Abstract
Sensitivity to social evaluation has been proposed as a potential marker or risk factor for depression, and has also been theorized to increase with pubertal maturation. This study utilized an ecologically valid paradigm to test the hypothesis that adolescents with major depressive disorder (MDD) would show altered reactivity to peer rejection and acceptance relative to healthy controls in a network of ventral brain regions implicated in affective processing of social information. A total of 48 adolescents (ages 11–17), including 21 with a current diagnosis of MDD and 27 age- and gender-matched controls, received rigged acceptance and rejection feedback from fictitious peers during a simulated online peer interaction during functional neuroimaging. MDD youth showed increased activation to rejection relative to controls in the bilateral amygdala, subgenual anterior cingulate, left anterior insula and left nucleus accumbens. MDD and healthy youth did not differ in response to acceptance. Youth more advanced in pubertal maturation also showed increased reactivity to rejection in the bilateral amygdala/parahippocampal gyrus and the caudate/subgenual anterior cingulate, and these effects remained significant when controlling for chronological age. Findings suggest that increased reactivity to peer rejection is a normative developmental process associated with pubertal development, but is particularly enhanced among youth with depression.
Keywords: depression, neuroimaging, social exclusion, rejection, adolescence
INTRODUCTION
Rates of depression increase dramatically during adolescence, with one in seven adolescents experiencing an episode of depression prior to adulthood (Kessler, 1994; Beesdo et al., 2009a). This increase begins after mid-puberty and has been linked to the rise in testosterone and estradiol (Angold et al., 1998; Angold et al., 1999; Joinson et al., 2012). Pubertal maturation also appears to encompass a period of neural plasticity, particularly for some kinds of socio-affective learning (Crone and Dahl, 2012), thus, it may be an opportune time to modify neurobehavioral risk factors in ways that could potentially have a positive impact on the life course trajectory. For these reasons, there is a critical need for research that advances mechanistic understanding of normal and abnormal development of social and affective processes (and their neurobehavioral underpinnings) during adolescence, in ways that can inform early prevention and intervention approaches at this vulnerable time in the life course trajectory.
Theorists have proposed that increased sensitivity to social rejection during adolescence may be one factor that can help to explain the increase in depression during the teen years (Prinstein and Aikins, 2004; Davey et al., 2008; Stroud et al., 2009; Silk et al., 2012a). During adolescence, normative changes in the social context along with maturational changes in neural and endocrine systems that influence processing of motivational and socio-affective information could contribute to increased sensitivity to social evaluation, creating a potential window of vulnerability for depression during adolescence. Adolescents begin to spend more time with their peers and these peer relationships take on increased affective and motivational salience (Larson and Asmussen, 1991; Steinberg and Morris, 2001). It is not known to what extent neural and endocrine changes contribute to the increased salience of peer social status during adolescence, but it is likely that changes such as remodeling of the fronto-striatal dopaminergic systems and a puberty-linked rise in sex hormones could contribute to an increase in motivations to obtain and defend social status among peers (Nelson et al., 2005; Blakemore, 2008; Steinberg, 2008; Crone and Dahl, 2012). Although these changes are normative, they also may lead to increased risk for depression among youth who are particularly reactive to social evaluation, and/or experience high levels of peer rejection and low levels of peer acceptance.
Recently, researchers have begun to examine the neural response to peer acceptance and rejection using virtual peer paradigms such as the Cyberball virtual ball tossing task (Eisenberger et al., 2003) and the Chatroom Task (Guyer et al., 2008). Findings indicate that exclusion/rejection in adolescents activates a ventral affective salience network including the amygdala, medial prefrontal cortex, ventral and dorsal anterior cingulate cortex (ACC), and anterior insula, as well as ventrolateral areas of the prefrontal cortex (VLPFC) involved in the regulation of social distress (Eisenberger et al., 2003; Guyer et al., 2008; Masten et al., 2009; Bolling et al., 2011a; Sebastian et al., 2011). In particular, several studies of adolescents have implicated the subgenual ACC (sgACC), as well as a larger ventral portion of the ACC, in responding to social exclusion and rejection (Masten et al., 2009; Bolling et al., 2011a; Sebastian et al., 2011). Peer feedback tasks have also indicated that social acceptance activates regions involved in reward processing, particularly the nucleus accumbens (NAcc) (Davey et al., 2010; Gunther Moor et al., 2010).
Emerging data from these studies is consistent with the idea that neural response to social evaluation may increase during adolescence. For example, several studies have shown age-related increases across childhood and adolescence in neural response to peer evaluation in regions of this affective processing network, including the NAcc and insula, striatum, medial PFC and ventral ACC (Guyer et al., 2009; Gunther Moor et al., 2010; Bolling et al., 2011a). One study that compared response to social exclusion on the Cyberball task among early and middle adolescents and young adults found that activity in the sgACC in response to exclusion was strongest among early adolescents compared with mid adolescents and adults, possibly suggesting a period of peak sgACC reactivity to social rejection during early adolescence (Gunther Moor et al., 2012). There is also evidence that VLPFC activity to social evaluation increases across childhood and adolescence (Gunther Moor et al., 2010; Bolling et al., 2011a), but is decreased in adolescents compared with adults (Sebastian et al., 2011), potentially suggesting that adolescents are less effective at recruiting regulatory resources in response to social threat.
These apparent increases in sensitivity to social evaluation during adolescence may be linked to neurodevelopment of fronto-striatal-limbic systems that respond to social and emotional stimuli (see Paus et al., 2008; Pfeifer and Blakemore, 2012). Gaining a better understanding of how, specifically, pubertal development influences neural responses to social feedback may be helpful in understanding mechanisms associated with the pubertal increased risk for depression, as well as other emotional and behavioral health problems that increase in this maturational period, such as substance abuse and risky behaviors (Steinberg, 2005). Current theoretical models (Nelson et al., 2005; Steinberg, 2008) suggest that changes in socio-emotional behavior during adolescence may be mediated by the influence of sex hormones on neural circuits that support the processing of social and emotional stimuli. Sex hormones are known to play a role in remodeling and activating fronto-limbic-striatal circuits during adolescent brain development (Sisk and Foster, 2004). Consistent with this model, a small body of emerging data has linked self-reported pubertal maturation to increases in sensation-seeking (Martin et al., 2002), physiological and subjective reactivity to emotional words (Silk et al., 2009), and neural response to affective faces (Forbes et al., 2011; Moore et al., 2012). In this study, we examined whether self-reported pubertal maturation is related to neural response to social evaluation.
In addition, there has been little research examining neural response to social evaluation in adolescents with depression. Behavioral studies indicate that rejection from peers often precedes depressive symptoms (Nolan et al., 2003; Rudolph and Conley, 2005). Rejection via social media and mobile technologies (i.e. facebook, text messaging) is increasingly prevalent, and has been linked to teen suicide and depression (O’Keeffe et al., 2011; Luxton et al., 2012). Yet, little is known about the neural response to social evaluation during adolescence in youth with depression, especially in clinical samples. Existing research with depressed youth suggests that adolescents with major depressive disorder (MDD) show altered amygdala reactivity in response to threatening faces (Roberson-Nay et al., 2006; Beesdo et al., 2009b) and decreased striatal response to monetary reward (Forbes et al., 2006). One recent Cyberball study conducted in a non-clinical sample of adolescents showed that sgACC activity to social exclusion predicted increases in depressive symptoms over 1 year (Masten et al., 2011), but neural response to rejection has not been investigated in clinically depressed adolescents. We also investigated whether adolescents with depression differ in response to peer acceptance. In the only study of which we are aware to address this question, Davey et al. (2011) provided positive or neutral feedback to 15- to 24-year olds with MDD about how fictitious peers rated their likability. Contrary to findings from studies using monetary reward (Forbes et al., 2006), Davey et al. (2011) found that teens and young adults with depression showed increased amygdala response to acceptance compared with controls, highlighting the potential for important differences in neural response to social vs monetary rewards in depression.
In this study, we utilized a new virtual peer interaction task, the Chatroom Interact Task (Silk et al., 2012b), to probe the neural responses to rejection and acceptance from virtual peers during live simulated interaction in a sample of clinically depressed youth and healthy controls. Unlike an earlier Chatroom Task (Guyer et al., 2008), in which adolescents evaluate and receive feedback from virtual peers about whether they would like to participate in an online chat, in the Chatroom Interact Task, participants engage in a live online interaction with virtual participants during which they are repeatedly selected (accepted) and not selected (rejected) to discuss various topics of interest to teens. The task was designed to increase ecological validity and participant engagement with the virtual peers.
First we hypothesized that, relative to healthy controls, youth with current MDD would show increased reactivity to peer rejection in a network of ventral brain regions implicated in affective processing of social information, including the amygdala, sgACC, anterior insula, ventral ACC and VLPFC. We also explored whether depressed youth would show altered reactivity to peer acceptance or rejection relative to controls in regions typically associated with reward processing, such as the NAcc and mPFC, but were unsure whether to expect blunted or increased reactivity given conflicting initial findings on response to monetary and social reward in depressed youth (Forbes et al., 2006, 2009; Davey et al., 2011). We further hypothesized that youth more advanced in pubertal development would show increased neural response to peer rejection and acceptance (above and beyond the effects of age) in regions involved in social and affective processing. Finally, we explored whether the association between pubertal status and neural response to peer rejection and acceptance would differ for depressed youth and healthy controls.
METHODS
Participants
Participants were 48 adolescents (34 female, ages 11–17, M[s.d.]age = 15.48 [1.68]). Twenty-one adolescents had a current primary diagnosis of MDD based on DSM-IV (American Psychiatric Association, 1994) criteria and 27 were low-risk controls (CON) with no psychiatric history. MDD and CON adolescents did not differ in age, pubertal status, gender, race, or maternal education (all P’s > 0.45). Because the groups were matched on gender, and MDD is more common among females than males (Kessler et al., 2001), both groups had a higher proportion of females than males.
Youth were recruited from pediatrician’s offices and community advertisements. MDD youth were also referred from University and community mental health clinics. Adolescents’ lifetime and present DSM-IV (American Psychiatric Association, 1994) diagnoses were assessed using the Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime version (Kaufman et al., 1997). MDD youth were included if they were on a stable dose of SSRI medication but still met criteria for MDD (N = 2). Participants were excluded if they were taking psychoactive medications other than SSRI’s or had metal objects in their body. CON youth were excluded if they met current or lifetime DSM-IV diagnosis for any Axis 1 disorder. MDD youth were excluded if they had a current diagnosis of obsessive–compulsive disorder, post-traumatic stress disorder, conduct disorder, substance abuse or dependence and ADHD combined type or predominantly hyperactive–impulsive type, or a lifetime diagnosis of bipolar disorder, psychotic depression, schizophrenia, schizoaffective disorder, or a pervasive developmental disorder. Nine MDD youth had a current or past diagnosis of one or more comorbid anxiety disorders (N’s for each anxiety diagnosis were: panic disorder = 1; specific phobia = 4; generalized anxiety disorder = 6; social phobia = 1; separation anxiety disorder = 3; agoraphobia = 1). One MDD youth had a comorbid diagnosis of ADHD inattentive only subtype and one MDD youth had a diagnosis of oppositional defiant disorder. Informed consent/assent was obtained from participants and their parents at the initial assessment, and all research procedures were approved by the University of Pittsburgh Institutional Review Board.
Chatroom Interact Task
The Chatroom Interact Task was designed to investigate reactions to social acceptance and rejection from virtual peers in an on-line setting (Silk et al., 2012b). On Day 1, participants were shown photographs and fictitious biographical profiles for potential virtual peers. Participants were asked to choose the top five males and top five females that they would be interested in interacting with online at their next visit. Selections were made from within sets of 30 photographs for each age (9–11, 12–14 or 15–17) and gender grouping. Participants also provided their own biographical profile and photograph.
On Day 2 (approximately 2 weeks later), participants returned to the laboratory and were told that they had been matched with four other youth (two males and two females) selected from the first visit and that these youth were ready to participate in a ‘chat game’ online. They reviewed biographical profiles for selected peers prior to the task. The task takes the form of a structured online interaction, rather than a free-form ‘chat’, in order to give the impression that subjects and virtual peers are interacting in real time while maintaining sufficient standardization across subjects and sufficient repetition across trials to conduct analyses. During neuroimaging, pictures of the peers and participant were projected on the screen 2 at a time, as the subject and virtual peers took turns selecting who they would rather talk to about a series of teen interests (e.g. music, friends; Figure 1). The task proceeded in five blocks, each containing 15 trials in which a person was chosen or not chosen to discuss each topic (total run time 16.7 min). Stimuli were presented using E-prime 1.0 software (Psychology Software Tools, Pittsburgh, PA). Each block began with an instruction about who would be making choices for that block (agent). The photograph of the agent was shown at the bottom left corner of the screen and the photographs of the other two players were shown next to each other in the middle of the screen, as in Figure 1. At the beginning of each trial, the question ‘Who would you rather talk to about …’ with the selected topic for that trial (i.e. … ‘music?’) appeared on the screen for 3.34 s (task component durations were chosen to be multiples of our TR, 1.67 s). Feedback was then provided about which person was chosen (the subject or the virtual peer) for 10.02 s. The photograph of the person who was not chosen was superimposed with an ‘X’ and the photograph of the person who was chosen was highlighted around the border. To maintain engagement in the task, in all trials in which the participant was not the agent, he/she was asked to press a button to indicate whether the person on the left or the right was chosen.
Fig. 1.
Depiction of an example trial on the Chatroom Interact Task.
As in Silk et al. (2012b), trials were arranged in blocks so that participants experienced two ‘accept’ blocks in which they were chosen two-third of the time (one same-gender and one opposite-gender) and two ‘reject’ blocks in which they were rejected two-third of the time (one same-gender and one opposite-gender). Topics were presented randomly and repeated in each block, but with a different ‘agent’ for each block. The first three blocks were played with the two same gender virtual peers and the last two blocks were played with the two opposite gender virtual peers. In block 1, the subject was the ‘agent’ and made choices among the two same gender virtual peers. Analyses focus on blocks 2–5, in which the subject was chosen/not chosen by the virtual peers (first same gender, then opposite-gender). Due to time constraints, we did not include a sixth block in which the subject could make choices among the two opposite gender peers. Blocks 2–5 included 60 trials (30 accept and 30 reject), with half of all accept and reject trials from a same gender peer and half from an opposite gender peer. The order of accept and reject blocks and trials were randomized within gender grouping. Participants were not led to believe that they would have additional interaction with the virtual peers beyond the structured ‘chat game’ (i.e. although they chose which participants they would be more interested in discussing topics with, they were not led to expect to engage in an open discussion on these topics with the virtual peers).
Debriefing questionnaire
Participants rated how they felt along six dimensions (happy, sad, angry, nervous, included and excluded) on a 1–5 point scale following completion of the Chatroom Interact task. Ratings were made after completing the task to determine whether depressed and control subjects differed in mood following completion of the task; therefore, mood ratings were not specific to accept or reject trials. Subjects were debriefed at the conclusion of the task and informed that they had been playing with a preset computer program. Upon questioning, two participants (one MDD and one CON) reported suspicion that the other participants were not real. Analyses were re-run excluding these two participants, and the pattern and significance of findings reported below remained unchanged.
Pubertal status
Self-reported pubertal status was assessed using the Pubertal Development Scale (PDS; Petersen et al., 1988), scored to provide two 5-point scales that differentially capture gonadal and adrenal hormonal signs of pubertal development (Shirtcliff et al., 2009). Physical maturation in humans is marked by independent maturation of the adrenal glands (adrenarche) and the gonads (gonadarche). It is not yet clearly understood how adrenal and gonadal aspects of pubertal maturation may differentially influence neural and behavioral changes during adolescence; therefore, we explored the potential influence of adrenal and gonadal signs of pubertal maturation separately. Signs associated with adrenarche include pubic hair, skin changes and body odor, whereas signs associated with gonadarche include the growth spurt and breast development and menarche (in girls). Scoring takes into account different signs of pubertal development in boys and girls. Scores ranged from 2 to 5 in the present sample.
BOLD functional MRI acquisition, preprocessing and analysis
Imaging acquisition
Images were acquired on a 3T Trio scanner. Thirty-two 3.2 mm slices were acquired parallel to the AC–PC line using a posterior-to-anterior echo planar (EPI) pulse sequence (T2*-weighted imaged depicting BOLD signal; TR = 1670 ms, TE = 29 ms, FOV = 205 mm, flip angle = 75). Thus, there were eight scans per 13.36 s trial. High-resolution T1-weighted MPRAGE images (1 mm, axial) were also collected for use in cross-registration.
Functional MRI data preprocessing
Functional MRI (fMRI) analyses were conducted using NeuroImaging software (NIS) (Fissell et al., 2003), Analysis of Functional Neuroimaging (AFNI) (Cox, 1996) and custom Matlab routines. Functional imaging data were corrected for motion using 3dVolReg implemented in AFNI using the first image as a reference. Quadratic trends within runs were removed and outliers over 1.5 interquartile range from the 25th or 75th percentiles were Windsorized using niscorrect from NIS. Data were temporally smoothed using a 4-point Gaussian filter and converted to %-change based on the median of all imaging data. Data were co-registered to the Colin-27 Montreal Neurological Institute (MNI) template using AIR’s 32-parameter non-linear automated warping algorithm (Woods et al., 1998) and spatially smoothed using a 6 mm FWHM filter.
Plan of analyses
We conducted Region of Interest (ROI) analyses on a priori regions specified using AFNI’s Talairach atlas including the sgACC, bilateral anterior insula, bilateral NAcc, bilateral ventrolateral prefrontal cortex (VLPFC), ventral ACC (vACC) and medial prefrontal cortex (mPFC). Because AFNI’s Talairach atlas ROI incorporates a smaller volume than the anatomical boundaries of the amygdala, the amygdala ROI was anatomically defined by hand tracing on the MNI Colin 27 brain (as in Siegle et al., 2007). The long duration of each trial enabled slow event related model-free analysis (i.e. examining the empirical shape of the hemodynamic response using scan-within-trial as a repeated measure) and thus eliminated the need for event deconvolution. BOLD activity in ROIs was extracted and effects were tested using mixed-effect analyses with participants as a random factor, valence (acceptance vs rejection) and scan-within-trial (eight scans within each 13.36 s trial) as repeated measures, and group as a fixed factor, assuming an AR1 covariance structure using restricted maximum likelihood estimation. As is standard for slow-event-related analyses, trial-related responses were ‘baseline-corrected’, i.e. considered with activity at the first scan subtracted from the rest of the scan yielding activity uniquely associated with the trial rather than activity lingering from previous trials. Type I error was controlled using a Bonferroni correction (P < 0.005) for mixed effects analyses. None of the a priori regions showed group × valence × scan interaction effects, indicating that group differences were primarily a function of mean differences in BOLD amplitude across the timecourse. Therefore, results presented below highlight group × valence interactions, with BOLD activity averaged across scans for each valence (but see Supplementary Figure S1 for waveforms depicting BOLD activity across scans). We also examined whether any of the group × valence effects were additionally moderated by gender of the virtual peers (same vs opposite gender) by testing for group × valence × gender interactions in the mixed effects models. To identify additional brain areas that differed between MDD and healthy youth in response to rejection or acceptance, a supplemental whole-brain voxelwise ANOVA (analysis of variance) was conducted with participant as a random factor, and group, valence and scan-within-trial as fixed factors. To control type 1 error at P < 0.05 across the whole brain for each family of tests (i.e. <5% chance that even one voxel was identified in error), voxelwise whole-brain tests at a given statistical threshold (P < 0.001) were subjected to empirically determined contiguity thresholds based on the spatial autocorrelation of statistical maps using AFNI’s AlphaSim program.
Given the lack of existing data on how pubertal development might influence neural response to peer acceptance and rejection, puberty effects were examined using a whole-brain analysis rather than an ROI approach. A whole-brain regression using AFNI’s 3dRegana was conducted to identify areas showing a main effect of pubertal status on response to rejection and/or acceptance controlling for the effects of chronological age. To examine whether the relationship between pubertal status and neural response to social evaluation differed for healthy youth and controls, similar whole brain regression analyses were conducted to identify areas showing group × pubertal status interaction effects . Analyses were conducted separately using adrenal and gonadal PDS subscales.
RESULTS
Subjective ratings
As shown in Table 1, MDD youth rated themselves as feeling more ‘sad’, ‘nervous’ and ‘excluded’, and less ‘happy’ immediately following the Chatroom Interact Task compared with CON youth. The two groups did not differ on ratings of feeling ‘included’.
Table 1.
Group differences in pubertal status and post-task subjective ratings
| CON M (s.d.) | MDD M (s.d.) | t | df | Cohen’s d | |
|---|---|---|---|---|---|
| Pubertal status | |||||
| Adrenal | 4.35 (0.97) | 4.55 (0.83) | −0.77 | 44 | −0.22 |
| Gonadal | 3.96 (0.66) | 3.80 (0.75) | 0.77 | 44 | 0.23 |
| Post-task ratings | |||||
| Happy | 3.24 (1.08) | 2.43 (0.76) | 2.93** | 46 | 0.87 |
| Sad | 1.35 (0.43) | 1.93 (0.78) | −3.26** | 46 | −0.92 |
| Nervous | 1.44 (0.68) | 2.29 (1.19) | −3.08** | 46 | −0.88 |
| Included | 3.17 (1.00) | 2.86 (0.78) | 1.17 | 46 | 0.35 |
| Excluded | 1.52 (0.63) | 2.17 (0.78) | −3.19** | 46 | −0.92 |
**P < 0.01.
fMRI: ROI analyses of group × valence effects
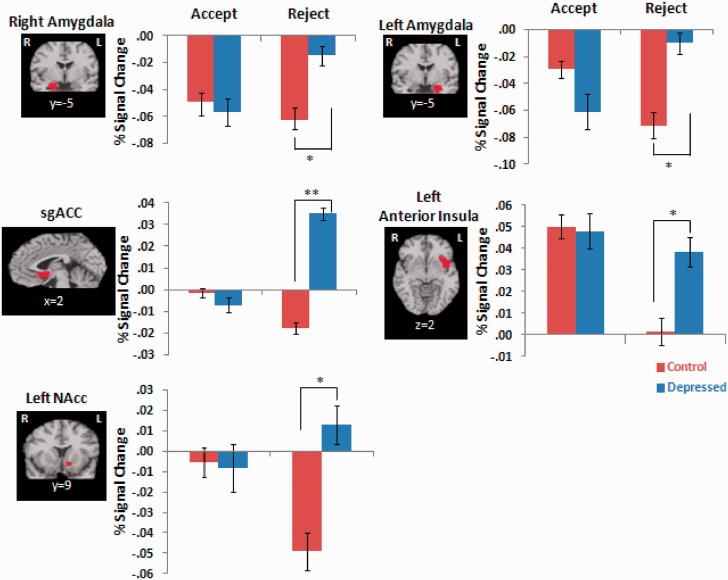
As shown in Table 2, there were significant group × valence interaction effects in our a priori regions including bilateral amygdala, sgACC, left anterior insula, left NAcc, bilateral VLPFC and vACC, but not the mPFC. Pairwise comparisons also shown in Table 2 revealed that MDD youth showed increased brain activity to rejection trials compared with CON youth in the bilateral amygdala, sgACC, left anterior insula and left NAcc, but did not differ in response to acceptance trials. As shown in Figure 2, relative to CON, MDD youth showed increased activation to rejection in the sgACC and left anterior insula. Activations in the left NAcc were also higher during rejection trials in MDD youth relative to CON youth, but this was driven by a deactivation (i.e. decreased activation from a pre-trial baseline) of the NAcc in response to rejection among the CON but not MDD youth. There was also deactivation of the bilateral amygdala in response to rejection compared with baseline among healthy controls that was attenuated in MDD youth. Pairwise t-tests comparing MDD youth to controls were not significant in the bilateral VLPFC or vACC, thus these results were not further considered (Supplementary Figure S2). There was also a significant group × valence × gender (same vs opposite) interaction effect in the bilateral amygdala, shown in Supplementary Figure S3.
Table 2.
ROI analyses: group × valence interaction effects
| A priori regions | Mixed-effects analysis group × valence interaction | Pairwise comparisons: MDD > CON |
|
|---|---|---|---|
| Accept | Reject | ||
| R amygdala | F(1, 305) = 17.91***, η2 = 0.06 | t(46) = 0.35, P = 0.73 | t(46) = 2.13, P = 0.04*, d = 0.62 |
| L amygdala | F(1, 274) = 28.91***, η2 = 0.10 | t(46) = 1.26, P = 0.22 | t(46) = 2.21, P = 0.03*, d = 0.64 |
| sgACC | F(1, 298) = 28.01***, η2 = 0.09 | t(46) = 0.32, P = 0.75 | t(46) = 3.06, P = 0.00**, d = 0.89 |
| R anterior insula | F(1, 422) = 3.24, η2 = 0.01 | t(46) = 0.04, P = 0.97 | t(46) = 0.94, P = 0.35, d = 0.27 |
| L anterior insula | F(1, 393) = 19.40***, η2 = 0.05 | t(46) = 0.13, P = 0.90 | t46) = 2.03, P = 0.05*, d = 0.59 |
| R NAcc | F(1, 326) = 3.94, η2 = 0.01 | t(46) = 0.55, P = 0.59 | t(46) = 2.04, P = 0.05*, d = 0.59 |
| L NAcc | F(1, 320) = 16.15***, η2 = 0.05 | t(46) = 0.10, P = 0.92 | t(46) = 2.47, P = 0.02*, d = 0.72 |
| R VLPFC | F(1, 375) = 13.29***, η2 = 0.03 | t(46) = 0.32, P = 0.75 | t(46) = 1.68, P = 0.10, d = 0.50 |
| L VLPFC | F(1, 408) = 11.29***, η2 = 0.03 | t(46) = 0.14, P = 0.89 | t(46) = 1.59, P = 0.12, d = 0.46 |
| mPFC | F(1, 368) = 2.05, η2 = 0.01 | t(46) = 0.87, P = 0.39 | t(46) = 1.86, P = 0.07, d = 0.54 |
| vACC | F(1, 314) = 8.36***, η2 = 0.03 | t(46) = 0.10, P = 0.92 | t(46) = 1.68, P = 0.10, d = 0.50 |
***P < 0.005 (Bonferroni corrected P-value for mixed effects F-tests), **P < 0.01, *P < 0.05; d, Cohen’s d effect size (0.20 = small, 0.50 = medium, 0.8 = large); η2, eta-squared effect size (0.01 = small, 0.06 = medium, 0.14 = large). ROI analyses were repeated excluding the two participants taking SSRI medications and all results were replicated, with the exception of the group × valence interaction effect in the vACC, which was no longer significant [F(1, 302) = 5.98, P = 0.02, η2 = 0.02].
Fig. 2.
ROI analyses revealing significant group × valence (acceptance vs rejection) interaction effects in the bilateral amygdala, sgACC, left anterior insula and bilateral NAcc (corrected P < 0.005). Pairwise comparisons show that MDD youth had signficiantly greater brain activity to rejection trials compared with CON youth (*P < 0.05, **P < 0.01). No significant group differences were found in acceptance trials.
Although not a primary focus of the study, we also found valence main effects in several of the ROIs, including the bilateral insula, bilateral VLPFC, mPFC and vACC (all corrected P’s < 0.005). As shown in Supplementary Figure S4, there was greater activation to acceptance compared with rejection in each of these areas across the entire sample.
fMRI: supplemental whole-brain analyses
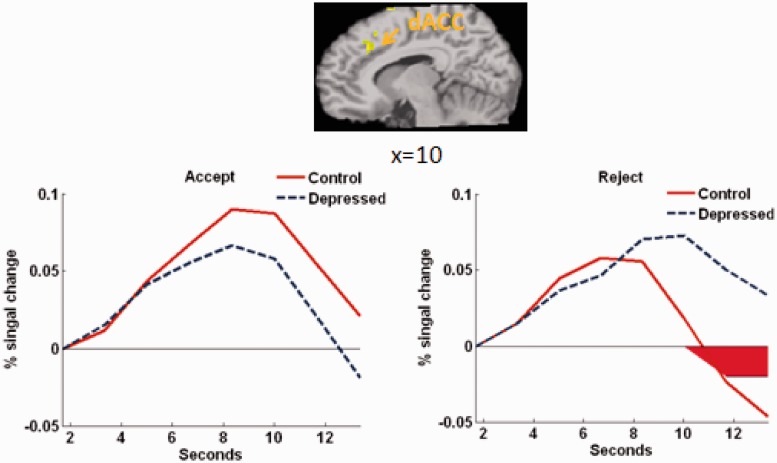
Supplemental whole-brain analyses revealed no additional group × valence effects, but several group × valence × scan interactions shown in Supplementary Table S1. Notably, MDD youth showed sustained activation in the dorsal ACC (dACC) relative to controls during the later 3 s of the feedback phase during rejection trials (Figure 3). There were also several valence main and valence × scan interaction effects, shown in Supplementary Table S2.
Fig. 3.
Time-course in the dACC showing group × valence × scan interaction effect in whole-brain analysis (P < 0.001, 18 voxels contiguity). MDD youth showed increased dACC activation to rejection compared with controls from 11.69 to 13.36 s [t(46) = 3.23, P < 0.01; significant scans shown in red].
fMRI: Whole-brain analysis of pubertal development
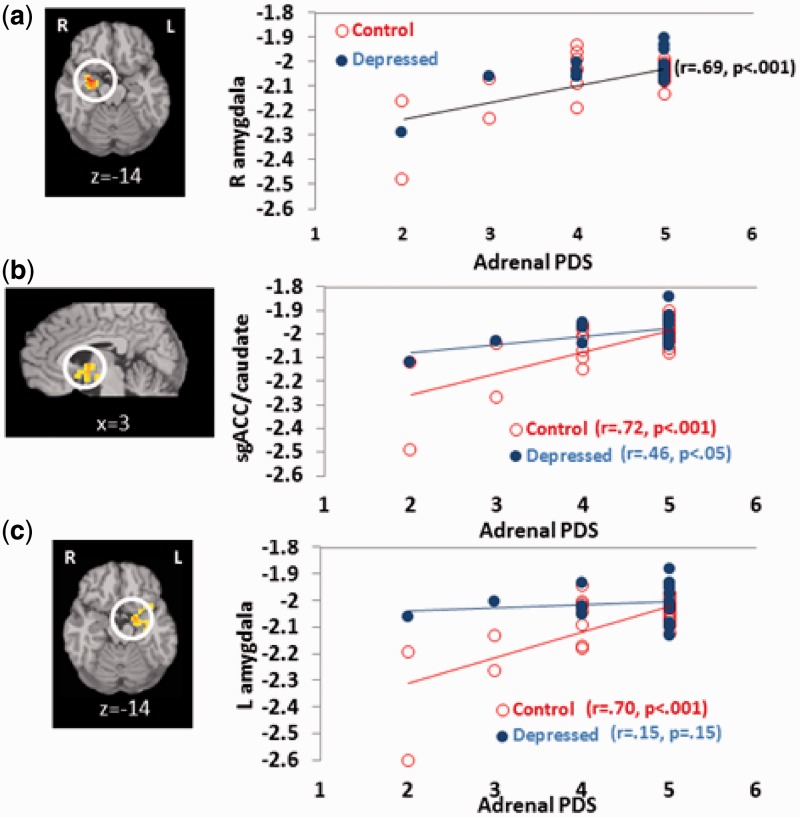
Whole-brain regression analyses revealed that more advanced pubertal status, specifically signs associated with adrenarche (i.e. pubic hair and body odor), were associated with increased activity (i.e. less deactivation) to rejection above and beyond the effects of age in the right amygdala/parahippocampal gyrus (Figure 4a) and the left amygdala/parahippocampal gyrus, as well as the caudate extending into the sgACC (P < 0.001, 22 voxels contiguity). There were no other areas that showed a main effect for pubertal status. These effects were qualified by group × pubertal status interaction effects (P < 0.001, 30 voxels contiguity) indicating that, contrary to hypotheses, the relationship between pubertal maturation and response to rejection in the left amygdala and caudate/sgACC was stronger among healthy controls than MDD youth. As shown in Figure 4b and c, pubertal maturation was associated with increased sgACC and amygdala activity in controls, but not among depressed youth, who showed greater sgACC and amygdala response to rejection earlier in puberty. There were no significant main or interaction effects for gonadal signs of pubertal maturation on response to rejection, and no effects for either measure of pubertal maturation on response to acceptance.
Fig. 4.
Relationships between pubertal maturation and response to rejection (negative numbers reflect deactivation from a pre-trial baseline). (a) Significant main effects of adrenal PDS on brain response to rejection were found in the right amygdala, controlling for age; (b, c) significant interaction effects between group and adrenal PDS on brain response to rejection were found in the sgACC/caudate and left amygdala/parahippocampal gyrus, respectively.
DISCUSSION
There is little developmental affective neuroscience research to guide strategies for early intervention or prevention of depression during adolescence. The results of this study suggest that although increased neural response to rejection appears to be normative across adolescence, this response is particularly heightened among youth with depression. Relative to healthy controls, MDD youth displayed a potentiated response to peer rejection in a ventral network of brain regions involved in the identification of emotional and social stimuli and the generation of affective states (Phillips et al., 2003), including the sgACC, anterior insula, amygdala and NAcc. These findings highlight potential neural mechanisms that may contribute to the relationship between peer rejection and MDD.
The finding of heightened sgACC activity to social rejection in adolescents with MDD is consistent with a recent Cyberball study conducted in a non-clinical sample of adolescents that showed that sgACC activity to social exclusion predicted increases in depressive symptoms over 1 year (Masten et al., 2011). The sgACC has been suggested to play a role in monitoring, modulating and/or generating negative emotions (Mayberg, 2003; Siegle et al., 2012) and increases in adults during sadness inductions (Mayberg et al., 1999). Adults with MDD show a reduction in gray matter volume and elevated metabolic activity of the sgACC (Drevets et al., 1997; Mayberg et al., 1999), and sgACC activity is predictive of response to cognitive therapy and medication in adult depression (Mayberg et al., 1997; Keedwell et al., 2010; Siegle et al., 2012). It is interesting that although Masten et al. (2009) and Sebastian et al. (2011) found increased sgACC response to exclusion (relative to inclusion) in healthy youth, we found increased sgACC response to rejection in depressed compared with healthy adolescents. Although the studies are not directly comparable given the use of different tasks and different analytic models (i.e. exclusion compared with inclusion vs bold activation under the curve from baseline to rejection), findings suggest that studies of sgACC response to exclusion in healthy youth may benefit from consideration of levels of depressive symptomatology in the sample. Findings also suggest that investigation of sgACC response to social stimuli in adolescence is an important avenue for future research on adolescent depression.
Whole-brain analyses also revealed greater activity in the dACC in MDD compared with healthy youth to social exclusion, but only from 7 to 9 s after receiving rejection feedback. Although social exclusion in adults typically activates the dACC (Eisenberger et al., 2003, 2007), most studies with adolescents have not shown dACC activation to social exclusion or rejection (Masten et al., 2009; Bolling et al., 2011a; Sebastian et al., 2011; Guyer et al., 2012). This study suggests that dACC activation may be more evident during sustained processing of exclusion/rejection and/or among adolescents higher in depressive symptoms.
We found an interesting pattern of differential activation of the anterior insula to acceptance and rejection for depressed and healthy youth. Although the insula was activated in response to acceptance in both depressed and healthy youth, it was only activated in response to rejection for depressed youth. There is evidence that the insula is involved in the experience of all emotions, including happy and positive emotions (Damasio et al., 2000; Phan et al., 2002) as well as negative affective states, such as anger (Denson et al., 2008), disgust (Phillips et al., 1997; Jabbi et al., 2008), physical pain (Aziz et al., 2000) and ‘social pain’ (Eisenberger et al., 2003; Masten et al., 2009). This is consistent with theoretical models suggesting that the insula is responsible for representing current internal physical and emotional states and generating the experience of ‘feelings’ (Damasio et al., 2000; Craig, 2009; Singer et al., 2009). Singer et al. (2009) propose that the anterior insula integrates external and internal physiological signals to generate a dominant feeling state that modulates social and motivational behavior. It appears that acceptance feedback was particularly salient to both depressed and healthy youth in this study. In contrast, our finding that the insula activated in response to rejection in depressed youth but not controls may suggest that the depressed youth experienced rejection trials as more affectively and motivationally salient and/or painful than healthy youth. This finding is also consistent with recent evidence of increased insula activity to rejection among anxious youth (Lau et al., 2012), suggesting that insula response to rejection could be a shared risk factor for anxiety and depression.
The salience of acceptance feedback in both groups is consistent with general evidence for a valence effect across most of our ROIs, in which acceptance more strongly activated ventral areas including not only the insula but also the VLPFC, mPFC and vACC compared with rejection. Although we previously found greater pupil dilation to rejection compared with acceptance in healthy adolescents on this task (Silk et al., 2012b), other adolescent neuroimaging studies have found greater neural responses to acceptance, compared with rejection, in response to virtual peer feedback (Gunther Moor et al., 2010; Guyer et al., 2012). This pattern of results differs from studies that have employed social inclusion/exclusion tasks, such as the Cyberball task, which have typically found greater neural response to exclusion compared with inclusion in adolescents (Masten et al., 2009; Bolling et al., 2011b; Sebastian et al., 2011). The reasons for these differences are unclear and warrant further investigation.
Also consistent with data from a similar virtual peer feedback task (Guyer et al., 2008; Lau et al., 2012), we found bilateral deactivation of the amygdala relative to baseline in response to rejection in healthy controls. This deactivation was attenuated in MDD youth, suggesting that amygdala activity in response to rejection was higher in depressed compared with healthy youth. The pattern of amygdala deactivation could be explained by (i) relatively high levels of baseline amygdala activity in the scanning environment and/or in anticipation of peer responses and (ii) rejection feedback delivered in the context of smiling faces. Social proximity and interaction are known to attenuate threat reactivity in humans (Beckes and Coan, 2011), thus smiling faces of purported interaction partners may be sufficient to reduce threat and deactivate the amygdala in healthy controls, even when conveying rejection feedback. In contrast, the rejection feedback may be more salient than smiling faces for MDD youth. Again, this finding converges with evidence of increased amygdala reactivity to rejection in anxious youth (Guyer et al., 2008; Lau et al., 2012), supporting amygdala reactivity to social rejection as a potential shared biomarker that could help to explain high levels of comorbidity between anxiety and depression in youth (Silk et al., 2012a).
Surprisingly, we found that depressed youth did not differ from controls in neural response to peer acceptance in reward-processing regions, such as the mPFC and NAcc. This differs from several studies that have shown decreased striatal response to monetary reward in adolescent MDD (Forbes et al., 2006, 2009), suggesting that social reward may be more salient than monetary reward for depressed youth. In fact, Davey et al. (2011) found increased amygdala response to social acceptance in an older sample of adolescents and young adults with MDD compared with healthy controls. These discrepant findings highlight the need for additional research on neural response to social vs monetary rewards in adolescents with depression.
Although we did not find group differences in reward-related brain activity in response to peer acceptance, we did find differences in bilateral NAcc response to peer rejection. Specifically, the NAcc displayed a pattern of decreased activation in response to peer rejection among healthy controls that was not present in MDD youth. Although the NAcc has received more attention for its role in the brain’s reward circuit (Knutson and Cooper, 2005), it also plays a role in encoding aversive events and punishment (McCutcheon et al., 2012). Other forms of social loss, such as complicated grief, have been shown to activate the NAcc (O’Connor et al., 2008). NAcc activation to peer rejection in MDD youth may suggest that MDD youth experience peer rejection as a form of loss, punishment, or as more strongly aversive than healthy controls.
Researchers have proposed that increased risk for behavioral and emotional health problems in adolescence may be a function of increased reactivity to social and emotional stimuli as a function of pubertal maturation (Nelson et al., 2005; Steinberg, 2005), but little empirical data exist in human adolescents to support this model. This study provides evidence that adolescents more advanced in self-reported pubertal status show more activation to simulated peer rejection in the sgACC and bilateral amygdala, key areas involved in the processing of social affective stimuli and social threat. These findings were driven by a pattern of deactivation in these areas earlier in puberty that was attenuated with pubertal maturation. This is consistent with earlier evidence of increased physiological reactivity to peer rejection on the Chatroom Interact Task among older compared with younger adolescents (Silk et al., 2012b), as well as data showing increased neural response to threatening faces in more pubertally advanced adolescents (Forbes et al., 2011; Moore et al., 2012). These associations remained significant controlling for chronological age, suggesting that the effects may be specific to pubertal maturation. Given high densities of steroid hormone receptors in the amygdala and cerebral cortex (Simerly et al., 1990; Sarkey et al., 2008), changes in reactivity to peer rejection in these regions with puberty could be mediated by the rise of basal levels of sex hormones during pubertal development. These influences could result from direct effects of sex hormones on limbic circuitry, or changes in socio-affective behavior in response to changes in physical appearance. It was interesting, in this study, that adrenal, but not gonadal hormonal signs of pubertal development were associated with neural response to peer rejection. There are several possible explanations for this finding. First, it may be that self rating of the questions assessing adrenarche (i.e. acne and axillary hair) is more accurate at capturing pubertal changes than questions assessing gonadarche (i.e. growth spurt), which can be difficult to self-rate. It is also possible that higher scores on the adrenarche scale may be a marker for early puberty, as adrenarche is the earliest event in the pubertal maturation process. Early pubertal maturation, particularly in girls, is known to be associated with increased psychosocial stress and adversity (Ge et al., 1996), which could help to explain the link between higher adrenarche scores and greater amygdala/ACC hyper-reactivity to rejection. These findings could also suggest a specific role for didehydroepiandrosterone/didehydroepiandrosterone sulfate DHEA/DHEA(S), which are the primary sex hormones associated with adrenarche. Little is known about the function of DHEA during adolescence. DHEA has been shown to have anxiolytic and antidepressant effects (Schmidt et al., 2005; Malkesman and Weller, 2009; Sripada et al., 2013); however, other studies have shown elevated DHEA in populations exposed to stress, such as individuals exposed to childhood trauma (Kellner et al., 2010), suggesting a potential compensatory role for elevated levels of DHEA. Furthermore, DHEA is precursor to both testosterone and estradiol; thus, the effects of increased DHEA could be mediated through effects on gonadal hormones. The collection of data on basal and task-related sex hormone responses in adolescents in future peer evaluation studies will be important in order to delineate specific mechanisms through which pubertal maturation influences sensitivity to peer rejection.
Interestingly, contrary to our hypotheses, the relationship between left amygdala and caudate/sgACC activity with pubertal maturation was stronger among healthy youth than depressed youth. Controls showed greater deactivation of the left amygdala and caudate/sgACC compared with depressed youth in the earlier stages of puberty, but by late puberty responses to rejection were similar in both healthy youth and controls. This suggests that greater response to rejection “earlier” in pubertal development may be an important risk factor, or marker for depression. Alternatively, it may be that pubertal steroids have less of an influence on reactivity to rejection in depressed youth because the salience of peer rejection is already maximized in this group.
This study has several limitations. First, because the study is cross-sectional, it is unclear whether increased neural response to peer rejection is a risk factor or correlate of adolescent depression. It may be that adolescents vulnerable to depression enter adolescence with greater sensitivity to social evaluation, or it may be that the experience of frequent peer rejection serves to sensitize or heighten activity in these regions. Future prospective longitudinal research with children and adolescents at high risk for depression may help to address this question. In particular, additional research that incorporates EMA, observational and/or sociometric data on real-world peer relationships with neuroimaging data (as in Eisenberger et al., 2007; Masten et al., 2012) would be valuable in addressing the interplay between social experience and neural response to social rejection during adolescence.
Second, because we did not include an adult comparison group, we do not know whether the present findings are unique to adolescents or may generalize to adults with depression. Third, there is evidence of gender differences in rates of depression (Kessler et al., 2001) as well as interpersonal sensitivity (Rudolph, 2002); however, given our relatively small sample size (particularly for boys), we were not able to investigate gender differences in reactivity to peer acceptance and rejection. Relatedly, there are limitations in combining boys and girls in analyses of the effects of pubertal development, as self-reported pubertal status is based on different criteria for boys and girl, pubertal changes occur along a different timeline for boys and girls, and the effects of pubertal hormones on brain activity may differ for boys and girls. Thus, it is possible that the effects of pubertal development on brain response could differ for males and females based on differences in both the timing of puberty and the levels of different pubertal hormones (Sisk and Foster, 2004). Future research is needed to investigate puberty-specific effects on neural response to social evaluation within larger samples of girls and boys. Furthermore, as gender differences in depression rates appear to emerge during puberty (Angold et al., 1999), further investigation of the interrelationships between puberty, gender and response to social evaluation may contribute to a better understanding of the mechanisms behind gender differences in depression. Finally, although the majority of participants (92%) did not show a clear preference for one virtual peer over the other in the first block, it remains possible that participants’ own ratings of the virtual peers during the first block might have influenced their expectations of acceptance and rejection from these peers during later blocks.
Despite these limitations, the study also has several strengths. First, the study is based on a well-characterized clinical sample of youth in a current episode of MDD. The study also utilized a newly developed virtual peer interaction paradigm that included live interaction with age-matched virtual peers. This allowed us to probe responses to ecologically valid social evaluative stimuli likely to be emotionally salient for adolescents. Additionally, data were obtained on pubertal status, allowing us to address potential puberty-driven developmental effects. Findings highlight neural sensitivity to peer rejection as an important feature of adolescent depression that could be better targeted in intervention and prevention approaches for this prevalent disorder. This need is particularly pressing in the current adolescent ‘cyber-culture’ which includes increased rates of rejection via social media and mobile technologies, which have been linked to suicide and depression (O’Keeffe et al., 2011; Luxton et al., 2012). Although some current psychosocial interventions for adolescent depression include social skills training (Kennard et al., 2009) or discussion of problematic interpersonal relationships (Mufson et al., 1999), treatments may benefit from an increased focus on augmenting skills for dealing with the ubiquitous experience of peer social evaluation. For example, virtual reality technologies have been used to improve exposure treatments for posttraumatic stress disorder and social phobia (Rothbaum et al., 2001; Anderson et al., 2003), and could potentially be incorporated into adolescent depression interventions as a way to enhance exposure and coping responses to negative peer evaluation. Furthermore, although, once thought to be detrimental for adolescents, recent data suggest that more frequent Internet-based social interaction actually has a positive effect on social connectedness and well-being among youth (Valkenburg and Peter, 2009). It may be possible to harness the effects of positive online social interactions to improve depression interventions. In support of this notion, a recent study showed that a brief period of online interaction with an unknown peer improved reports of self-esteem and perceived relational value and decreased negative affect in adolescents who had just experienced social exclusion via the Cyberball task (Gross, 2009). Targeting skills for coping with negative social interactions among prepubertal youth with neurobiological vulnerabilities for depression may also help to prevent the onset of depression during the transition through adolescence.
FINANCIAL DISCLOSURES
Dr G.J.S. is an unpaid consultant for TrialIQ. The other authors report no conflicts of interest.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This research was supported by National Institute of Drug Abuse grant R21DA024144 (J.S.S./R.E.D., PI’s), the Clinical and Translational Science Institute at the University of Pittsburgh (NIH/NCRR/CTSA Grant UL1 RR024153) and the National Institute of Mental Health intramural research program. The authors are grateful to Daniel Pine, MD for his input and assistance on this project; Marcie Walker, Katie Burkhouse and Karen Garelik for their assistance in data acquisition; Harvey Iwamoto for task related computer programming; and Ruth Stroud and Jennifer Sears for assistance with photography. They also thank the participants and their families.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, D.C: 1994. American Psychiatric Association. [Google Scholar]
- Anderson P, Rothbaum BO, Hodges LF. Virtual reality exposure in the treatment of social anxiety. Cognitive and Behavioral Practice. 2003;10:240–7. [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychological Medicine. 1999;29:1043–53. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. Journal of Clinical Neurophysiology. 2000;17:604–12. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Beckes L, Coan JA. Social baseline theory: the role of social proximity in emotion and economy of action. Social and Personality Psychology Compass. 2011;5:976–88. [Google Scholar]
- Beesdo K, Höfler M, Leibenluft E, Lieb R, Bauer M, Pfennig A. Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disorders. 2009a;11:637–49. doi: 10.1111/j.1399-5618.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009b;66:275–85. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, Mayes LC, Pelphrey KA. Development of neural systems for processing social exclusion from childhood to adolescence. Developmental Science. 2011a;14:1431–44. doi: 10.1111/j.1467-7687.2011.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. NeuroImage. 2011b;54:2462–71. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M. Being liked activates primary reward and midline self-related brain regions. Human Brain Mapping. 2010;31:660–8. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Yucel M. Increased amygdala response to positive social feedback in young people with major depressive disorder. Biological Psychiatry. 2011;69:734–41. doi: 10.1016/j.biopsych.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Denson TF, Pedersen WC, Ronquillo J, Nandy AS. The angry brain: neural correlates of anger, angry rumination, and aggressive personality. Journal of Cognitive Neuroscience. 2008;21:734–44. doi: 10.1162/jocn.2009.21051. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–54. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fissell K, Tseytlin E, Cunningham D, et al. Fiswidgets: a graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–25. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, May CJ, Siegle GJ, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry. 2006;47:1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology. 2011;36:429–52. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Coming of age too early: pubertal influences on girls’ vulnerability to psychological distress. Child Development. 1996;67:3386–400. [PubMed] [Google Scholar]
- Gross EF. Logging on, bouncing back: an experimental investigation of online communication following social exclusion. Developmental Psychology. 2009;45:1787–93. doi: 10.1037/a0016541. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, Güroğlu B, Op de Macks ZA, Rombouts SARB, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. NeuroImage. 2012;59:708–17. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5:461–482. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–15. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3:e2939. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joinson C, Heron J, Araya R, et al. Association between pubertal development and depressive symptoms in girls from a UK cohort. Psychological Medicine. 2012;42:2579–89. doi: 10.1017/S003329171200061X. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips ML. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. Journal of Affective Disorders. 2010;120:120–5. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Kellner M, Muhtz C, Peter F, Dunker S, Wiedemann K, Yassouridis A. Increased DHEA and DHEA-S plasma levels in patients with post-traumatic stress disorder and a history of childhood abuse. Journal of Psychiatric Research. 2010;44:215–9. doi: 10.1016/j.jpsychires.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Kennard BD, Clarke GN, Weersing VR, et al. Effective components of TORDIA cognitive-behavioral therapy for adolescent depression: preliminary findings. Journal of Consulting & Clinical Psychology. 2009;77:1033–41. doi: 10.1037/a0017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Lifetime and 12-month prevalence of DSM-III–R psychiatric disorders in the United States: results from the National Comorbidity Study. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas KR. Mood disorders in children and adolescents: an epidemiologic perspective. Biological Psychiatry. 2001;49:1002–14. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Larson RW, Asmussen L. Anger, worry, and hurt in early adolescence: an enlarging world of negative emotions. In: Colten ME, Gore S, editors. Adolescent Stress: Causes and Consequences. Hawthorne, NY: Aldine de Gruyter; 1991. pp. 21–41. [Google Scholar]
- Lau JYF, Guyer AE, Tone EB, et al. Neural responses to peer rejection in anxious adolescents. International Journal of Behavioral Development. 2012;36:36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxton DD, June JD, Fairall JM. Social media and suicide: a public health perspective. American Journal of Public Health. 2012;102(Suppl. 2):S195–200. doi: 10.2105/AJPH.2011.300608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkesman O, Weller A. Two different putative genetic animal models of childhood depression—a review. Progress in Neurobiology. 2009;88:153–69. doi: 10.1016/j.pneurobio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, et al. Sensation seeking, puberty and nicotine, alcohol and marijuana use in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1495–502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Development and Psychopathology. 2011;23:283–92. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience. 2012;7:106–14. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Frontiers in Neuroscience. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE, III, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson L, Weissman MM, Moreau D, Garfinkel R. Efficacy of interpersonal psychotherapy for depressed adolescents. Archives of General Psychiatry. 1999;56:573–9. doi: 10.1001/archpsyc.56.6.573. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nolan SA, Flynn C, Garber J. Prospective relations between rejection and depression in young adolescents. Journal of Personality and Social Psychology. 2003;85:745–55. doi: 10.1037/0022-3514.85.4.745. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Wellisch DK, Stanton AL, Eisenberger NI, Irwin MR, Lieberman MD. Craving love? Enduring grief activates brain’s reward center. NeuroImage. 2008;42:969–72. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe GS, Clarke-Pearson K, Council on Communications and Media The impact of social media on children, adolescents, and families. Pediatrics. 2011;127:800–4. doi: 10.1542/peds.2011-0054. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and inital norms. Journal of Youth and Adolescence. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Blakemore SJ. Adolescent social cognitive and affective neuroscience: past, present, and future. Social Cognitive and Affective Neuroscience. 2012;7:1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Aikins JW. Cognitive moderators of the longitudinal association between peer rejection and adolescent depressive symptoms. Journal of Abnormal Child Psychology. 2004;32:147–58. doi: 10.1023/b:jacp.0000019767.55592.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: an fMRI study. Biological Psychiatry. 2006;60:966–73. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Hodges LF, Ready D, Graap K, Alarcon RD. Virtual reality exposure therapy for Vietnam veterans with posttraumatic stress disorder. Journal of Clinical Psychiatry. 2001;62:617–22. doi: 10.4088/jcp.v62n0808. [DOI] [PubMed] [Google Scholar]
- Rudolph KD. Gender differences in emotional responses to interpersonal stress during adolescence. Journal of Adolescent Health. 2002;30:3–13. doi: 10.1016/s1054-139x(01)00383-4. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Conley CS. The socioemotional costs and benefits of social-evaluative concerns: do girls care too much? Journal of Personality. 2005;73:115–38. doi: 10.1111/j.1467-6494.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Hormones & Behavior. 2008;53:753–64. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Archives of General Psychiatry. 2005;62:154–62. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Tan GC, Roiser JP, Viding E, Dumontheil I, Blakemore SJ. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage. 2011;57:686–94. doi: 10.1016/j.neuroimage.2010.09.063. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: correspondence between hormonal and physical development. Child Development. 2009;80:327–37. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson WK, Collier A, et al. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Archives of General Psychiatry. 2012;69:913–24. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Davis S, McMakin DL, Dahl RE, Forbes EE. Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychological Medicine. 2012a;42:2095–107. doi: 10.1017/S0033291712000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko L, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21:7–16. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Social Cognitive and Affective Neuroscience. 2012b;7:93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. Journal of Comparative Neurology. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–2. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, et al. DHEA enhances emotion regulation neurocircuits and modulates memory for emotional stimuli. Neuropsychopharmacology. 2013;38:1798–807. doi: 10.1038/npp.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A Social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, et al. Stress response and the adolescent transition: performance versus peer rejection stressors. Development and Psychopathology. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkenburg PM, Peter J. Social consequences of the internet for adolescents: a decade of research. Current Directions in Psychological Science. 2009;18:1–5. [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22:153–65. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.