Abstract
An intense fear of abandonment or rejection is a central feature of social relationships for individuals with borderline personality disorder (BPD). A total of 20 unmedicated BPD patients and 20 healthy participants (HC, matched for age and education) played a virtual ball-tossing game including the three conditions: exclusion, inclusion and a control condition with predefined game rules, whereas cerebral activity was assessed using functional magnetic resonance imaging. Subjective experiences of exclusion were assessed after each blocked condition. Both groups felt similarly excluded during the exclusion condition; however, BPD subjects felt more excluded than HC during the inclusion and control conditions. In all three conditions, BPD patients showed a stronger engagement of the dorsal anterior cingulate and medial prefrontal cortex. For HC, activation in several cerebral regions such as the insula and the precuneus differed depending on the interaction situation, whereas for BPD subjects activation in these regions was not modulated by experimental conditions. Subjects with BPD differed from HC in both their subjective reactions to and their neural processing of social interaction situations. Our data suggest that individuals with BPD have difficulty in discriminating between social situations, and tend to hypermentalize during social encounters that are not determined by the intentions of others.
Keywords: borderline personality disorder, social exclusion, social interaction, cyberball, hypermentalizing
INTRODUCTION
Borderline personality disorder (BPD) is a severe psychiatric disorder affecting about 3% of the adult population (Trull et al., 2010). Core features of BPD are affective dysregulation, identity disturbances and problems in social interaction (Lieb et al., 2004), with an intense fear of loss, abandonment, or rejection by social partners (Diagnostic and Statistical Manual of Mental Disorders (DSM-V); APA, 2013). Clinical experience suggests that social rejection and solitude can trigger states of aversive tension in individuals with BPD, and that these conditions often precede self-injurious behaviors (Herpertz, 1995; Stiglmayr et al., 2005).
Staebler et al. (2011a) found that BPD patients scored higher than either healthy controls or patients with anxiety disorders, including social phobia, or mood disorders, on a questionnaire measuring rejection sensitivity (Downey and Feldman, 1996). Compared with either of the other groups, they reported a greater tendency to both expect and perceive rejection in social situations and to react more strongly to those experiences. These findings were confirmed in a study employing the ‘cyberball’ game in which the experience of social rejection is induced under experimental conditions (Staebler et al., 2011b). In this paradigm, participants engage in an online ball-tossing game with partners who they believe to be co-participants, but in fact are pre-programmed virtual players. In the ‘inclusion’ condition, all players receive the same number of ball tosses, whereas in the ‘exclusion’ condition the co-players stop tossing the ball to the subject, thereby excluding him from the game. BPD patients felt more rejected than did healthy controls (HCs) independent of the experimental conditions, i.e. they felt more rejected even when they were being equally included.
Only one study, using a small sample (10 BPD patients), has directly examined cerebral correlates of social inclusion and exclusion in BPD (Ruocco et al., 2010). In this study, BPD patients played a card game with two real partners while undergoing functional near-infrared spectroscopy. The card game was adapted from the cyberball paradigm. Findings showed altered processing in the frontolimbic regions during social rejection. Specifically, activity in the medial prefrontal cortex (mPFC) was increased and was correlated with general rejection and abandonment fears.
In contrast, numerous studies have investigated the cerebral processing of social rejection in healthy individuals. Several of these found enhanced activation during social exclusion in the dorsal anterior cingulate cortex (dACC) that partly correlated with an enhanced subjective experience of social exclusion (Eisenberger et al., 2003; Kross et al., 2007, 2011; Kawamoto et al., 2012). Eisenberger and Lieberman (2004) suggested that activity within this region is linked to the activation of a ‘neural alarm system’ relevant for the detection of social exclusion through conflict monitoring. The ventral anterior cingulate cortex (vACC) and the insula have been identified as two other cerebral structures that are essential for the processing of social exclusion. Following the general model of emotion regulation (Ochsner and Gross, 2005), an enhanced activation of these structures has been linked to the affective value of the experience of social exclusion (Eisenberger et al., 2003; Somerville et al., 2006; Onoda et al., 2009; Bolling et al., 2011; Kross et al., 2011; Moor et al., 2012). A positive co-variation of insula and vACC activation with self-reported experience of exclusion supports this idea (Kross et al., 2007; Onoda et al., 2009; Way et al., 2009; Moor et al., 2012). Enhanced activation during exclusion has also been observed in brain areas, such as the mPFC, the posterior cingulate cortex (PCC) and the precuneus (Kross et al., 2007; Onoda et al., 2009; Bolling et al., 2011; DeWall et al., 2012; Kawamoto et al., 2012). These regions have been linked to self-referential processes, to mentalizing, to evaluation of responses to negative affective stimuli and to episodic memory retrieval (Ochsner et al., 2004; Spreng et al., 2009). It has been suggested that they modulate the affective response to social exclusion. Further, the regulation of the experience of social rejection is also associated with activation in the dorsolateral prefrontal cortex (dlPFC) and the ventrolateral prefrontal cortex (vlPFC) (Eisenberger et al., 2003; Somerville et al., 2006; Kross et al., 2007; Bolling et al., 2011; DeWall et al., 2012; Kawamoto et al., 2012; Moor et al., 2012).
Differences in cerebral processing of social exclusion correlated with rejection sensitivity and with self-esteem. Under conditions of social exclusion, Kross et al. (2007) found that subjects with a high rejection sensitivity showed less activation in the dlPFC and vlPFC regions than did subjects with a low sensitivity, whereas Onoda et al. (2010) found that subjects with low self-esteem showed an enhanced activation of the dACC, the vACC, the mPFC, the insula and the precuneus. These findings suggest that similar alterations during the processing of social exclusion might be observed in BPD, i.e. a reduced activation in dlPFC and vlPFC linked to the high rejection sensitivity in this disorder along with increased engagement of the dACC, vACC, the mPFC, the insula and the precuneus due to the reduced self-esteem described in BPD patients (Ruesch et al., 2007; Lynum et al., 2008; Staebler et al., 2011a).
Findings of studies on the effects of rejection sensitivity and self-esteem during social exclusion in healthy subjects correspond to cerebral alterations having been described in neuroimaging studies on BPD and which are summarized in a neurobiological model of disturbed top-down cognitive control of emotion processing in BPD (e.g. Koenigsberg et al., 2009; Niedtfeld and Schmahl, 2009, 2012; Mauchnik and Schmahl, 2010; O’Neill and Frodl, 2012; New et al., 2012; see also Ruocco et al., 2013). This model assumes that BPD patients exhibit hyperreactivity in limbic structures such as the amygdala and insula in combination with a reduced activation of prefrontal brain regions, with the ACC as a modulator of the bottom-up and top-down systems. These alterations are assumed to result in impairments of emotion processing that are clinically observable as a pronounced affective instability and enhanced sensitivity and reactivity to emotional information (Linehan, 1993; Koenigsberg, 2010).
The aim of this study was to gain further insight into the cerebral processing of social rejection in patients with BPD by using functional magnetic resonance imaging (fMRI) during experimentally induced experiences of social rejection. We hypothesized that in the cyberball paradigm, (i) BPD subjects would feel more excluded during social interactions than HCs, and (ii) that this would be linked to alterations in the activation of cerebral structures that are engaged in social rejection and modulated by rejection sensitivity and self-esteem. We expected enhanced activation in the dACC, insula and mPFC, as well as reduced activation in the vlPFC and dlPFC in BPD patients as compared with HCs.
METHODS
Subjects
The sample consisted of 20 adult females, who met at least five of the nine DSM-IV criteria for BPD and have not been on psychotropic medication for at least 2 weeks, and 20 female HCs with no lifetime or current psychiatric diagnoses. The groups were matched for age and education (Table 1). We recruited the patients from our department database, whereas HCs were contacted by newspaper advertisement. All patients were outpatients at the time of the investigation. General exclusion criteria were a lifetime history of psychotic or bipolar I disorder, current major depressive episode, current substance abuse or addiction, current pregnancy, history of organic brain disease, skull or brain damage, or severe neurological illness. We also excluded participants who had any metal implants in their body, were left-handed, or who suffered from claustrophobia.
Table 1.
Demographic and clinical variables in patients with BPD and in HC with results of the t-tests (independent, two-tailed)
| BPD-patients |
HC |
BPD vs HC (independent t-test) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | s.d. | n | Mean | s.d. | T | P-value | |
| Age | 20 | 29.2 | 7.5 | 20 | 28.7 | 7.8 | −0.2 | 0.800 |
| Years of education | 20 | 12.1 | 1.5 | 20 | 12.1 | 1.5 | 0.0 | 1.000 |
| RSQ | 20 | 14.1 | 5.0 | 20 | 5.5 | 2.7 | −6.8 | <0.001 |
| BSL | 20 | 1.6 | 0.6 | 19 | 0.2 | 0.2 | −9.7 | <0.001 |
| SES | 20 | 12.1 | 5.9 | 20 | 27.1 | 3.0 | 10.2 | <0.001 |
| BDI | 20 | 18.1 | 9.9 | 20 | 2.3 | 2.9 | −6.8 | <0.001 |
| BSI | 20 | 1.3 | 0.5 | 19 | 0.2 | 0.2 | −8.4 | <0.001 |
| FDS | 20 | 23.2 | 13.5 | 20 | 4.0 | 4.0 | −6.1 | <0.001 |
| Comorbidity | Current | Lifetime | ||||||
|---|---|---|---|---|---|---|---|---|
| Major depression | 0 | 18 | ||||||
| Bipolar-II | 0 | 2 | ||||||
| PTSD | 5 | 5 | ||||||
| Panic disorder | 5 | 1 | ||||||
| Social phobia | 7 | 5 | ||||||
| Specific phobia | 6 | 5 | ||||||
| OCD | 4 | 1 | ||||||
| Bulimia | 4 | 1 | ||||||
| Anorexia | 4 | 0 | ||||||
| Substance Abuse/ dependence | 0 | 7 |
PTSD, post-traumatic stress disorder; OCD, obsessive–compulsive disorder.
The diagnosis of BPD according to DSM-IV was made by trained clinical psychologists using the International Personality Disorder Examination (Loranger, 1999), and Axis I disorders were assessed using the Structured Clinical Interview for DSM-IV (SCID-I; First et al., 1997). We additionally assessed BPD symptom severity using the Borderline Symptom List (BSL; Bohus et al., 2007), general psychopathology using the Brief Symptom Inventory (BSI; Derogatis, 1993), trait dissociation using the German adaptation (Fragebogen zu Dissoziativen Symptomen (FDS); Freyberger et al., 1998) of the Dissociative Experiences Scale (Bernstein and Putnam, 1986), depressive symptoms using the Beck Depression Inventory (BDI; Beck et al., 1961), rejection sensitivity using the Rejection Sensitivity Questionnaire (RSQ; Downey and Feldman, 1996), and self-esteem using the Rosenberg Self-Esteem Scale (SES; Rosenberg, 1965). For further sample characteristics, see Table 1 and for test descriptions see Supplementary Table S1. The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Research Ethics Board of the University of Heidelberg. Subjects provided written informed consent prior to study participation.
Functional and structural MRI acquisition
Brain images were collected using a Siemens TRIO-3T MRI scanner (Siemens Medical Systems, Germany). For each participant, a high resolution anatomical scan using T1-weighted 3-D magnetization-prepared rapid acquisition gradient echo (1 × 1 × 1 mm3 voxel size) was acquired and used as an individual template for normalization of functional data. The blood oxygen level-dependent signal was measured using T2-weighted gradient echo planar imaging (EPI) with the following protocol parameters: field of view = 192 × 192 mm, voxel size = 3 × 3 × 3 mm, echo time = 30 ms, repetition time (TR) = 2000 ms, number of slices = 36, matrix = 64 × 64. The first five scans were discarded to minimize T1 effects.
Experimental design and tasks
Subjects played a total of 18 rounds of the cyberball game that is well established to induce social rejection (Williams et al., 2000; Eisenberger et al., 2003; Williams and Jarvis, 2006). Six rounds were played in each of three experimental conditions: social exclusion, social inclusion and a control condition. All rounds were played with two virtual partners, who the subject was told were co-participants. To increase the ecological validity of the paradigm, photographs of the two virtual co-players were presented throughout the game, as proposed by Williams et al. (2000; Krill and Platek, 2009; Bolling et al., 2011). In the exclusion condition, the subject received the ball only once at the beginning of each round, and was then ostracized for the remainder of the round. In the inclusion condition, all players received an equal number of ball tosses. In the control condition, each player again received an equal number of ball tosses, but here, subjects were told that the direction of the toss was determined by a specific rule, whereby each player was obligated to toss only to the partner on the right or on the left. In this condition, therefore, other players’ actions could not be attributed to their intentions. We added this control condition since in the study by Staebler et al. (2011b), BPD patients reported a higher sense of exclusion during even the inclusion condition of the game. We hypothesized that compared with healthy subjects, BPD patients would feel more excluded and show stronger engagement of cerebral areas linked to the experience of social exclusion during both the exclusion and the inclusion conditions, but not during the control condition.
Each cyberball round had a duration of about 30 s. Before each round, subjects were told whether or not players could choose where to toss the ball, and if not, whether they were to throw to the player on their left or their right. The different conditions were presented in a pseudo-random sequence. Each round was followed with equal probability and in pseudo-random order by the administration of either a painful or non-painful temperature stimulus. Data relating to this variable are reported elsewhere.
After each round, the perceived percentage of received ball tosses (0–100%) was assessed, and subjects indicated on a visual scale (11 points, ranging from ‘not at all’ to ‘very strong’) their experiences of being excluded, of being included, the level of inner tension, the strength of dissociative symptoms and the painfulness of the temperature stimulus. At the end of the experiment, participants were asked to rate on an 11-point rating scale whether they had doubts about playing with real partners from the game start.
Data analysis
Behavioral data
Differences in subjective ratings were tested using 2 × 3 repeated measures analyses of variance (ANOVA), with the between-subject factor of ‘group’ (HC, BPD) and the within-subject factor of ‘ball tossing condition’ (exclusion, inclusion, control). Post hoc analyses were performed by pairwise comparisons (Bonferroni corrected for multiple testing). SPSS (version 20; SPSS Inc., USA) was used as the statistical software.
fMRI data
Functional imaging data were analyzed using standard procedures (SPM8; Welcome Department of Cognitive Neurology, London, UK). Preprocessing of the EPI time series was conducted following customary practice: slice time correction and spatial realignment to correct for head motion, and co-registration onto participants’ T1-scan; normalization to the standard brain of the Montreal Neurological Institute space; re-sampling to 3-mm3 voxels; and smoothing with a Gaussian kernel with a full-width at half of maximum of 9 mm. Six regressors were used to model the first-level analysis: three regressors for the ball tossing conditions of ‘exclusion’, ‘inclusion’ and ‘control’ together with two regressors for the pain and temperature blocks which followed the ball tossing conditions; and one for modeling key presses. The six realignment parameters were additionally modeled. A high-pass filter of 512 s was applied. Second-level analysis was carried out with a flexible-factorial model that included the first level contrasts for the three ball tossing conditions for BPD patients and HCs. The following factors were entered: ‘subject’, ‘group’ and the experimental condition ‘ball tossing condition’. We included the main-effect of subject, group and condition together with the interaction effect of group by condition as regressors in the design matrix. Following Lieberman and Cunningham (2009), we report voxels that met an uncorrected threshold of P < 0.001 and were part of a cluster larger than 10. Statistical analysis was carried out using a variance analytical approach to control the experiment-wise error rate within our 2 × 3-experimental design with the independent factor ‘group’ and the repeated measurement factor ‘ball tossing condition’ instead of calculating multiple pairwise comparison with t-contrasts. Following this reasoning, we report F-statistics for the main effect of ‘ball tossing condition’ and the interaction effect of this factor with the factor ‘group’ and thereby identify those brain regions for which activation differences above chance could be observed due to the influence of any of the different social encounters with and without modulation by group. To determine the exact nature of these effects, post hoc tests were calculated with the beta values of the peak-voxel within each cluster by means of independent and dependent t-tests (Bonferroni-corrected) using SPSS. As the flexible factorial model does not allow for the calculation of the main effect of the independent factor ‘group’, this effect was tested with a two-sample t-test to compare brain activation between groups independent of the ball tossing condition (McLaren et al., 2011). Additionally, t-contrasts are reported in the Supplementary Data (Supplementary Table S4).
RESULTS
Behavioral data
All participants of both groups reported on having no doubts on playing with real partners (mean score BPD: 1.55 s.d. 2.3, HC: 1.7 s.d. 2.0, t = 0.22, P = 0.824). The statistical analysis revealed a main effect ‘group’ for the experience of exclusion and inclusion assessed after each block of the cyberball game; however, its interpretability is restricted by the higher-order interaction effect ‘group’ and ‘condition’ (Table 2): compared with HCs, BPD subjects experienced a greater sense of exclusion and a lesser sense of inclusion during both the inclusion (P = 0.001, respectively P = 0.001) and the control conditions (P < 0.001, respectively P = 0.001), but not during the exclusion condition (all P > 0.5).
Table 2.
Mean and standard deviation of ratings after exclusion (EX), inclusion (IN) and control (C) condition of the cyberball game together with results of the 2 × 3-ANOVA
| Ratings | EX | IN | C | ‘Group’ | ‘Condition’ | ‘Group’ × ‘condition’ | |
|---|---|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | df1 = 1 | df1 = 2 | df1 = 2 | ||
| df2 = 38 | df2 = 76 | df2 = 76 | |||||
| ‘I was excluded’ | BPD | 7.7 (1.4) | 3.7 (1.5) | 3.0 (1.9) | F = 11.1 | F = 300.6 | F = 11.0 |
| HC | 8.0 (1.3) | 2.0 (1.4)* | 1.0 (1.1)* | P = 0.002 | P < 0.001 | P < 0.001 | |
 = 0.23 = 0.23 |
 = 0.89 = 0.89 |
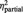 = 0.23 = 0.23 |
|||||
| ‘I felt related’ | BPD | 1.8 (1.1) | 5.6 (1.4) | 6.0 (2.2) | F = 12.8 | F = 206.1 | F = 9.4 |
| HC | 1.7 (1.1) | 7.2 (1.4)* | 8.3 (1.6)* | P = 0.001 | P < 0.001 | P < 0.001 | |
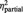 = 0.25 = 0.25 |
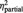 = 0.84 = 0.84 |
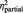 = 0.20 = 0.20 |
|||||
| % of ball tosses received | BPD | 16% (6.3) | 41% (11.0) | 40% (12.0) | F = 0.8 | F = 132.4 | F = 2.4 |
| HC | 13% (6.7) | 47% (13.0) | 44% (16.0) | P = 0.373 | P < 0.001 | P = 0.095 | |
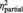 = 0.02 = 0.02 |
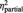 = 0.78 = 0.78 |
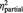 = 0.06 = 0.06 |
|||||
| Inner tension | BPD | 4.3 (2.3) | 4.2 (2.0) | 3.7 (2.3) | F = 13.1 | F = 5.6 | F = 0.1 |
| HC | 2.0 (2.0) | 2.1 (1.8) | 1.6 (1.6) | P = 0.001 | P = 0.005 | P = 0.870 | |
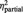 = 0.26 = 0.26 |
 = 0.13 = 0.13 |
 < 0.01 < 0.01 |
|||||
| Dissociation | BPD | 3.2 (2.7) | 2.8 (2.5) | 2.8 (2.6) | F = 16.7 | F = 6.5 | F = 6.7 |
| HC | 0.4 (0.8)* | 0.5 (0.9)* | 0.4 (0.8)* | P < 0.001 | P = 0.007 | P = 0.006 | |
 = 0.31 = 0.31 |
 = 0.15 = 0.15 |
 = 0.15 = 0.15 |
In case of significant interaction effects, means differing between groups are marked with * in the single ball tossing conditions.
There was a trend toward condition-dependent differences for the received ball tosses between groups (see ‘group’ × ‘condition’ interaction in Table 2). The difference in the perceived percentage of received ball tosses between the exclusion and inclusion conditions was more accentuated in the HCs than in the BPD subjects (P = 0.032).
Inner tension was increased across all experimental conditions in BPD subjects compared with HCs (see main effect ‘group’ in Table 2). BPD subjects reported more intensive dissociative symptoms than HCs during each of the ball tossing conditions (main effect group; Table 2). Additionally, the extent of dissociative symptoms was modulated by the ball tossing condition (see interaction by group and condition, Table 2): although the HCs reported similar dissociative symptoms across experimental conditions (all P > 0.1), BPD subjects reported more dissociative symptoms after the exclusion compared with the inclusion and control conditions (P = 0.002, respectively P = 0.001).
fMRI results
Main effect group
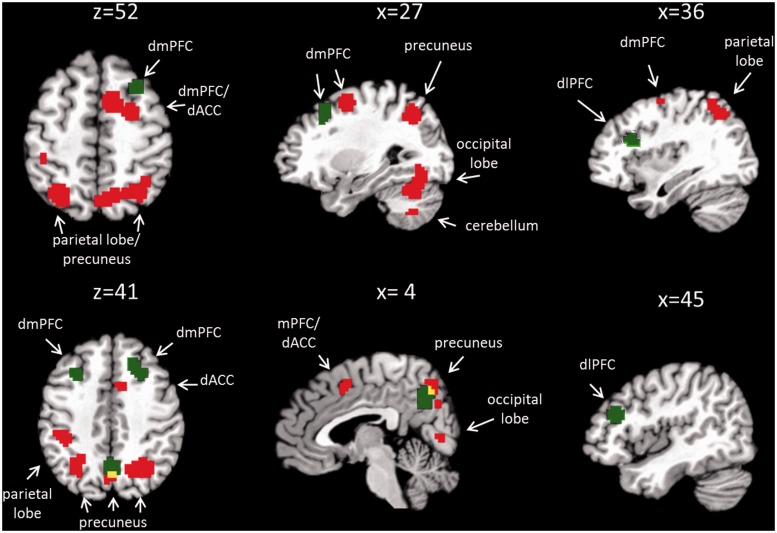
Whole-brain analysis revealed significantly higher activation in the BPD group in comparison to the HC group in several clusters, including the dorsal mPFC (dmPFC, BA6), the dACC (BA24 and 32), the precuneus (BA7), the superior and inferior parietal lobes (SPL, IPL; BA7, BA40), the occipital lobe (BA18, BA19) and the thalamus (independent t-test, P < 0.001 uncorrected, k > 10) (Figure 1, Supplementary Table S2). In the reverse contrast, we found no brain region with higher activation in HCs compared with BPD subjects.
Fig. 1.
Overlay of main effect of ‘group’ (red) and interaction effect of ‘group × condition’ (green). Red blobs correspond to brain regions with increased activation in the BPD group compared with HC independent of the social condition. Green blobs correspond to brain regions differing between groups depending on the type of social interaction condition. Overlap between main effect of ‘group’ and interaction effect of ‘group × condition’ is represented in yellow. dmPFC: dorsal medial prefrontal cortex, dlPFC: dorsolateral prefrontal cortex, dACC: dorsal anterior cingulate cortex.
Activation within all of these clusters was modulated by the ball tossing condition, which was confirmed by masking procedures. However, this modulating effect equaled for both group differences, i.e. there was no interaction effects between group and ball tossing condition.
Main effect condition
A main effect of condition was seen for several clusters (Supplementary Table S3): precuneus and parts of the IPL, the dmPFC (BA6), the dACC (BA 24, 32), the insula, the precentral gyrus, the PCC and two clusters in the middle temporal gyrus (MTG, BA 21, 22). Post hoc tests for the peak-voxel activations of each cluster revealed different effects of the ball tossing conditions within these regions:
The only cluster that was most strongly activated during exclusion was in the left MTG (BA22; Supplementary Figure S1g, P < 0.01). A cluster in the right MTG (BA21) was also more activated during exclusion compared with the control condition (Supplementary Figure S1e, P < 0.001), but this activation did not differ from the inclusion condition.
Higher activation during both the exclusion and the control conditions compared with the inclusion condition was found in the insula and the primary motor cortex (Supplementary Figure S1c and f, all P < 0.001). In the precuneus and the dmPFC, activation was most pronounced during social inclusion (inclusion > exclusion > control, all P < 0.01, Supplementary Figure S1a and b). In the PCC, the highest activation was found during the control condition (control > exclusion > inclusion, all P < 0.05, Supplementary Figure S1d).
Interaction effect group × ball tossing condition
Whole-brain analysis with the flexible factorial design revealed an interaction effect of ‘group’ and ‘ball tossing condition’ in several clusters (Figure 1, Table 3). One cluster was found in the right insula and expanded into the dlPFC (BA13/BA46). Additional clusters involved the right and left dmPFC (BA8 and BA6), and the precuneus (BA7) (Table 3).
Table 3.
Interaction effect ‘group’ × ‘ball tossing condition’: whole brain analysis, uncorrected, P < 0.001, k > 10
| BA | Anatomic label | Cluster size | MNI |
F | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BA 7 | R | Precuneus | 65 | 0 | −61 | 40 | 12.44 |
| BA 13 | R | Insula | 64 | 36 | 23 | 22 | 12.31 |
| BA 46 | R | Middle frontal gyrus | 48 | 32 | 19 | 11.80 | |
| BA 8 | R | Middle frontal gyrus | 50 | 30 | 20 | 49 | 10.79 |
| BA 6 | R | Medial frontal gyrus | 21 | 26 | 40 | 10.63 | |
| BA 8 | L | Middle frontal gyrus | 23 | −30 | 20 | 43 | 10.19 |
| – | L | Cerebellum | 20 | −15 | −55 | −35 | 9.57 |
| – | L | Cerebellum | 12 | −33 | −76 | −32 | 9.13 |
| – | L | Cerebellum | 14 | −15 | −79 | −35 | 9.00 |
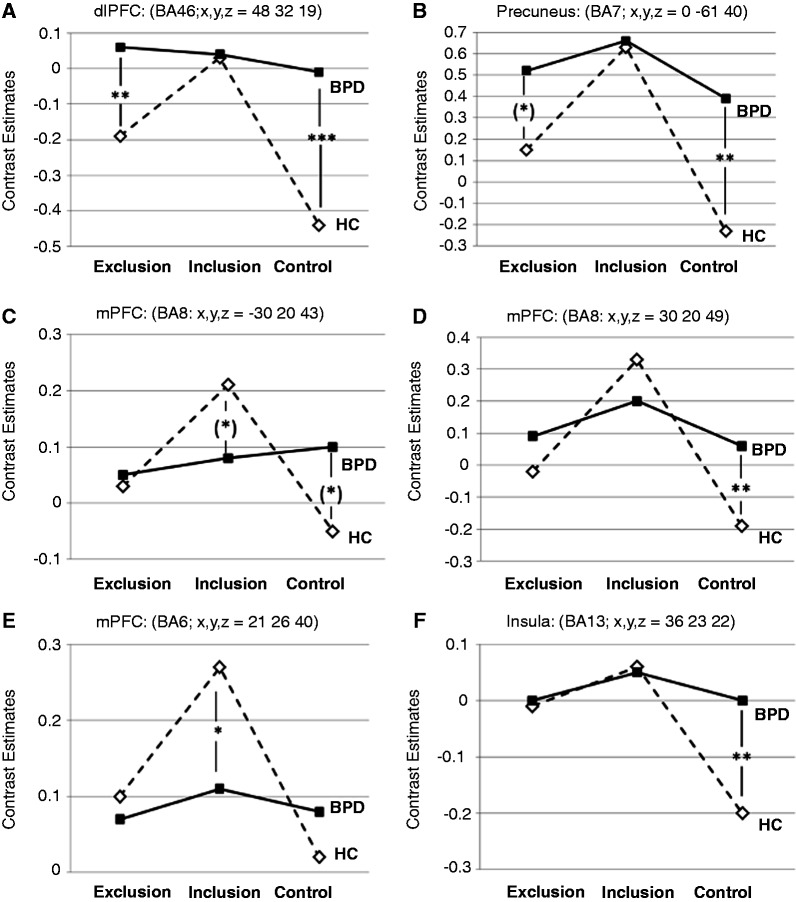
Figure 2 shows the activation of the peak-voxels of each of these clusters. Post hoc tests were calculated to compare activation between groups for the different experimental conditions: during the exclusion condition, post hoc tests revealed a stronger activation in the BPD group than in HCs for the peak-voxel within the right dlPFC (BA46) and—as a trend—within the precuneus region (Figure 2A and B).
Fig. 2.
Mean peak-voxel activation of the clusters in the interaction contrast for the three experimental conditions: (A) dorsolateral prefrontal cortex (dlPFC, BA46); (B) precuneus (BA7); (C, D) dorsal medial prefrontal cortex (dmPFC, BA8); (E) dorsal medial prefrontal cortex (dmPFC, BA6); (F) insula (BA13). Group differences are reported with ‘*’ for P < 0.05, ‘**’ for P < 0.01, ‘***’ for P < 0.001 and ‘(*)’ for P < 0.1 (corrected for multiple comparisons). BPD: borderline personality disorder; HC: healthy control subjects.
During the inclusion condition, peak-voxel activations were lower in BPDs than in HCs within the right dmPFC (BA6; Figure 2E) and—as a trend—for the left dmPFC (BA8; Figure 2C).
During the control condition, activation was higher in BPDs than in HCs for the peak-voxels of nearly all clusters. Exceptions were the left dmPFC (BA8; Figure 2C) for which this effect could be observed only as a trend, and the right dmPFC (BA6; Figure 2E).
In the HC group, post hoc analysis revealed higher peak-voxel activation during social exclusion than the control condition within nearly all brain regions for which an interaction by group and experimental condition was seen with the flexible factorial design. Only for the left dmPFC (BA8), no differences between both conditions could be confirmed statistically. However, compared with the inclusion condition, activation during exclusion was decreased in all regions except for the insula. Here, no difference between exclusion and inclusion was observed. Activation during inclusion was higher than the control condition in all brain regions (all P < 0.05).
In contrast in the BPD group, post hoc analysis revealed no differences in peak-voxel activation between ball tossing conditions (all P > 0.1). Only within the precuneus cluster (BA7; Figure 2B), peak-voxel activation was lower during the control condition than the inclusion condition (P = 0.014), whereas no difference was confirmed compared with the exclusion condition.
DISCUSSION
The aim of this study was to investigate the experience of social exclusion and its underlying neural processes in individuals with BPD. We expected that, compared with HCs, BPD subjects would experience stronger feelings of social exclusion during social interactions, and that these feelings would be accompanied by alterations in the engagement of brain regions relevant for social exclusion and emotion regulation. Our data indeed revealed higher exclusion ratings in BPD patients during the inclusion and control conditions. However, group differences in neural engagement were surprisingly most pronounced during social situations in which subjects were either included or in which interactions were determined by predefined rules.
Previous studies reported stronger feelings of social exclusion independent of the experimental condition in BPD, or found no differences between groups (Ruocco et al., 2010; Staebler et al., 2011b; Renneberg et al., 2012). In contrast, our data support the idea that BPD patients feel rejected by others even in situations in which they are actually being included (Staebler et al., 2011b) or in which social actions cannot be attributed to voluntary decisions of others.
Being accepted and included might be unexpected for most BPD patients, whose self-perception is characterized by negative beliefs of not being likable combined with an assumption that others are untrustworthy (Butler et al., 2002; Arntz et al., 2004; Baer et al., 2012). Festinger’s theory of cognitive dissonance (1957) assumes that when people encounter a mismatch between their expectations and actual experiences, they can resolve it by changing their perception to match the expectation. In this study, the bias seen in BPD subjects in judging social inclusion situations might be explained as an attempt to reduce cognitive dissonance, resulting in a stabilization of negative beliefs.
In contrast to our expectations, both groups felt similarly excluded during the exclusion condition. This finding might be caused by a ceiling effect in the self-reported reaction to exclusion which prevented a further increase in the rating scores in the BPD group. An alternative explanation may be that social exclusion is so threatening that even healthy individuals react strongly to such an experience. This is in line with a study by Lawrence et al. (2011), who reported no difference between BPD patients and HCs in the increase of negative emotions and decrease of positive emotions after social exclusion.
Beyond alterations in the subjective experience of social interactions in BPD, we identified an unspecific hyperactivation of several cerebral structures in the BPD group, and alterations that were dependent on the specific demands of the interaction situations. Independent of the ball tossing condition, BPD patients showed a hyperactivation in the dACC, the dmPFC and the precuneus. These structures have been linked to the experience of rejection in studies with healthy subjects (e.g. Eisenberger et al., 2003; Moor et al., 2012). In agreement with the literature, these structures were differentially activated during the different ball tossing conditions. The modulating effect was comparable in both BPD and HCs. In the context of social rejection, the dACC has been discussed as a ‘neural alarm system’ relevant for the detection of social exclusion by Eisenberger (2012; Eisenberger and Lieberman, 2004). The enhanced dACC-activation in BPD patients points to a higher sensitivity of this system and may correspond to the detection of social exclusion cues even in situations where such cues do not exist. In line with this, Etkin et al. (2011) propose in their framework of emotional processing that dACC and dorsal mPFC are relevant for the detection and appraisal of emotional conflicts. The higher activation of the dmPFC fits with the findings by Ruocco et al. (2010), although they reported higher activation in BPD patients in a more ventrally located part of the mPFC (BA9) and only during social exclusion. Additionally, activation of the mPFC and precuneus was previously linked to self-referential processes, mentalizing, and the evaluation of responses to negative affective stimuli (Ochsner et al., 2004; Spreng et al., 2009). Generally enhanced activation in these structures in the BPD group supports the idea of an altered modulation of the affective response to perceived rejection that is independent of the specific interaction situation, including encounters during which the individual is in fact being included by others.
It has to be mentioned that these group differences have to be interpreted with caution. All of these brain structures have previously been linked to various cognitive constructs that are not necessarily related to social–emotional processes. Although this restricts the interpretation of our findings, one might argue that the hyperactivation in the BPD group is indeed linked to social–emotional processing, as the activation in these brain regions was modulated by the ball tossing conditions. Nevertheless, further studies have to extend the experimental setting by a non-social control condition to gain further insight into the involved processes.
Apart from these general hyperactivations in BPD, we also found group differences that were linked to the ball tossing conditions. Unlike HCs whose cerebral activations were modulated by the specific experimental condition, BPD subjects showed similar brain activation in the different conditions. The most prominent differences between groups in brain activation were observed during the rule-driven control condition, during which BPD subjects showed significantly higher activation than HCs in nearly all brain areas relevant to the processing of social exclusion; namely the dlPFC, the dmPFC, the precuneus and the insula. Although activation could not be distinguished between the inclusion and control conditions in BPD subjects, HCs disengaged cerebral structures linked to the ‘social brain’ during the control condition. This fits well with the finding that BPD features in an adolescent inpatient sample were linked to ‘hypermentalizing’, defined as an over-attribution of intentions and emotions to social partners (Sharp et al., 2011). Hypermentalizing—in this case, attributing intentions to co-players even in situations where those could not influence the interaction—constitutes one explanation for the missing disengagement of these brain areas in BPD subjects during the control condition. Further studies are required to investigate whether BPD patients have difficulties in distinguishing intrinsic or extrinsic motivations for the behavior of social partners.
It seems worth noting that our data suggest differential alterations in subregions of the mPFC in BPD depending on the experimental conditions: within a region of the dmPFC covering BA6 and located close to the dACC, we found a general hyperactivation in BPD, whereas within a more anterior part of the dmPFC we conversely observed a reduced activation during social inclusion in BPD compared with HCs. In more anterior parts of the dmPFC corresponding to BA8, we observed a higher activation in the BPD group occurring only during the control condition. These findings strongly suggest a functional segregation within the mPFC in the context of the processing of social encounters, whereby different processes seem to be differentially affected by BPD. Following the model of Etkin et al. (2011), all of the alterations observed within this study are localized within the dorsal part of the mPFC which has been linked to the detection and appraisal of emotional conflicts and action monitoring, rather than emotion regulation processes related to the ventral mPFC. Nevertheless, the differential effects of the interaction conditions within the dmPFC suggest that a finer grained linkage to cognitive functions is necessary to adequately explain our findings. One might speculate whether, e.g. the higher engagement of the more anterior parts of the dmPFC in BPD reflects a more intense experience of uncertainty as one aspect of the appraisal of social encounters (Volz et al., 2005). This uncertainty may in particular be linked to BPD during social encounters in which the interaction behavior of social partners is not intrinsically motivated. However, further studies that manipulate these cognitive sub-functions experimentally are required to test whether they can be linked to BPD psychopathology.
Our unexpected finding of enhanced dlPFC activation during social exclusion in BPD contradicts our hypothesis of reduced prefrontal activation during social interaction in BPD, which would go along with the idea of a disturbed cognitive top-down control (Mauchnik and Schmahl, 2010), and would have been in accordance with a decreased activation of the dlPFC and vlPFC during social exclusion with increasing rejection sensitivity (Kross et al., 2007). However, following Etkin et al. (2011), the higher dlPFC activation together with a generally enhanced dACC and dmPFC activation fits with the interpretation of enhanced conflict evaluation processes in BPD.
It has to be mentioned that in most regions the highest activation was observed during the inclusion condition. This finding was observed particularly in the healthy individuals and might be caused by the characteristics of the cyberball task as applied in this study. For methodological reasons, we applied the cyberball paradigm as a blocked design with an alternating sequence of exclusion, inclusion and control situations which were played with the same virtual partners. Studies with a comparable design (Bolling et al., 2011; Kawamoto et al., 2012; Moor et al., 2012) also reported enhanced activation in the parietal lobe and mPFC during inclusion. As the co-players remained the same during all conditions while varying their behavior, subjects may have been continually uncertain on what kind of behavior to expect. Thus, they may have put special effort into monitoring their co-players’ behavior and detecting cues that signal social rejection. This monitoring would particularly be important during the inclusion condition since here, subjects could not rule out the possibility of being rejected at some later point. In contrast, during the exclusion condition, rejection already occurred close to kick-off; and during the control condition, it was downright prevented due to the predefined rules. Thus, the increased uncertainty about the partners’ behavior during inclusion might result in an enhanced activation of the dACC and IPL as these are regions involved in salience detection and the control of goal-directed attention (Singh-Curry and Husain, 2009). In line with this explanation of a lower uncertainty during the exclusion and control conditions, HCs also engaged regulatory or evaluative mechanisms linked to regions of the dlPFC especially during the inclusion condition. It seems surprising that the BPD patients would not react to the uncertainty regarding potential social rejection during the inclusion condition by increased engagement of the dlPFC. One explanation might lie therein that alterations in social interactions in BPD on the one hand involve a misattribution of the partners’ (negative) intentions independent of the partners’ possibilities to influence the social encounter. On the other hand, they could involve a lack in trying to gather social cues to confirm or contradict expectations regarding the course of an interaction. Further research is required to clarify whether these two alterations in the processing of social encounters can actually be differentiated and confirmed in BPD.
Beyond that, using the same co-players during all experimental conditions might have affected the tendency to over-attribute intentions as compared with studies which have only applied one round of inclusion and exclusion in a fixed sequence or have used a between-subject design (e.g. Eisenberger et al., 2003; Ruocco et al., 2010; Staebler et al., 2011b; Renneberg et al., 2012). Further studies are needed to investigate whether using different teams of co-players during inclusion and exclusion would affect the experience of being rejected, and whether it would differentially affect the engagement of cerebral structures potentially linked to coping with these experiences for BPD patients and healthy subjects.
Some shortcomings of this study have to be addressed. The methodological approach is only a first step toward understanding the experience of social exclusion in BPD. The observed alterations of processing during experimental conditions have to be linked to real-life problems in social functioning; and the specificity of the observed pattern of alterations for BPD remains to be investigated as we did not include a clinical control group. Beyond that, BPD patients are characterized by a multitude of different co-morbidities for which modulating effects on the experience of social rejection in different social interaction situations have yet to be investigated. The same holds true for the contribution of different symptom dimensions, as well as for the effect of prior history of bullying, physical abuse and emotional neglect. To rule out potential effects of depressive disorders, we had excluded subjects with current depressive episode. In addition, we assessed the effects of depressive symptoms as measured by the BDI corrected for borderline symptom severity on brain imaging data. None of the described alterations could be explained by the severity of depressive symptoms.
In summary, our data point toward an altered processing of social interaction situations in unmedicated female BPD patients. This difference was not manifested in a higher sensitivity to actual social exclusion, but rather in a bias toward perceiving social exclusion during interactions that involved social inclusion as well as ‘neutral’ social encounters. These subjective experiences were linked to alterations in cerebral processing within structures of the ‘social brain’ (Mars et al., 2012). In a recent review of social interaction in BPD, Lis and Bohus (2013) concluded that social interaction problems in BPD occur not only during challenging social situations such as provocation or rejection but also under ‘normal’ conditions. Our results strongly support this idea, as BPD patients appear to have problems in differentiating between different forms of social interaction and their meanings. This is in line with findings in BPD during economic exchange games. Similar to our data, King-Casas et al. (2008) observed a lack in the modulation of cerebral activation depending on the behavior of social partners in BPD, i.e. a lack in the modulation of insula activation dependent on the unfairness of co-players. This might point—as the authors proposed—to an insensitivity to the violation of social norms (see also Franzen et al., 2011), or more generally to a failure in the adaptation to characteristics of a social environment independently of its emotional valence. Such impairments might underlie the disorganization of behavior across social domains, which has been described by Hill et al. (2008). Their data suggest that social dysfunctioning in BPD cannot simply be characterized by an altered quality or quantity of social actions, but instead by an inadequacy of behavior in different social domains. Our data suggest that treatments of social interaction problems in BPD patients should target their attentiveness to negatively biased expectations together with awareness to social cues during interaction, and to correct these expectations if appropriate. A better ability to differentiate types of social interaction situations, together with the development of skills to build affiliations, might help to restore the balance between experiences of social exclusion and inclusion in the everyday lives of BPD patients.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (KFO-256 BO 1487/11-1). We thank all participants for their collaboration, and M. Riess, E. Pfeiffer, and S. Getto for their help with data acquisition.
REFERENCES
- APA, editor (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association.
- Arntz A, Dreessen L, Schouten E, Weertman A. Beliefs in personality disorders: a test with the personality disorder belief questionnaire. Behaviour Research and Therapy. 2004;42:1215–25. doi: 10.1016/j.brat.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Baer RA, Peters JR, Eisenlohr-Moul TA, Geiger PJ, Sauer SE. Emotion-related cognitive processes in borderline personality disorder: a review of the empirical literature. Clinical Psychology Review. 2012;32:359–69. doi: 10.1016/j.cpr.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock H, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease. 1986;174:727–35. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Bohus M, Limberger MF, Frank U, Chapman AL, Kuhler T, Stieglitz RD. Psychometric properties of the Borderline Symptom List (BSL) Psychopathology. 2007;40:126–32. doi: 10.1159/000098493. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. 2011;54:2462–71. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AC, Brown GK, Beck AT, Grisham JR. Assessment of dysfunctional beliefs in borderline personality disorder. Behaviour Research and Therapy. 2002;40:1231–40. doi: 10.1016/s0005-7967(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Minneapolis, MN: National Computer Systems; 1993. BSI, Brief Symptom Inventory: Administration, Scoring, and Procedures Manual, 4th edn. [Google Scholar]
- DeWall CN, Masten CL, Powell C, Combs D, Schurtz DR, Eisenberger NI. Do neural responses to rejection depend on attachment style? An fMRI study. Social Cognitive and Affective Neuroscience. 2012;7:184–92. doi: 10.1093/scan/nsq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70:1327–43. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Eisenberger N, Lieberman M. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences Trends in Cognitive Sciences. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews. Neuroscience. 2012;13:421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L. A Theory of Cognitive Dissonance. Evanston, IL: Row & Peterson; 1957. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin LS. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)—Clinical Version. Washington: American Psychiatric Press; 1997. [Google Scholar]
- Franzen N, Hagenhoff M, Baer N, et al. Superior ‘theory of mind’ in borderline personality disorder: an analysis of interaction behavior in a virtual trust game. Psychiatry Research. 2011;187:224–33. doi: 10.1016/j.psychres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Freyberger HJ, Spitzer C, Stieglitz RD, et al. Adaptation and psychometric properties of the German version of the Dissociative Experience Scale. Journal of Traumatic Stress. 1998;11:799–809. doi: 10.1023/A:1024457819547. [DOI] [PubMed] [Google Scholar]
- Herpertz SC. Self- injurious behaviour. Psychopathological and nosological characteristics in subtypes of self-injurers. Acta Psychiatrica Scandinavica. 1995;91:57–68. doi: 10.1111/j.1600-0447.1995.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Hill J, Pilkonis P, Morse J, et al. Social domain dysfunction and disorganization in borderline personality disorder. Psychological Medicine. 2008;38:135–46. doi: 10.1017/S0033291707001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Onoda K, Nakashima K, Nittono H, Yamaguchi S, Ura M. Is dorsal anterior cingulate cortex activation in response to social exclusion due to expectancy violation? An fMRI study. Frontiers in Evolutionary Neuroscience. 2012;4:11. doi: 10.3389/fnevo.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–10. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H. Affective instability: toward an integration of neuroscience and psychological perspectives. Journal of Personality Disorders. 2010;24:60–82. doi: 10.1521/pedi.2010.24.1.60. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Fan J, Ochsner KN, et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biological Psychiatry. 2009;66:854–63. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krill A, Platek SM. In-group and out-group membership mediates anterior cingulate activation to social exclusion. Frontiers in Evolutionary Neuroscience. 2009;1:1. doi: 10.3389/neuro.18.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6270–5. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. Journal of Cognitive Neuroscience. 2007;19:945–56. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- Lawrence KA, Chanen AM, Allen JS. The effect of ostracism upon mood in youth with borderline personality disorder. Journal of Personality Disorders. 2011;25:702–14. doi: 10.1521/pedi.2011.25.5.702. [DOI] [PubMed] [Google Scholar]
- Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364:453–61. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan MM. Cognitive-Behavioral Treatment of Borderline Personality Disorder. New York: The Guildford Press; 1993. [Google Scholar]
- Lis S, Bohus M. Social interaction in borderline personality disorder. Current Psychiatry Reports. 2013;15:338. doi: 10.1007/s11920-012-0338-z. [DOI] [PubMed] [Google Scholar]
- Loranger AW. International Personality Disorder Examination (IPDE): DSM-IV and ICD-10 Modules. Odessa: Psychological Assessment Resources; 1999. [Google Scholar]
- Lynum LI, Wilberg T, Karterud S. Self-esteem in patients with borderline and avoidant personality disorders. Scandinavian Journal of Psychology. 2008;49:469–77. doi: 10.1111/j.1467-9450.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain”. Frontiers in Human Neuroscience. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauchnik J, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Current Psychiatry Reports. 2010;12:46–55. doi: 10.1007/s11920-009-0089-7. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Schultz AP, Locascio JJ, Sperling EA, Atri A. 2011. Repeated-measures designs overestimate between-subject effects in fMRI packages using one error term. Poster on the 17th Annual Meeting of the Organization for Human Brain Mapping, June 26–30, 2011 in Québec City, Canada. [Google Scholar]
- Moor BG, Guroglu B, Op de Macks ZA, Rombouts SA, Van der Molen MW, Crone EA. Social exclusion and punishment of excluders: neural correlates and developmental trajectories. Neuroimage. 2012;59:708–17. doi: 10.1016/j.neuroimage.2011.07.028. [DOI] [PubMed] [Google Scholar]
- New AS, Perez-Rodriguez MM, Ripoll LH. Neuroimaging and borderline personality disorder. Psychiatric Annals. 2012;42:65–71. [Google Scholar]
- Niedtfeld I, Schmahl C. Emotion regulation and pain in borderline personality disorder. Current Psychiatry Reviews. 2009;5:48–54. [Google Scholar]
- Niedtfeld I, Schmahl C. Emotionale dysregulation bei der borderline-persönlichkeitsstörung. Zeitschrift für Psychiatrie, Psychologie und Psychotherapie. 2012;60:185–93. [Google Scholar]
- O'Neill A, Frodl T. Brain structure and function in borderline personality disorder. Brain Structure & Function. 2012;217:767–82. doi: 10.1007/s00429-012-0379-4. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, Nittono H, Ura M, Yamawaki S. Decreased ventral anterior cingulate cortex activity is associated with reduced social pain during emotional support. Social Neuroscience. 2009;4:443–54. doi: 10.1080/17470910902955884. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima K, et al. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Social Cognitive and Affective Neuroscience. 2010;5:385–91. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renneberg B, Herm K, Hahn A, Staebler K, Lammers CH, Roepke S. Perception of social participation in borderline personality disorder. Clinical Psychology & Psychotherapy. 2012;19:473–80. doi: 10.1002/cpp.772. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: University Press; 1965. [Google Scholar]
- Ruesch N, Lieb K, Gottler I, et al. Shame and implicit self-concept in women with borderline personality disorder. American Journal of Psychiatry. 2007;164:500–8. doi: 10.1176/ajp.2007.164.3.500. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: an activation-likelihood-estimation meta-analysis. Biological Psychiatry. 2013;73:153–60. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Ruocco AC, Medaglia JD, Tinker JR, et al. Medial prefrontal cortex hyperactivation during social exclusion in borderline personality disorder. Psychiatry Research. 2010;181:233–6. doi: 10.1016/j.pscychresns.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Sharp C, Pane H, Ha C, et al. Theory of mind and emotion regulation difficulties in adolescents with borderline traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:563–73. doi: 10.1016/j.jaac.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–48. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9:1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Staebler K, Helbing E, Rosenbach C, Renneberg B. Rejection sensitivity and borderline personality disorder. Clinical Psychology & Psychotherapy. 2011a;18:275–83. doi: 10.1002/cpp.705. [DOI] [PubMed] [Google Scholar]
- Staebler K, Renneberg B, Stopsack M, Fiedler P, Weiler M, Roepke S. Facial emotional expression in reaction to social exclusion in borderline personality disorder. Psychological Medicine. 2011b;41:1929–38. doi: 10.1017/S0033291711000080. [DOI] [PubMed] [Google Scholar]
- Stiglmayr CE, Grathwol T, Linehan MM, Ihorst G, Fahrenberg J, Bohus M. Aversive tension in patients with borderline personality disorder: a computer-based controlled field study. Acta Psychiatrica Scandinavica. 2005;111:372–9. doi: 10.1111/j.1600-0447.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. Journal of Personality Disorders. 2010;24:412–26. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz K, Schubotz R, Cramon YV. Variants of uncertainty in decision-making and their neural correlates. Brain Research Bulletin. 2005;67:403–12. doi: 10.1016/j.brainresbull.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15079–84. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD, Cheung CK, Choi W. Cyberostracism: effects of being ignored over the Internet. Journal of Personality and Social Psychology. 2000;79:748–62. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: a program for use in research on interpersonal ostracism and acceptance. Behavior Research Methods. 2006;38:174–80. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.