Abstract
Processing self-related material recruits similar neural networks regardless of whether the self-relevance is made explicit or not. However, when considering the neural mechanisms that distinctly underlie cognitive and affective components of self-reflection, it is still unclear whether the same mechanisms are involved when self-reflection is explicit or implicit, and how these mechanisms may be modulated by individual personality traits, such as self-esteem. In the present functional MRI study, 25 participants were exposed to positive and negative words that varied with respect to the degree of self-relevance for each participant; however, the participants were asked to make a judgment about the color of the words. Regions-of-interest analysis showed that medial prefrontal cortex (mPFC) and posterior cingulate cortex were associated with gauging the self-relevance of information. However, no main effect of valence or an interaction effect between self-relevance and valence was observed. Further, positive correlations were observed between levels of self-esteem and response within dorsal mPFC (dmPFC) both in the contrast positive-high in self-relevance trials vs positive-low in self-relevance trials and in the contrast negative-low in self-relevance trials vs positive-low in self-relevance trials. These results suggested that the activation of dmPFC may be particularly associated with the processes of self-positivity bias.
Keywords: self-esteem, implicit self-relevant processing, dorsal medial prefrontal cortex (dMPFC), self-positivity bias
INTRODUCTION
Previous studies exploring neural correlates of participants’ self-reflection have shown that cortical midline structures are implicated in self-referential thought regardless of whether the self-relevance was made explicit or not (Macrae et al., 2004; Northoff and Bermpohl, 2004; Moran et al., 2009; Rameson et al., 2010). Given that the information that is processed about the self is often valenced, previous studies have also shown that there are distinct neural circuits that subserve cognitive (self vs non-self distinction) and emotional (positive vs negative) aspects of self-processing (Moran et al., 2006). However, the majority of these studies that have examined valenced self-processing has done so by using explicit paradigms (Moran et al., 2006). Yet, a portion of the information that we process about our environment and about ourselves is done implicitly (Greenwald and Farnham, 2000). Therefore, in this study, we aimed to investigate whether the distinct neural circuits that were previously found to underlie cognitive and affective components of self-processing in explicit tasks would also be recruited when participants are completing an implicit self-relevance task. In addition, it should be noted that the valenced information that we process about ourselves is also influenced by our own implicit biases and traits such as self-esteem (Tao et al., 2012). Thus, the second aim of the study was to assess whether levels of self-esteem may modulate activity in regions underlying implicit self-processing.
Previous studies on explicit self-processing have shown that the medial prefrontal (ventral and dorsal) and medial parietal/posterior cingulate (anterior and posterior) cortices are engaged during tasks which require making specific judgments about one’s own traits compared with judgments of others or semantic judgments (Craik et al., 1999; Johnson et al., 2002; Kelley et al., 2002; D'Argembeau et al., 2005; Ochsner et al., 2005). Because explicit judgment of the self-descriptiveness of trait adjectives can result in self-presentational bias, in which people rate themselves as possessing more positive personality traits and displaying more positive behaviors than others, a few studies have also employed indirect ways to assess self-relevant processing (Moran et al., 2009; Rameson et al., 2010; Frewen et al., 2013). Results from these studies that have investigated neural correlates when individuals implicitly process self vs other information suggest that processing self-related material recruited similar neural networks to when the self-relevance is made explicit (Rameson et al., 2010).
An additional aspect to processing self-relevant information is emotion (Heatherton, 2011). This particularly may have importance when we consider one’s vulnerability to mental health ills such as depression. Depressed patients are inclined to ruminate about negative self-relevant information and make negative attributions to themselves (Grunebaum et al., 2005; Northoff, 2007). Previous studies have looked at this in an explicit task and found that medial prefrontal cortex (MPFC) underlined processing of personal relevance of information, whereas adjacent ventral anterior cingulated cortex (vACC) was able to distinguish emotional valence of this material (Moran et al., 2006). With respect to studies employing an implicit task to explore neural processing of valenced self, to authors’ knowledge, there is only one priming study that has looked at this. Results showed that self-negative trials compared with self-positive trials were associated with greater response within two regions: the posterior mid-cingulate and right superior parietal cortex (Frewen et al., 2013). All the other studies examining implicit self-relevant processing did not consider the valence of the stimuli (Moran et al., 2009; Rameson et al., 2010).
Another factor that may impact on how we process implicit information about self and the valence of such information is self-esteem. Self-esteem is a broadly defined personality variable referring to the degree to which an individual values and accepts him- or herself (Pruessner et al., 2005). The neural mechanism underlying the association between levels of self-esteem and processing of explicit self-relevant information has been investigated. In our recent study, we observed a significant negative correlation between levels of self-esteem and changes in activation of dorsal anterior cingulate cortex (dACC) in response to evaluating self-relevant information (Yang et al., 2012). However, the study design precluded us from investigating the association between self-esteem levels and processing of the valence of self-referential information. Another study investigating this topic in female participants used self-descriptiveness of words presented, as well as task-induced affective response, as proxies for trait self-esteem and found that response to valenced self-relevant material within dorsal and ventral medial prefrontal cortex (dmPFC and vmPFC), cingulate cortex and left temporoparietal cortex varied with individual differences in self-esteem proxy measures (Frewen et al., 2013). However, it still remains unclear how trait self-esteem levels as measured by a standardized self-esteem scale may impact the neural correlates of implicit self-relevant processing in a group of both male and female participants.
Therefore, the goal of this study is to first investigate whether brain regions that were previously found to be involved in processing cognitive and affective components of self would also be recruited by an implicit task; and second, to observe whether self-esteem may modulate activity in these regions. Therefore, we focused our investigation on brain regions which have been found to be involved in self-relevant processing in previous neuroimaging studies; these regions include mPFC, dmPFC, vmPFC, vACC, dACC, and posterior cingulate cortex (PCC). We hypothesized that mPFC and PCC will be recruited to process the personal relevance of information and that the activity in vACC would distinguish between the valence of the self-relevant material (Moran et al., 2006). Further, as people with high self-esteem are more likely to have positive self-views than people with low self-esteem (Tao et al., 2012), and dmPFC has been shown to be particularly involved in reflective processes that is positive in nature (van der Meer et al., 2010; Heatherton, 2011), we hypothesized that people’s self-esteem level would be correlated with the activity levels in dmPFC when participants make positive self-reflections.
METHODS
Participants
Twenty-nine healthy university students enrolled at Southwest University, China participated in the study. Four participants were excluded due to excessive head motion (>1 mm between successive image acquisitions) during scanning which resulted in a final functional MRI (fMRI) dataset of 25 (12 male, mean age = 22.5). Participants did not have a history of psychiatric or neurological disorders, significant physical illness, head injury or alcohol/drug abuse. After participants were given a complete explanation of the study, written informed consent was obtained. Participants were paid for their participation.
Questionnaire
Participants completed the Rosenberg self-esteem scale (RSE), a questionnaire that assesses a person’s overall evaluation of his or her self-worth (Rosenberg, 1965). The RSE is made up of 10 items such as ‘On the whole, I am satisfied with myself’ or ‘I feel I do not have much to be proud of’ and is coded on a four-point scale ranging from 1 (strongly disagree) to 4 (strongly agree), with the negative items needing to be reverse scored.
Stimuli and procedure
A total of 460 personality-trait adjectives were selected from established personality-trait adjective pools (Huang and Zhang, 1992), each of which consisted of three Chinese characters. Half of the words were positive adjectives and the other half was negative.
Upon arrival, participants completed the RSE. They then underwent a scanning session to complete the experimental task. While undergoing the scanning session, participants viewed 460 personality-trait adjectives, written in red or green, and made a non-emotional judgment about the word (‘is the word in red or in green?’). Adjectives were presented for 2 s each. Null events consisting of a fixation cross for 2000, 4000 and 6000 ms and these were pseudorandomly interspersed to introduce jitter into the fMRI time series. Participants were required to press the appropriate keys to indicate the color of the adjectives (‘1’ for red and ‘2’ for green) as soon as the adjectives appeared on the screen. There were five runs in the whole experiment and in each run, there were 92 trials. Order of presentation of the trials was randomized.
In order to create sets of positive and negative words that varied with respect to degree of self-relevance for each subject, immediately after completing the experimental task and exiting the scanner, participants then viewed the same 460 adjectives in white print on a black background and were instructed to change their response. Specifically, they were instructed to now indicate their response to each adjective by answering the question ‘How much does this adjective describe me’ using the scale 1 (not at all like me) through 4 (most like me). For further analysis, items with a response of 1 or 2 were considered low in self-relevance, whereas items with a response of 3 or 4 were considered high in self-relevance.
Imaging data acquisition
Images were acquired in a 3T Siemens TRIO MRI scanner. Functional data comprised 1405 volumes acquired with T2*-weighted gradient echo planar imaging sequences. We obtained 32 echo planar images per volume sensitive to blood oxygenation level-dependent (BOLD) contrast [TR = 2000 ms; TE = 30 ms; 3 × 3 mm in-plane resolution; field of view (FOV) = 192]. Slices were acquired in an interleaved order and oriented parallel to the AC–PC plane, with thickness of 3 mm, 0.99 mm gap. High-resolution T1-weighted 3D fast-field echo sequences were obtained for anatomical reference (176 slices, TR = 1900 ms; TE = 2.52 ms; slice thickness = 1 mm; FOV = 250; voxel size = 1 × 1 × 1 mm).
Imaging data analyses
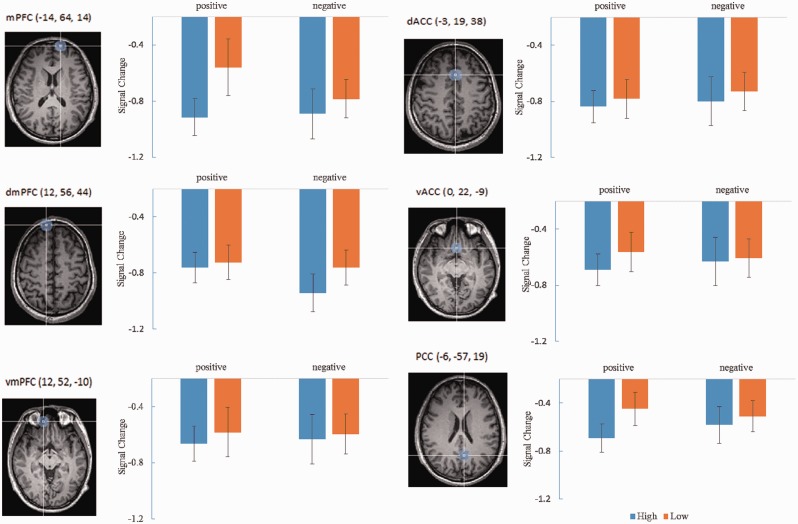
Data were analyzed using Brain Voyager QX v2.3 software (Brain Innovation, The Netherlands). Functional scans were realigned within and across runs to correct for head motion, and co-registered with each participant’s anatomical data. Functional data were then normalized into standard stereotactic Talairach space, resliced into a voxel size of 3 mm × 3 mm × 3 mm and smoothed with a 4 mm Gaussian kernel to increase signal-to-noise ratio. All regions of interest (ROIs) were defined using prior functional-defined ROIs, with a 10 mm radius sphere centered at Talariach coordinates. Given that some previous studies on neural correlates of self-processing concentrated on the cognitive components of self-processing, whereas other studies concentrated on the valence components of self-processing, we included ROIs reported in several studies in order to be able to assess all the possible areas previously suggested to underlie this process. The mPFC (x = −14, y = 64, z = 14), dmPFC (x = 12, y = 56, z = 44), and vmPFC (x = 12, y = 52, z = −10) from Rameson et al. (2010), the vACC (x = 0, y = 22, z = −9) and dACC (x = −3, y = 19, z = 38) from Moran et al. (2006) and the PCC (x = −6, y = −57, z = 19) from Moran et al. (2009) were defined in further analysis. First level effects were estimated using the general linear model and employing a canonical hemodynamic response function convolved with the experimental design. Group analyses were conducted using random-effects models in order to enable population inferences.
In the second-level analysis, trials were sorted into four conditions based on the valence of each trait and the participants’ responses to those traits. A general linear model incorporated these four task effects (comprising the four cells of the 2 × 2 analysis of variance (ANOVA): positive-high in self-relevance (PH), positive-low in self-relevance (PL), negative-high in self-relevance (NH), negative-low in self-relevance (NL). The beta values of the four conditions for each ROI were extracted to compute both main effects (self-relevance and valence) and their interactions. To identify whether the effects of valence within self, and the effects of self within valence were associated with the self-esteem scores of participants, the self-esteem scores derived from the RSE questionnaire were correlated with the beta values for each ROI in the following four contrasts separately: PH relative to PL trials, PH relative to NH trials, NH relative to NL trials, and NL relative to PL trials. Further, in order to identify whether the self-positive bias was associated with the self-esteem scores of participants, the beta values for each ROI in the interaction contrast [PH + NL] > [NH + PL] were correlated with the self-esteem scores. The Bonferroni correction was employed when performing multiple statistical tests simultaneously in all the analyses.
RESULTS
Behavioral results
Participants’ judgments were collapsed into high (three and four responses) and low (one and two responses) self-relevance categories and analyzed with a 2 × 2 ANOVA examining the main effects of self-relevance (high vs low) and valence (positive vs negative) both on reaction times and on proportion of items endorsed. For reaction times, results revealed no significant main effects of valence or self-relevance, nor a significant valence × self-relevance interaction effect (Figure 1A). For the proportion of items endorsed, results revealed a self-relevance × valence interaction, F(1, 24) = 208.9, P < 0.001. Greater proportion of positive words was endorsed as high self-descriptive than low self-descriptive; greater number of negative words was rated as being low in self-relevance than high in self-relevance (Figure 1B), which is consistent with prior work (Moran et al., 2006).
Fig. 1.
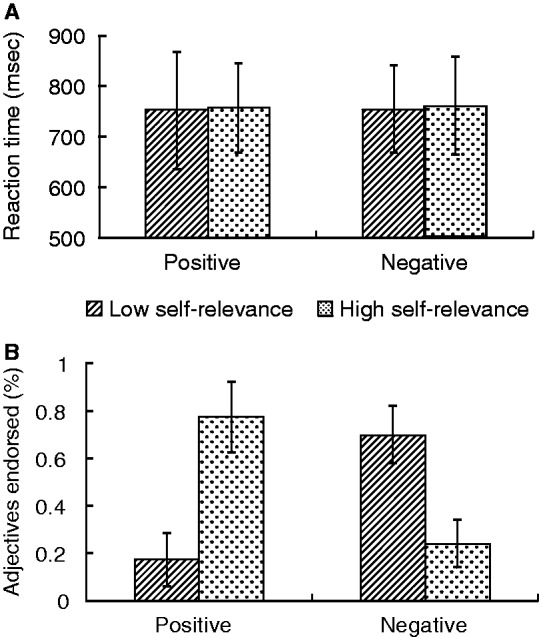
Reaction time (A) and adjectives endorsed (%) (B) for positive and negative words that were high self-relevant and low self-relevant for each participant. The error bars indicate the standard deviation of the mean.
fMRI results
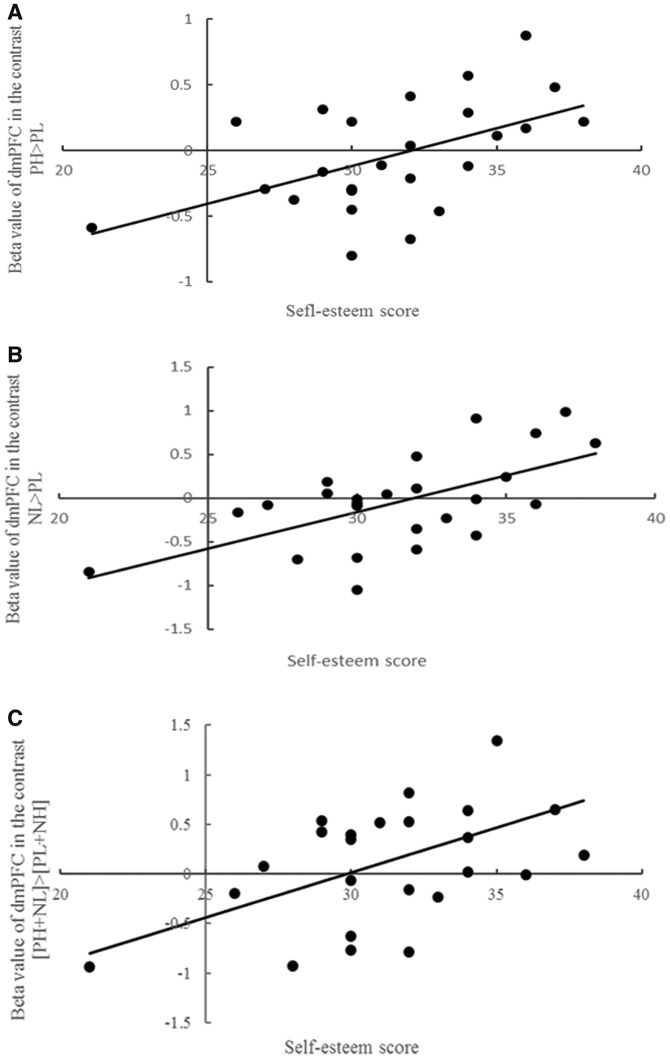
The main effect of self-relevance was observed in mPFC [F(1, 24) = 17.307, P < 0.05, corrected] and PCC [F(1, 24) = 10.902, P < 0.05, corrected] (Figure 2). No main effect of valence or an interaction effect between self-relevance and valence was observed. We then examined correlations between self-esteem scores and response in dmPFC ROI during PH relative to PL trials, PH relative to NH trials, NH relative to NL trials and NL relative to PL trials separately. Concerning the contrast PH > PL, positive correlations were observed between levels of self-esteem and response within dmPFC, r = 0.521, P < 0.01, corrected (Figure 3A). Concerning the contrast NL > PL, a positive correlation was observed between levels of self-esteem and response within dmPFC, r = 0.604, P < 0.01, corrected (Figure 3B). Concerning the interaction contrast [PH + NL] > [NH + PL], a positive correlation was only found in dmPFC, r = 0.531, P < 0.05, corrected (Figure 3C). There were no significant correlations between self-esteem and the remaining ROIs.
Fig. 2.
Axial sections display mPFC (left top), dmPFC (left middle), vmPFC (left bottom), dACC (right top), vACC (right middle) and PCC (right bottom) spherical ROIs superimposed on a normalized anatomic image. Graphs to the right of each image display signal change (parameter estimates) across each condition. Error bars indicate standard error of the mean.
Fig. 3.
(A) The curve estimation between beta value of dmPFC in the contrast of PH > PL and the self-esteem score in 25 participants. (B) The curve estimation between beta value of dmPFC in the contrast of NL > PL and the self-esteem score in 25 participants. (C) The curve estimation between beta value of dmPFC in the contrast of [PH + NL] > [NH + PL] and the self-esteem score in 25 participants.
Whole-brain analysis results regarding BOLD signal changes associated with the main effects of self-relevance, valence and the self-relevance by valence interaction (Supplementary Table S1), and correlations between self-esteem scores and five specified contrasts (Supplementary Table S2) can be found in the Supplementary Data.
DISCUSSION
The main goal of this research was to investigate whether brain regions that were previously found to be involved in processing cognitive and affective components of self would also be recruited by an implicit task. Results suggest that mPFC and PCC underlie processing of implicit self-relevance of information. These findings are consistent with the work of Moran et al. (2009) and Rameson et al. (2010). However, the findings are inconsistent with the results of the study by Frewen et al. (2013), in which the direct contrast of self-relevant condition compared with other-relevant condition did not show differential recruitment of any brain area. We speculate that the discrepancy between the findings may be due to the choice of the control condition. For example, in studies by Moran et al. (2009), Rameson et al. (2010) and in this study, highly self-relevant information was compared directly with low self-relevant information. However, in the study of Frewen et al. (2013), self-relevant information was compared with information related to ‘other’. Specifically, in the study of Frewen et al. (2013), most participants endorsed positive views of both themselves and others, which may have lead to the other-relevant information being processed as affectively salient as the self-relevant information; therefore, few differences were observed between self-relevant processing and other-relevant processing conditions (Frewen et al., 2013). In addition, it has been suggested that choosing a third figure for the other-evaluation condition may differentially affect participant’s ratings of a variety of unintentional processes (Schmitz et al., 2004). Therefore, it may be that comparing self-relevant information with non-self-relevant information (rather than other-relevant information) would be a more appropriate contrast to specifically tap the neural correlates underlying self-referential processing.
In regard to the neural correlates of affective components of self, results showed that processing the valenced components of the implicit self neither activated vACC nor other brain regions. It was inconsistent with the results of Frewen et al. (2013), the only study, to our knowledge, that directly investigated the neural bases of valence differences in implicit self-relevant processing. In that study, self-negative trials activated the posterior mid-cingulate and right superior parietal cortex to a greater extent than self-positive trials (Frewen et al., 2013). Authors in that study used the Visual–Verbal Self-Other Referential Processing Task (VV-SORP-T), which implicitly encourages attention to the association between self and a valenced word. Therefore, it is possible that the VV-SORP-T encourages affective processing to a greater extent than this study. In this study, the stimulus presentations or task instructions did not attempt to emphasize participants’ introspection or interoception, and thus might not elicit strong affective responses, limiting our ability to find a difference between conditions.
The second goal of this research was to investigate the neural correlates underlying the association between levels of self-esteem and processing of implicit valenced self-relevant information. Results showed that positive correlations were observed between levels of self-esteem and response within dmPFC in the contrast PH trials vs PL trials. These findings are consistent with two previous important findings. First, the activation of dmPFC has been shown to be associated with self-referential processing that is positive (van der Meer et al., 2010; Heatherton, 2011), and dmPFC has also been found to respond to positive self-relevant processing particularly in women who experienced greater positive affect during a self-relevant condition (Frewen et al., 2013). Second, this contrast effectively assessed one aspect of the self-positivity bias (regarding positive information as more self-relevant compared with negative information). Studies have shown that those with high self-esteem are more likely to show self-positivity bias than those with low self-esteem (Tao et al., 2012); in fact, maintenance of self-esteem relies on self-positivity bias to a certain extent (Lin et al., 2003). Present findings of a positive correlation between self-esteem levels and change in dmPFC activity in a contrast that captures one aspect of the positivity bias are consistent with the previous findings.
Furthermore, an important feature of self-positivity bias is that people not only tend to attribute positive traits or outcomes to stable, internal and global personal characteristics but also tend to attribute negative traits or outcomes as unrelated to personal characteristics (Watson et al., 2008). In this study, the NL trials are representative of that aspect of the self-positivity bias (where negative information is rated as low in self-relevance). To that end, the finding showing the positive correlation between levels of self-esteem and change in activity within dmPFC concerning the contrast NL trials vs PL trials further suggests that dmPFC may be involved in processing both components of the self-positivity bias (for those with high self-esteem, self is highly associated with positive but has low association with negative adjectives).
As both the study of Frewen et al. (2013) and this study focus on the individual differences in valenced self-referential processing, we would like to further compare these two fMRI studies. An important advantage of the task in Frewen et al. (2013) is that this task combines indirect and direct measurement of valenced self-relevant processing within a single methodology. In this study, the study task allows for an indirect measurement of valenced self-relevant processing, but the direct measurement of individual difference is provided by the RSE, which cannot separate the distinct cognitive and affective components of self-reflection. Further, the indirect measurement of valenced self-reference processing in Frewen et al. (2013) produces an association between self and valence and encourages attention to that association, which happens to be the disadvantage of this study. However, the task in this study encourages self-reflection by comparing self-relevant information with non-self-relevant information, which is more appropriate than the task of Frewen et al. (2013), in which self-relevant information is compared with ‘other’ information.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
This work was supported by the Key Discipline Fund of National 211 Project, China (NSKD11017). K.D. is supported by Canadian Institutes of Health Research Postdoctoral Fellowship.
REFERENCES
- Craik F, Moroz T, Moscovitch M, et al. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lundberg E, Brimson-Theberge M, Theberge J. Neuroimaging self-esteem: a fMRI study of individual differences in women. Social Cognitive and Affective Neuroscience. 2013;8:546–55. doi: 10.1093/scan/nss032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald AG, Farnham SD. Using the implicit association test to measure self-esteem and self-concept. Journal of Personality and Social Psychology. 2000;79(6):1022–38. doi: 10.1037//0022-3514.79.6.1022. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp J, Li S, et al. Symptom components of standard depression scales and past suicidal behavior. Journal of Affective Disorders. 2005;87(1):73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang S. Desirability, meaningfulness and familiarity ratings of 562 personality-trait adjectives. Psychological Science (In Chinese) 1992;5:17–22. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Lin YC, Lin CH, Raghubir P. Avoiding anxiety, being in denial, or simply stroking self-esteem: why self-positivity? Journal of Consumer Psychology. 2003;13(4):464–77. [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14(6):647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Social Neuroscience. 2009;4(3):197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18(9):1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G. Psychopathology and pathophysiology of the self in depression—neuropsychiatric hypothesis. Journal of Affective Disorders. 2007;104(1–3):1–14. doi: 10.1016/j.jad.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8(3):102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, et al. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28(4):815–26. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50(2):701–8. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Tao R, Zhang S, Li Q, Geng H. Modulation of self-esteem in self- and other-evaluations primed by subliminal and supraliminal faces. PLoS One. 2012;7(10):e47103. doi: 10.1371/journal.pone.0047103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34(6):935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Watson LA, Dritschel B, Jentzsch I, Obonsawin MC. Changes in the relationship between self-reference and emotional valence as a function of dysphoria. British Journal of Psychology. 2008;99(Pt 1):143–52. doi: 10.1348/000712607X248689. [DOI] [PubMed] [Google Scholar]
- Yang J, Dedovic K, Chen W, Zhang Q. Self-esteem modulates dorsal anterior cingulate cortical response in self-referential processing. Neuropsychologia. 2012;50(7):1267–70. doi: 10.1016/j.neuropsychologia.2012.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.