this study possible. This study was supported by the START-Program (112/05) of the Medical Faculty of the RWTH Aachen University, the German Research Foundation (DFG; Initiative of Excellence: ZUK 32/1) and the Brain Imaging Facility of the Interdisciplinary Centre for Clinical Research of the Medical Faculty of the RWTH Aachen University.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984. [Google Scholar]
- Barrowclough C, Tarrier N, Humphreys L, Ward J, Gregg L, Andrews B. Self-esteem in schizophrenia: relationships between self-evaluation, family attitudes and symptomatology. Journal of Abnormal Psychology. 2003;112:92–9. [PubMed] [Google Scholar]
- Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12:106. doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, Lombardo MV, Bhanji JP. Roles of medial prefrontal cortex and orbitofrontal cortex in self-evaluation. Journal of Cognitive Neuroscience. 2010;22:2108–19. doi: 10.1162/jocn.2009.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research. 2009;114:154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. NeuroImage. 2010;50:1340–9. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Self-responsibility and the self-serving bias: an fMRI investigation of causal attributions. NeuroImage. 2003;20:1076–85. doi: 10.1016/S1053-8119(03)00331-8. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychological Medicine. 2004;34:591–6. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Oakley DA, Frith CD. Delusions of alien control in the normal brain. Neuropsychologia. 2003;41:1058–67. doi: 10.1016/s0028-3932(02)00313-5. [DOI] [PubMed] [Google Scholar]
- Brent BJ, Coombs G, Keshavan MS, Seidman LJ, Moran JM, Holt DJ. Subclinical delusional thinking predicts lateral temporal cortex responses during social reflection. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss129. . 2012 Dec 19. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Lysaker PH. Self-esteem and insight as predictors of symptom change in schizophrenia: a longitudinal study. Clinical Schizophrenia & Related Psychoses. 2012;6:69–75. doi: 10.3371/CSRP.6.2.4. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cornoldi C, De Beni R, Venneri A. Left mediotemporal structures mediate the retrieval of episodic autobiographical mental images. NeuroImage. 2006;30:645–55. doi: 10.1016/j.neuroimage.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant ‘default mode’ functional connectivity in schizophrenia. The American Journal of Psychiatry. 2007;164:450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Grigg O, Grady CL. The default network and processing of personally relevant information: Converging evidence from task-related modulations and functional connectivity. Neuropsychologia. 2010;48:3815–23. doi: 10.1016/j.neuropsychologia.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero AG, Lysaker PH. Socially naïve self-appraisal moderates the relationship between cognitive insight and positive symptoms in schizophrenia. Schizophrenia Research. 2013;143:97–101. doi: 10.1016/j.schres.2012.10.037. [DOI] [PubMed] [Google Scholar]
- Habel U, Chechko N, Pauly K, et al. Neural correlates of emotion recognition in schizophrenia. Schizophrenia Research. 2010;122:113–23. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:118–22. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F. Theory of mind and self. Annals of the New York Academy of Sciences. 2003;1001:134–44. doi: 10.1196/annals.1279.008. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold R, Feldmann Á, Simon M, et al. Regional gray matter reduction and theory of mind deficit in the early phase of schizophrenia: a voxel-based morphometric study. Acta Psychiatrica Scandinavica. 2009;119:199–208. doi: 10.1111/j.1600-0447.2008.01297.x. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biological Psychiatry. 2011;69:415–23. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophrenia Research. 2006;82:153–62. doi: 10.1016/j.schres.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pins D, Lafargue G, et al. Increased overlap between the brain areas involved in self-other distinction in schizophrenia. PLoS One. 2011;6:e17500. doi: 10.1371/journal.pone.0017500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, et al. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Archives of General Psychiatry. 2003;60:1069–77. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Arnold MC, Bayen UJ, McEvoy JP, Wilson WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophrenia Research. 2002;57:51–67. doi: 10.1016/s0920-9964(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrea CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, et al. Towards a functional anatomy of self-processing: effects of faces and words. Cognitive Brain Research. 2000;10:133–44. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Gur RE, Gur RC. Recognition of facial emotions in neuropsychiatric disorders. CNS Spectrums. 2004;9:267–74. doi: 10.1017/s1092852900009202. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Kang DH, Kim C-W, et al. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Research. 2010;181:121–9. doi: 10.1016/j.pscychresns.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B Manual. Erlangen: Perimed Fachbuch-Verlagsgesellschaft; 1989. [Google Scholar]
- Leube D, Pauly K. Ich-Störung—Psychologie. In: Kircher T, Gauggel S, editors. Neuropsychologie der Schizophrenie. Heidelberg: Springer; 2008. pp. 484–95. [Google Scholar]
- Li H, Chan RCK, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophrenia Bulletin. 2010;36:1029–39. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2009;22:1623–35. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Longe O, Maratos FA, Gilbert P, et al. Having a word with yourself: neural correlates of self-criticism and self-reassurance. NeuroImage. 2010;49:1849–56. doi: 10.1016/j.neuroimage.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences USA. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mashiach-Eizenberg M, Hasson-Ohayon I, Yanos PT, Lysaker PH, Roe D. Internalized stigma and quality of life among persons with severe mental illness: the mediating roles of self-esteem and hope. Psychiatry Research. 2013;208:15–20. doi: 10.1016/j.psychres.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;18:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Buchsbaum MS. Cortical intercorrelations of temporal area volumes in schizophrenia. Schizophrenia Research. 2005;76:207–29. doi: 10.1016/j.schres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25:295–305. doi: 10.1037/a0021747. [DOI] [PubMed] [Google Scholar]
- Montag C, Neuhaus K, Lehmann A, et al. Subtle deficits of cognitive theory of mind in unaffected first-degree relatives of schizophrenia. European Archives of Psychiatry and Clinical Neurosciences. 2012;262:217–26. doi: 10.1007/s00406-011-0250-2. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Ohnishi T, Lane RD, et al. Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage. 2006;32:1472–82. doi: 10.1016/j.neuroimage.2006.04.186. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Brent BK, Benton M, et al. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophrenia Research. 2010;116:252–8. doi: 10.1016/j.schres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, Liddle PF. Appreciating symptoms and deficits in schizophrenia: right posterior insula and poor insight. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:523–7. doi: 10.1016/j.pnpbp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Park IH, Ku J, Lee H, et al. Disrupted theory of mind network processing in response to idea of reference evocation in schizophrenia. Acta Psychiatrica Scandinavica. 2011;123:43–54. doi: 10.1111/j.1600-0447.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- Pauly K, Finkelmeyer A, Schneider F, Habel U. The neural correlates of positive self-evaluation and self-related memory. Social and Cognitive Affective Neuroscience. 2013;8:878–86. doi: 10.1093/scan/nss086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly K, Habel U. Neural substrates of emotion dysfunctions in patients with schizophrenia spectrum disorders. In: Ritsner MS, editor. Textbook of Schizophrenia Spectrum and Related Disorders. Volume I: Conceptual Issues and Neurobiological Advances. Dordrecht: Springer; 2011. pp. 405–29. [Google Scholar]
- Pauly K, Kircher T, Weber J, Schneider F, Habel U. Self-concept, emotion and memory performance in schizophrenia. Psychiatry Research. 2011;186:11–7. doi: 10.1016/j.psychres.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. Common and unique neural correlates of autobiographical memory and theory of mind. Journal of Cognitive Neuroscience. 2009;22:1095–111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indication of organic brain damage. Perceptual and Motor Skills. 1958;8:271–6. [Google Scholar]
- Royet J-P, Zald D, Versace R, et al. Emotional responses to pleasant and unpleasant olfactory, visual and auditory stimuli: a Positron Emission Tomography study. Journal of Neuroscience. 2000;20:7752–9. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, et al. Association of enhanced limbic response to threat with decreased cortical facial recognition memory response in schizophrenia. American Journal of Psychiatry. 2010;167:418–26. doi: 10.1176/appi.ajp.2009.09060808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22:941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schnell K, Heekeren K, Daumann J, et al. Correlation of passivity symptoms and dysfunctional visuomotor action monitoring in psychosis. Brain. 2008;131:2783–97. doi: 10.1093/brain/awn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, et al. Reduced cortical thickness in first episode schizophrenia. Schizophrenia Research. 2010;116:204–9. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Seidel E-M, Eickhoff SB, Kellermann T, et al. Who is to blame? Neural correlates of causal attribution in social situations. Social Neuroscience. 2010;5:335–50. doi: 10.1080/17470911003615997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Matsuda T, Koeda M, et al. Brain activations during judgments of positive self-conscious emotion and positive basic emotion: pride and joy. Cerebral Cortex. 2008;18:898–903. doi: 10.1093/cercor/bhm120. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Soulsby B, et al. Follow-up MRI study of the insular cortex in first-episode psychosis and chronic schizophrenia. Schizophrenia Research. 2009;108:49–56. doi: 10.1016/j.schres.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okuboc Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–74. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove MM, Markowitsch HJ, Mertens M, Woermann FG. Bi-hemispheric engagement in the retrieval of autobiographical episodes. Behavioural Neurology. 2005;16:203–10. doi: 10.1155/2005/460745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. Journal of Neuroscience. 2010;30:15915–26. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer L, de Vos AE, Stiekema AP, et al. Insight in schizophrenia: involvement of self-reflection networks? Schizophrenia Bulletin. 2013;39:1288–95. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Hunyadi E, Gruppe DW, Connors CM, Schultz RT. Self, mother and abstract other: an fMRI study of reflective social processing. NeuroImage. 2008;41:1437–46. doi: 10.1016/j.neuroimage.2008.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm BA, Taylor ANW, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf (27 November 2013, date last accessed) [Google Scholar]
- Weinberg D, Shahar G, Noyman G, Davidson L, McGlashan TH, Fennig S. Role of the self in schizophrenia: a multidimensional examination of short-term outcomes. Psychiatry. 2012;75:285–97. doi: 10.1521/psyc.2012.75.3.285. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Zaudig M, Fydrich T. SKID-I/II: Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe; 1997. [Google Scholar]
- Witthaus H, Kaufmann C, Bohner G, et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Research. 2009;173:163–9. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.